Abstract
The questions of individuality and stability of cues to identity in vocal signals are of considerable importance from theoretical and conservation perspectives. While individuality in alarm calls has been reported for many sciurids, it is not well-documented that the vocal identity encoded in the alarm calls is stable between different encounters with predators. Previous studies of two obligate hibernating rodents, speckled ground squirrels Spermophilus suslicus, and yellow ground squirrels Spermophilus fulvus demonstrated that, after hibernation, most individuals could not be identified reliably by their alarm calls. Moreover, in most speckled ground squirrels, individual patterns of alarm calls changed progressively over as little as 2 weeks. However, these previous data have been obtained using the collection of alarm calls from trapped animals. Here, we examined ten free-ranging dye-marked yellow ground squirrels to determine whether their alarm calls retain the cues to individuality between two encounters of surrogate predators (humans), separated on average by 3 days. Discriminant function analysis showed that the alarm calls of individual yellow ground squirrels were very similar within a recording session, providing very high individual distinctiveness. However, in six of the ten animals, the alarm calls were unstable between recording sessions. Also, we examined ten dye-marked individuals for consistency of acoustic characteristics of their alarm calls between the encounters of humans, differing in techniques of call collection, from free-ranging vs trapped animals. We found differences only in two variables, both related to sound degradation in the environment. Data are discussed in relation to hypotheses explaining the adaptive utility of acoustic individuality in alarm calls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retention of individual vocal traits over time has been studied across a variety of taxa for various reasons. Most studies with nonpasserine birds have aimed to identify stable individual vocal signatures as fingerprints for monitoring rare or secretive species in nature (Lengagne 2001; Puglisi and Adamo 2004; Terry et al. 2005; Grava et al. 2008; Klenova et al. 2009). For whales and dolphins, the main research focus was on stable vocalizations that serve individual recognition (Sayigh et al. 1990; Tyack 1997) or as indicators of group identity (Ford 1991; Rendell and Whitehead 2003). Studies with non-human primates have concentrated on the effects of the social environment on the sustainability of vocal structures (Jones et al. 1993; Snowdon and Elowson 1999; Rukstalis et al. 2003). For ground-dwelling sciurids, the question of stability in vocal signature over time (Matrosova et al. 2009, 2010) has been discussed mainly in relation to the abilities of conspecifics to discriminate individuals by their alarm calls based solely on the acoustic characteristics (Hare 1998; Blumstein and Daniel 2004).
Besides their primary function of warning conspecifics of predators (Sherman 1977; Blumstein 2007) or warning predators that they have been detected by a caller (Woodland et al. 1980; Sherman 1985; Hasson 1991; Digweed and Rendall 2009a,b), alarm calls of at least two species of marmots and five species of ground squirrels also provide concomitant information about the identity of the caller (Nikol’skii and Suchanova 1994; McCowan and Hooper 2002; Blumstein and Munos 2005; Matrosova et al. 2009, 2010; Schneiderová and Policht 2010). However, selection pressures driving the evolution of individual distinctiveness in mammalian alarm calls are not yet clear (Blumstein et al. 2004; Blumstein 2007). Two explanatory hypotheses have been advanced in this regard, the caller reliability hypothesis and the multiple calling hypothesis. According to the caller reliability hypothesis, the callers producing false alarms in the absence of real danger should be considered as unreliable, and their alarms should be ignored by conspecifics (Cheney and Seyfarth 1988; Hare and Atkins 2001; Blumstein et al. 2004). According to the multiple calling hypothesis, simultaneous alarms of multiple callers should result in higher responsiveness of conspecifics compared with those of a single caller, as they signal enhanced urgency of response (Robinson 1981; Weary and Kramer 1995; Blumstein et al. 2004; Sloan and Hare 2006, 2008).
Both hypotheses share the common implicit assumption that the alarm calls convey correct information concerning the identity of a caller. The caller reliability hypothesis also rests on the assumption that individual vocal signatures should be stable at least for some time; otherwise conspecifics could not memorize and distinguish between reliable and unreliable callers. However, most individual speckled and yellow ground squirrels Spermophilus suslicus and Spermophilus fulvus showed unstable alarm calls over a year (Matrosova et al. 2009, 2010) and among individual speckled ground squirrels calls proved unstable between predatory events separated by 2 days or by 2 weeks (Matrosova et al. 2009). Group inherent features, like age or sex, did not have significant effects on the retention of a stable alarm call structure in yellow ground squirrels (Matrosova et al. 2010). However, these findings were obtained with animals emitting alarm calls toward a human from live-traps. Although the collection of alarm calls from animals sitting in live-traps is generally accepted for examining individuality in alarm calls (e.g., Koeppl et al. 1978; Hanson and Coss 2001; Blumstein and Munos 2005), the documented temporal instability in the alarm calls of the two ground squirrel species could be affected by a procedure of recording calls from trapped animals. Also, alarm calls produced by captive squirrels at least have the potential to be very different from free-ranging squirrels. These methodological points could not be addressed earlier in that the retention of the vocal signature has yet to be studied in any free-ranging terrestrial mammal. Also, the alarm calls produced by trapped and free-ranging ground squirrels has not been compared.
Free-living yellow ground squirrels represent convenient subjects to study individuality encoded in alarm calls. Yellow ground squirrels are the largest of the Spermophilus species, with a body length without tail of 230–370 mm, a body mass at emergence from the hibernation of 600–900 g, and a body mass before hibernation of 1,600–2,000 g. These squirrels live in patchy vegetation and thus can be observed visually (Ismagilov 1969; Efimov 2005; Matrosova et al. 2007; Vasilieva and Tchabovsky 2009). Information regarding the individual identity conveyed by alarm calls is potentially important for this species, as yellow ground squirrels demonstrate a male dominance hierarchy during the mating period (Bokshtein et al. 1989), social play between litter mates, and prolonged affiliative contacts between mothers and offspring, up to hibernation (Vasilieva et al. 2009).
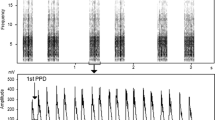
The alarm call of the yellow ground squirrel is the loudest and most common call type and does not vary in structure when produced in response to raptors, terrestrial predators, and humans (Nikol’skii 1979). It consists of frequency-modulated tonal notes, each of about 70 ms duration, with a maximum fundamental frequency of 5–6 kHz emitted in clusters of two to 16 notes (Fig. 1), produced with inter-cluster intervals substantially longer than cluster duration (Nikol’skii 1979; Matrosova et al. 2007, 2010). Here, we examine individual distinctiveness and the short-term stability in the acoustic structure of the alarm call of free-ranging yellow ground squirrels. Also, we examine individual characteristics in alarm calls, produced by the same individual yellow ground squirrels when trapped and when freely ranging.
Materials and methods
Subjects, study site, and dates of work
We recorded alarm calls from 13 yellow ground squirrels (seven adult females, four male juveniles, and two female juveniles, estimated to be between 60 and 70 days postpartum) in their natural colony in Saratov province, Russia (50°43′88″N, 46°46′04″E), from the end of May to mid-June 2008. Five female adults and two female juveniles were recorded three times, once live-trapped, and twice as free-ranging. One female adult and two male juveniles were recorded twice, firstly as live-trapped and secondly as free-ranging. One other female adult and two male juveniles were recorded twice, both times as free-ranging; given that we had two samples, each of ten individuals (with seven individuals presented in both). The sample “first field vs second field” included six female adults and two male and two female juveniles, recorded twice as free-ranging. The sample “live-trapped vs free-ranging” included six female adults and two male and two female juveniles, recorded firstly as live-trapped and secondly as free-ranging. Adult males were not recorded because during these dates they actively foraged before entering hibernation at the end of June and responding to danger escaped to their burrows without calls. Before the study, all the animals were captured either with loops or with wire-mesh live-traps of original construction without bait (see details in Matrosova et al. 2010) and individually marked with both microchips (Bayer AG, Leverhusen, Germany) and dye marks; urzol black D for fur (p-phenylenediamine; Rhodia, Paris, France) for individual identification in the field. For acoustic recording, the live-trapped subjects were placed singly in wire-mesh hutches 30 × 15 × 15 cm. All acoustic recordings from animals sitting in hutches were made within 1 h of capture.
Call recording
The free-ranging subjects emitted alarm calls toward a researcher approaching, standing, or sitting within 3–50 m (Supplementary movie). On average, a recording session lasted until a target caller ceased its vocal activity or dropped into its burrow. The live-trapped subjects emitted calls from the hutch toward a human observer, sitting, or moving within 2–12 m. The range of distances during recordings from free-ranging and trapped animals was within the range of natural variation of propagation of alarm calls throughout the colony to potential conspecific or predatory receivers. On average, a recording session lasted 3–4 min and provided 30–40 alarm call clusters per animal. For recordings (48 kHz sampling frequency), we used a CF Marantz PMD-660 recorder with Sennheiser K6 ME-64 (Sennheiser electronic, Wedemark, Germany) cardioid electret condenser microphone (frequency response of 40–20,000 Hz). The level of recording was adjusted for distance and relative SPL.
Call samples
Two call sets were created, according to the two samples of subjects and recordings: first field vs second field and live-trapped vs free-ranging. Call samples first field vs second field and live-trapped vs free-ranging overlapped partially (for seven animals, first field individual call sets were used also as free-ranging call sets), but were used in separate analyses.
The call sample first field vs second field contained calls taken from two successive recordings per each of ten free-ranging individuals, separated by \( {\hbox{mean}}\pm {\hbox{SD}} = 2.9\pm 1.4\,{\hbox{days}} \), hereafter referred to as first field and second field call samples respectively. From each recording, we took measurements from ten randomly selected alarm call clusters of good quality, i.e., not disrupted by wind and non-overlapped by noise (six recordings provided only three to six clusters). From the total number of 20 recordings, we took measurements from 173 clusters; 89 clusters for the first field and 84 clusters for the second field recordings.
The call sample live-trapped vs free-ranging contained calls taken from ten individuals, two recordings per animal, separated by \( {\hbox{mean}}\pm {\hbox{SD}} = 3.9\pm 2.7\,{\hbox{days}} \), hereafter referred to as live-trapped and free-ranging call samples, respectively. From each recording, we took measurements from ten randomly selected alarm call clusters of good quality. From the total number of 20 recordings, we took measurements from 189 clusters; 100 clusters for the live-trapped and 89 clusters for the free-ranging recording conditions.
Call analysis
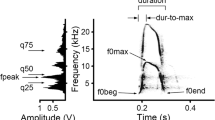
Calls were analyzed spectrographically using Avisoft SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany) at 24 kHz sampling frequency, 16-bit resolution after high-pass filtration at 1 kHz to remove background noise. Spectrograms were created with Hamming window, FFT 1,024 points, frame 50%, and overlap 96.87%. From each recording, we took measurements of six note parameters and two cluster parameters (Fig. 1). These parameters were selected previously as the least correlated of 18 parameters on the basis of the principal component analysis (Matrosova et al. 2010). Since many clusters consisted of only two notes and the first note in a cluster typically showed slightly higher within individual acoustic variability compared with all subsequent notes, which were very similar to each other, we took all the note parameters from the second note in each cluster (Matrosova et al. 2010). From the screen with the reticule cursor, we measured the following note parameters: three fundamental frequency parameters (f0 st, the fundamental frequency at start of a note; f0 max, the maximum fundamental frequency of a note; f0 end, the fundamental frequency at the end of a note), two duration parameters (dur st-max, the time period from the beginning of a note to the point of maximum fundamental frequency of a note; dur max-end, the time period from the point of maximum fundamental frequency to the end of a note), and one power spectrum parameter (quart 1, the lower quartile of a note; Fig. 1). For each cluster, we measured the period from the start of the first to the start of the second note (period 1–2) and calculated the difference between the maximum fundamental frequencies of the first and the second note (df max 1–2). All measurements were exported automatically to Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Statistical analyses
All statistical analyses were made with STATISTICA, v. 6.0 (StatSoft, Tulsa, OK, USA), all tests were two-tailed, and differences were considered significant where p < 0.05. We applied parametrical tests, as a Kolmogorov–Smirnov test showed that distributions of parameter values departed from normality (p < 0.05) in only two of 160 (1.25%) comparisons. We used discriminant function analysis (DFA) to calculate the probability of the assignment of alarm calls to the correct individual for each call sample (of first field, second field, live-trapped and free-ranging recordings). We included all eight alarm call parameters in the DFA. We classified calls from the test sets (of second field and of free-ranging recordings) with DFA function derived from the training call sets (of first field and of live-trapped recordings, respectively), considering the value of the correct cross-validation as a measure of retention of individuality over time (Tripp and Otter 2006; Klenova et al. 2009; Matrosova et al. 2009, 2010). With a 2 × 2 χ 2 test, we compared the obtained values of correct assignment of calls from each recording. We used a repeated-measures ANOVA to compare the individual mean parameter values for calls from the first field vs second field recordings and from live-trapped vs free-ranging recordings, as a Kolmogorov–Smirnov test showed that distributions of parameter values did not depart from normality (p > 0.20) in all comparisons.
We calculated the expected level of correct classification with DFA if the calls we analyzed were randomly distributed among individuals (Solow 1990). To perform each randomization analysis, 500 permutation procedures with macros, specially created for STATISTICA software, were used. Using a distribution obtained by the permutation, we noted whether the observed value exceeded 95% or 99% of the values within the distribution (Solow 1990; Klenova et al. 2008; Matrosova et al. 2010).
Results
First field vs second field recordings
DFA showed 98.9% correct assignment to individual of alarm calls for the first field recording (Wilks' lambda = 0.00002; F 72,445 = 30.92; p < 0.001) and 98.8% correct assignment of alarm calls for the second field recording (Wilks' lambda = 0.00015; F 72,415 = 18.78; p < 0.001) (Table 1, Fig. 2a). The correct assignment values did not differ significantly from each other \( \left( {\chi_1^2 = 0.45;\,p = 0.51} \right) \), and both significantly (p < 0.01) exceeded the random values (33.6% and 33.5%, respectively) calculated using the randomization procedure.
DFA values for assignment of alarm calls to individual (white bars); cross-validation values (striped bars); and random values, calculated with a randomization procedure (black bars). Comparisons with χ 2 test between observed and random values are shown by solid-line brackets and comparisons for first field vs second field (a) and live-trapped vs free-ranging (b) recordings and cross-validation values are shown by dashed brackets
Cross-validation of the test set (second field recording) with discriminant functions of the training set (first field recording) showed a strong and significant decrease in the correct assignment of calls to individuals to as few as 38.1% \( \left( {\chi_1^2 = 75.13;\,p < 0.001} \right) \) (Table 1). The value of correct assignment received with cross-validation did not differ significantly (p = 0.18) from the random value (33.6%), calculated with the randomization procedure (Fig. 2a).
Correct assignment of alarm calls to individual with DFA always exceeded 90% (Table 1). However, after cross-validation of calls of the second field recording with discriminant functions derived from calls of the first field recording, alarm calls of only four of the ten subject animals (three adult and one juvenile) could be distinguished from the total call sample with a probability exceeding 50%. Alarm calls of the remaining six individuals after cross-validation showed correct assignment values lower than the random value (Table 1).
Comparison of the individual mean parameter values between alarm calls of the first field and of the second field recordings with repeated-measures ANOVA showed a significant increase of values only for the lower quartile of a note (Table 2). Overall then, there was little evidence of a directional shift in the alarm call parameter values between the first field and second field recordings.
Live-trapped vs free-ranging recordings
DFA showed 100% correct assignment to individual of alarm calls for the live-trapped recording (Wilks' lambda = 0.00007; F 72,512 = 27.12; p < 0.001) and 97.8% correct assignment of alarm calls for the free-ranging recording (Wilks' lambda = 0.00006; F 72,445 = 24.12; p < 0.001) (Table 3, Fig. 2b). The correct assignment values did not differ significantly from each other \( \left( {\chi_1^2 = 0.63;\,p = 0.43} \right) \), and both significantly (p < 0.001) exceeded the random values (29.6% and 32.0%, respectively), calculated using the randomization procedure.
Cross-validation of the test set (free-ranging recording) with discriminant functions of the training set (live-trapped recording) showed a strong and significant decrease in the correct assignment of calls to individuals to as few as 14.6% \( \left( {\chi_1^2 = 139.3;\,p < 0.001} \right) \) (Table 3, Fig. 2b). The value of correct assignment received with cross-validation was significantly lower (p < 0.01) than the random value (29.6%), calculated with the randomization procedure (Fig. 2b).
Correct assignment of alarm calls to individual with DFA always exceeded 90% (Table 3). However, after cross-validation of calls of the free-ranging recording with discriminant functions derived from calls of the live-trapped recording, alarm calls of only two of the ten subject animals could be distinguished from the total call sample with a probability of 100%. Alarm calls of the remaining eight individuals after cross-validation showed the correct assignment value 0% (Table 3).
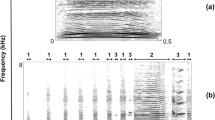
Comparison of the individual mean alarm call parameter values with repeated-measures ANOVA showed significantly lower values for the lower quartile of a note and for the time period from the point of maximum fundamental frequency to the end of the note. Also, it showed significantly higher values for the fundamental frequency at the end of the note for free-ranging compared with live-trapped recordings (Table 2). Differences in values of the last two parameters were related to degradation of calls due to the larger average distance to the microphone for free-ranging vs live-trapped recordings (Fig. 3).
Spectrogram of the alarm call cluster for a live-trapped (distance to microphone 2 m) and b free-ranging (distance to microphone 20 m) recordings, collected from the same female no. 1281. Due to degradation of the end parts of the alarm call notes during propagation through the environment, the point of measuring the fundamental frequency at the end of the note (f0 end) is shifted, what results in the lower f0 end and shorter duration of call notes recorded from the field compared with those from live-trapped animals
Discussion
This study is the first examining the short-term stability of the alarm call structure in a free-ranging mammal. Also, this is the first study examining the consistency of the alarm call characteristics between free-ranging and live-trapped callers. Individuality encoded in alarm calls in yellow ground squirrels was well-expressed within recordings, but decreased between recordings, approaching the random value of correct classification to individual callers. These data are consistent with the results obtained from previous studies conducted with trapped yellow and speckled ground squirrels (Matrosova et al. 2009, 2010) in that alarm calls are very similar within recordings, but show significant structural variation between recordings even over short time periods. We discuss our current findings in relation to two hypotheses advanced to explain the adaptive utility of individuality encoded in the structure of mammalian alarm calls.
Both the caller reliability (Cheney and Seyfarth 1988; Hare and Atkins 2001; Blumstein et al. 2004) and multiple calling hypotheses (Blumstein et al. 2004; Sloan and Hare 2006, 2008) predict that the alarm call structure should exhibit greater similarity within individuals than between individuals. Our data support this prediction. Also, they support the multiple calling hypothesis, as it does not necessarily assume that calls convey caller identity, but rather that calls of multiple signalers can be discriminated as emanating from unique individuals at the time of their production (i.e., that they are discriminated but not identified). Besides yellow ground squirrels, strong keys to individuality in alarm call structure within recordings are reported for Belding's ground squirrels Spermophilus beldingi (Leger et al. 1984; McCowan and Hooper 2002), speckled ground squirrels (Volodin 2005; Matrosova et al. 2009), European ground squirrels Spermophilus citellus and Taurus ground squirrels Spermophilus taurensis (Schneiderová and Policht 2010), steppe marmots Marmota bobak (Nikol'skii and Suchanova 1994) and yellow-bellied marmots Marmota flaviventris (Blumstein and Munos 2005). Also, for Richardson's ground squirrels Spermophilus richardsonii and yellow-bellied marmots, receivers discriminate among individual alarm signalers in playbacks of recorded alarm calls (Hare 1998; Blumstein and Daniel 2004). Thus, our data, together with the related data on other ground-dwelling sciurids, support the multiple calling hypothesis in that strong inter-individual differences in alarm call structure should allow listeners to readily estimate the number of individuals calling simultaneously.
The caller reliability hypothesis also assumes that individual alarms retain their acoustic structure at least for some time, otherwise conspecifics will not be able to memorize individual characteristics of their voices and to distinguish between the reliable and unreliable callers (Hare and Atkins 2001; Blumstein et al. 2004). Our data provide, however, only partial support for the caller reliability hypothesis, as the alarm call structure was kept stable between recordings in only four of ten (40%) of the freely ranging yellow ground squirrels. Comparable low proportions of individuals with stable alarms were also found in studies with trapped ground squirrels, six of 20 (30%) for the speckled ground squirrel (Matrosova et al. 2009) and six of 22 (27%) for the yellow ground squirrel (Matrosova et al. 2010).
Given that, pro and contra the caller reliability hypothesis rests mainly on the question of whether ground squirrels are able to update caller identity information. However, updating the identity information of a caller has yet to be studied in any animal. In case members of a social group are able to update their knowledge about changes in individuals' call parameters in very short time spans, the flexibility in vocal signals might provide reliability only for group members and thus exclude conspecifics which are not frequently present in the direct vicinity. This consideration seems reasonable because yellow ground squirrel groups are mainly based on female kinship (Matrosova et al. 2008). At the same time, the relative lack of stability between alarm call episodes suggests that it should be difficult for an individual ground squirrel to learn the identity of individual callers. If 60% of individuals did not retain stable alarm calls for as few as 3 days, then individuals would have to constantly re-learn the identity of callers on a daily or even hourly basis, what seems unlikely.
Thus, it is reasonable to expect that the alarm calls of the same individual ground squirrel will be very similar in structure within a single encounter with a predator but can differ between predatory events. This feature of alarm communication of ground squirrels can potentially confound the results of playbacks designed for testing the caller reliability hypothesis. Ideally, the effect of this factor can be addressed by taking alarm calls from multiple recording sessions of the same caller in each trial of the “reliable” playback treatment and by taking alarm calls from other recording sessions of the same caller in each trial of the “unreliable” playback treatment, instead of taking alarm calls from different parts of the same recording session within treatment (Hare and Atkins 2001). Also, the facultative stability of individual alarm calls precludes the attractive idea of censuses and individual vocal monitoring of rare European ground squirrels in their highly fragmented colonies inhabited by a small number of individuals (Koshev 2008; Mateju et al. 2008).
Humans as surrogate predators represent traditional objects to provoke alarm calling in ground-dwelling sciurids (Nikol'skii 1979; Slobodchikoff et al. 1991; McCowan and Hooper 2002; Koshev and Pandourski 2008). For many species of ground-dwelling sciurids, people are potential predators, hunting them for food, fur, or as agricultural pests (Slobodchikoff et al. 1991; Shekarova et al. 2008). On the study grids during this work, the appearance of a human in vicinity of the squirrels evoked immediate alarm calling by one or even a few individuals (our unpublished data).
The current findings suggest that the method of acoustic recordings from trapped ground-dwelling sciurids is valid. Significant differences in the lower quartile values between repeated recordings were found in both the experimental designs, in first field vs second field and in live-trapped vs free-ranging. However, alarm call notes recorded from live-trapped yellow ground squirrels were longer in duration and lower in fundamental frequency at the end of the note compared with those of free-ranging squirrels. We can explain this by differences in the distance from a caller to the microphone. In alarm calls of yellow ground squirrels, the end part of a note, exhibiting the fast fall of the fundamental frequency, is rather low in intensity compared with the remaining part of a note (Fig. 3). Accordingly, the end part of alarm call notes is nearly invisible on spectrographic representations of calls recorded distantly from free-ranging individuals, because of substantial environmental degradation. The values of all other alarm call parameters did not show differences induced by the procedures of recording from live-trapped or from free-ranging individuals.
Researchers in the field of vocal communication of ground-dwelling sciurids often apply a method of collecting acoustic recordings from trapped animals, calling toward a human from wire-mesh traps. This method has been applied to reveal individual, age and sex-specific information contained in alarm calls of yellow-bellied marmots (Blumstein and Munos 2005) and speckled ground squirrels (Volodin 2005), to study relations between alarm calling and fecal cortisol in yellow-bellied marmots (Blumstein et al. 2006), to prepare playback stimuli for examination of age differences in responses of California ground squirrels Spermophilus beecheyi (Hanson and Coss 2001), and to examine acoustic properties of Richardson's, Uinta Spermophilus armatus, Columbian Spermophilus columbianus, Wyoming Spermophilus elegans ground squirrels and hybrids between these species (Koeppl et al. 1978). Balph and Balph (1966) reported that all six call types described for freely ranging Uinta ground squirrels occurred also in captured animals in live-traps and during handling. The structural similarity of alarm calls produced by trapped animals toward humans and toward predators under natural conditions suggests that the collection of calls from trapped ground squirrels may serve as a good alternative to the collection of acoustic recordings from free-ranging animals.
References
Balph DM, Balph DF (1966) Sound communication of Uinta ground squirrel. J Mammal 47:440–450
Blumstein DT (2007) The evolution of alarm communication in rodents: structure, function, and the puzzle of apparently altruistic calling in rodents. In: Wolff JO, Sherman PW (eds) Rodent societies. U Chicago Press, Chicago, pp 317–327
Blumstein DT, Daniel JC (2004) Yellow-bellied marmots discriminate among the alarm calls of individuals and are more responsive to the calls from juveniles. Anim Behav 68:1257–1265
Blumstein DT, Munos O (2005) Individual, age and sex-specific information is contained in yellow-bellied marmot alarm calls. Anim Behav 69:353–361
Blumstein DT, Verneyre L, Daniel JC (2004) Reliability and the adaptive utility of discrimination among alarm callers. Proc R Soc B 271:1851–1857
Blumstein DT, Patton ML, Saltzman W (2006) Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol Lett 2:29–32
Bokshtein FM, Kucheruk NV, Tupikova NV (1989) The use of territory and the interrelationships in the yellow ground squirrels (Citellus fulvus Licht, 1823). Russ J Ecol 20(5):45–50
Cheney DL, Seyfarth RM (1988) Assessment of meaning and the detection of unreliable signals in vervet monkeys. Anim Behav 36:477–486
Digweed SM, Rendall D (2009a) Predator-associated vocalizations in North American red squirrels, Tamiasciurus hudsonicus: are alarm calls predator specific? Anim Behav 78:1135–1144
Digweed SM, Rendall D (2009b) Predator-associated vocalizations in North American red squirrels (Tamiasciurus hudsonicus): to whom are alarm calls addressed and how do they function? Ethology 115:1190–1199
Efimov VI (2005) Yellow ground squirrel (Spermophilus fulvus Lichtenstein, 1823). In: Kucheruk VV, Khlyap LA (eds) Lagomorpha and rodents of the Middle Asia’s deserts. GEOS, Moscow, pp 40–65
Ford JKB (1991) Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can J Zool 69:1454–1483
Grava T, Mathevon N, Place E, Balluet P (2008) Individual acoustic monitoring of the European eagle owl Bubo bubo. Ibis 150:279–287
Hanson MT, Coss RG (2001) Age differences in the response of California ground squirrels (Spermophilus beecheyi) to conspecific alarm calls. Ethology 107:259–275
Hare JF (1998) Juvenile Richardson’s ground squirrels, Spermophilus richardsonii, discriminate among individual alarm callers. Anim Behav 55:451–460
Hare JF, Atkins BA (2001) The squirrel that cried wolf: reliability detection by juvenile Richardson’s ground squirrels (Spermophilus richardsonii). Behav Ecol Sociobiol 51:108–112
Hasson O (1991) Pursuit-deterrent signals: communication between prey and predators. Trends Ecol and Evol 6:325–329
Ismagilov MI (1969) Yellow ground squirrel—Citellus fulvus. In: Sludskiy AA (ed) Mammals of Kazakhstan, v 1, rodents (marmots and ground squirrels). Nauka, Alma-Ata, pp 120–159
Jones BS, Harris DHR, Catchpole CK (1993) The stability of the vocal signature in phee calls of the common marmoset Callithrix jacchus. Am J Primatol 31:67–75
Klenova AV, Volodin IA, Volodina EV (2008) Duet structure provides information about pair identity in the red-crowned crane (Grus japonensis). J Ethol 26:317–325
Klenova AV, Volodin IA, Volodina EV (2009) Examination of pair-duet stability to promote long-term monitoring of the endangered red-crowned crane (Grus japonensis). J Ethol 27:401–406
Koeppl JW, Hoffmann RS, Nadler CF (1978) Pattern analysis of acoustical behavior in four species of ground squirrels. J Mammal 59:677–696
Koshev YS (2008) Distribution and status of the European ground squirrel (Spermophilus citellus) in Bulgaria. Lynx (Praha), ns 39:251–261
Koshev Y, Pandourski I (2008) Structure and variability of alarm calls of European ground squirrel (Rodentia: Spermophilus citellus L. 1766) from West Bulgaria. Acta zoologica Bulgarica 60:99–105
Leger WD, Berney-Key SD, Sherman PW (1984) Vocalizations of Belding`s ground squirrels (Spermophilus beldingi). Anim Behav 32:753–764
Lengagne T (2001) Temporal stability in the individual features in the calls of eagle owls (Bubo bubo). Behaviour 138:1407–1419
Mateju J, Nova P, Uhlikova J, Hulova S, Cepakova E (2008) Distribution of the European ground squirrel (Spermophilus citellus) in the Czech Republic in 2002–2008. Lynx (Praha), ns 39:277–294
Matrosova VA, Volodin IA, Volodina EV, Babitsky AF (2007) Pups crying bass: vocal adaptation for avoidance of age-dependent predation risk in ground squirrels? Behav Ecol Sociobiol 62:181–191
Matrosova VA, Volodin IA, Volodina EV (2008) Does kinship affect the alarm call structure in the yellow ground squirrel Spermophilus fulvus? Lynx (Praha) n s 39:295–303
Matrosova VA, Volodin IA, Volodina EV (2009) Short-term and long-term individuality in speckled ground squirrel alarm calls. J Mammal 90:158–166
Matrosova VA, Volodin IA, Volodina EV, Vasilieva NA, Kochetkova AA (2010) Between-year stability of individual alarm calls in the yellow ground squirrel Spermophilus fulvus. J Mammal 91(3) in press
McCowan B, Hooper SL (2002) Individual acoustic variation in Belding’s ground squirrel alarm chirps in the High Sierra Nevada. J Acoust Soc Am 111:1157–1160
Nikol’skii AA (1979) Species specificity of alarm call in sousliks (Citellus, Sciuridae) of Eurasia. Zoologicheskii Zhurnal 58:1183–1194
Nikol’skii AA, Suchanova MV (1994) Individual variability of alarm call in steppe marmot (Marmota bobac Mull., 1776). In: Rumiantsev VY (ed) Actual problems of marmots investigation. ABF Publishing House, Moscow, pp 169–181
Puglisi L, Adamo C (2004) Discrimination of individual voices in male great bitterns (Botaurus stellaris) in Italy. Auk 121:541–547
Rendell L, Whitehead H (2003) Vocal clans in sperm whales (Physeter macrocephalus). Proc R Soc B 270:225–231
Robinson SR (1981) Alarm communication in Belding`s ground squirrels. Z Tierpsychol 56:150–168
Rukstalis M, Fite JE, French JA (2003) Social change affects vocal structure in a callitrichid primate (Callitrix kuhlii). Ethology 109:327–340
Sayigh LS, Tyack PL, Wells RS, Scott MD (1990) Signature whistles of free-ranging bottlenose dolphins Tursiops truncatus: stability and mother-offspring comparisons. Behav Ecol Sociobiol 26:247–260
Schneiderová I, Policht R (2010) Alarm calls of the European ground squirrel Spermophilus citellus and the Taurus ground squirrel S. taurensis encode information about caller identity. Bioacoustics 19(3) in press
Shekarova ON, Neronov VV, Savinetskaya LE (2008) Speckled ground squirrel (Spermophilus suslicus): current distribution, population dynamics and conservation. Lynx (Praha) ns 39:317–322
Sherman PW (1977) Nepotism and the evolution of alarm calls. Science 197:1246–1253
Sherman PW (1985) Alarm calls of Belding’s ground squirrels to aerial predators: nepotism or self-preservation? Behav Ecol Sociobiol 17:313–323
Sloan JL, Hare JF (2006) Adult Richardson’s ground squirrels (Spermophilus richardsonii) ignore rate changes in juvenile alarm calls: age-differential response urgency perception? Ethology 112:896–902
Sloan JL, Hare JF (2008) The more the scarier: adult Richardson’s ground squirrels (Spermophilus richardsonii) assess response urgency via the number of alarm signalers. Ethology 114:436–443
Slobodchikoff CN, Kiriazis J, Fischer C, Creef E (1991) Semantic information distinguishing individual predators in the alarm calls of Gunnison’s prairie dogs. Anim Behav 42:713–719
Snowdon CT, Elowson AM (1999) Pygmy marmosets modify call structure when paired. Ethology 105:893–908
Solow AR (1990) A randomization test for misclassification probability in discriminant analysis. Ecology 71:2379–2382
Terry AMR, Peake TM, McGregor PK (2005) The role of vocal individuality in conservation. Front Zool 2(10): doi:10.1186/1742-9994-2-10
Tripp TM, Otter KA (2006) Vocal individuality as a potential long-term monitoring tool for Western screech-owls, Megascops kennicottii. Can J Zool 84:744–753
Tyack PL (1997) Development and social functions of signature whistles in bottlenose dolphins, Tursiops truncatus. Bioacoustics 8:21–46
Vasilieva NA, Tchabovsky AV (2009) Relationships between social activity, date of emergence and body mass of juveniles in the long-teeth ground squirrel (Spermophilus fulvus, Rodentia, Sciuridae). Zoologicheskii Zhurnal 88:588–595
Vasilieva NA, Savinetskaya LE, Tchabovsky AV (2009) Large body size and short period of activity do not impede fast growth in the long-teeth ground squirrel (Spermophilus fulvus). Zoologicheskii Zhurnal 88:339–343
Volodin IA (2005) Individuality in the alarm call of the speckled suslik Spermophilus suslicus (Rodentia, Sciuridae). Zoologicheskii Zhurnal 84:228–235
Weary DM, Kramer DL (1995) Response of eastern chipmunks to conspecific alarm calls. Anim Behav 49:81–93
Woodland DJ, Jafaar Z, Knight ML (1980) The “pursuit deterrent” function of alarm signals. Amer Natur 115:748–753
Acknowledgements
We thank Prof. S.A. Shilova, Dr. A.V. Tchabovsky, L.E. Savinetskaya, and N.S. Vasiliev for help with data collection. We are sincerely grateful to Dr. A.A. Lisovsky and Dr. V.S. Lebedev for help with statistics and to the four anonymous reviewers for their detailed and encouraging comments and for the correction of English. We are sincerely grateful to Stephen Pollard for his courteous and most helpful corrections of writing and language at the final stage of the revision of the manuscript. During our work, we adhered to the “Guidelines for the treatment of animals in behavioral research and teaching” (Anim. Behav., 2006, 71:245–253) and to the laws of Russian Federation, the country where the research was conducted. This study was supported by the Russian Foundation for Basic Research grants 09-04-00416 (for V.M., I.V., and E.V) and 08-04-00507 (for N.V.), and Russian Science Support Foundation (for N.V).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Movie of a juvenile female yellow ground squirrel producing a few clusters of alarm calls toward a human. Black “collar” is a dye mark. (MPG 8748 kb)
Rights and permissions
About this article
Cite this article
Matrosova, V.A., Volodin, I.A., Volodina, E.V. et al. Stability of acoustic individuality in the alarm calls of wild yellow ground squirrels Spermophilus fulvus and contrasting calls from trapped and free-ranging callers. Naturwissenschaften 97, 707–715 (2010). https://doi.org/10.1007/s00114-010-0686-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0686-7