Abstract
Analyses of the elytral hydrocarbons from male and female emerald ash borer, Agrilus planipennis Fairmaire, that were freshly emerged vs. sexually mature (>10 days old) revealed a female-specific compound, 9-methyl-pentacosane (9-Me-C25), only present in sexually mature females. This material was synthesized by the Wittig reaction of 2-decanone with (n-hexadecyl)-triphenylphosphonium bromide followed by catalytic reduction to yield racemic 9-Me C25, which matched the natural compound by gas chromatography/mass spectrometry (retention time and EI mass spectrum). In field bioassays with freeze-killed sexually mature A. planipennis females, feral males spent significantly more time in contact and attempting copulation with unwashed females than with females that had been washed in n-hexane to remove the cuticular lipids. Hexane-washed females to which 9-Me-C25 had been reapplied elicited similar contact time and percentage of time attempting copulation as unwashed females, indicating that 9-methyl-pentacosane is a contact sex pheromone component of A. planipennis. This is the first contact sex pheromone identified in the Buprestidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The waxy layer on the cuticle of insects provides a hydrophobic barrier that prevents desiccation (Gibbs 1998) and may also provide patterns for mimicry or camouflage, repel excess rainwater, reflect solar radiation, and give species-specific olfactory cues (Gullan and Cranston 2005). Some of the hydrocarbon components also function as contact pheromones (Howard and Blomquist 2005). There is good evidence that in the Cerambycidae, mate recognition is mediated by contact chemoreception with this waxy layer wherein males align their body and mount females only after antennal contact with the cuticle of the female (live or freeze-killed) with subsequent copulatory behavior and coupling to the females' genitalia (Kim et al. 1993; Fukaya et al. 1996, 1997, 2000; Wang 1998; Ginzel et al. 2003a, b; Ginzel and Hanks 2003; Barbour et al. 2007). Whether or not contact chemoreception is important in the Buprestidae is unknown.
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is an invasive buprestid species originating from Asia that has caused extensive mortality of ash trees (Fraxinus spp. L.; Oleaceae) since its introduction into the USA and Canada (Haack et al. 2002; Cappaert et al. 2005; Poland and McCullough 2006) and its continued spread in North America threatens all native ash species (Wei et al. 2004; Poland and McCullough 2006). Movement of infested firewood and nursery stock has exacerbated the natural spread of EAB (Marchant 2006), and large-scale devastation of ash trees has occurred in both urban and rural environments. Monitoring of this rapid spread has proven challenging because it is very difficult to detect low to moderate densities of EAB using visual surveys (Poland and McCullough 2006), and evidence to date suggests that EAB uses visual cues rather than pheromones to locate mates (Otis et al. 2005; Rodriguez-Saona et al. 2007; Lelito et al. 2007, 2008). Trap trees, consisting of girdled ash trees with sticky bands, have detected new outlier EAB infestations but they are labor intensive (Poland and McCullough 2006). Purple-colored sticky traps catch EAB at a higher rate than any other color tested (Francese et al. 2005), particularly when these traps are baited with host volatiles, either sesquiterpenes found in ash bark (Crook et al. 2008) or ash leaf volatiles (Rodriguez-Saona et al. 2006; de Groot et al. 2008). Although an antennally active compound emitted primarily by female EAB has been identified (Bartelt et al. 2007), its biological activity has yet to be demonstrated. Improved detection methods and management tools are urgently required but there is a paucity of information on the chemical ecology of this insect.
The mating biology of the EAB is poorly understood. For other buprestids, it has been suggested that individuals locate a susceptible host and then search for mates using visual and tactile cues (Carlson and Knight 1969; Gwynne and Rentz 1983). Dunn and Potter (1988) observed greater catches of male twolined chestnut borer, Agrilus bilineatus (Weber), in cages containing conspecific females than in empty cages and suggested that the attraction could be due either to a pheromone or to auditory cues produced by the females. Visual cues have clearly been demonstrated to be important in orienting EAB males to potential mates (Otis et al. 2005; Lelito et al. 2007, 2008). Lelito et al. (2007) coined the term “paratrooper copulation” for the way in which hovering EAB males would rapidly descend from heights of 0.3–1.0 m above the foliage directly onto the backs of EAB females. Dead EAB adults of either sex, whether they had been washed in solvent or not, elicited the same number of approaches by feral male EAB; in addition, males spent comparatively more time investigating unwashed females than males or washed females, suggesting the presence of a contact pheromone on the cuticle of female EAB (Lelito et al. 2007). The identity or chemical composition of this putative contact pheromone was not determined.
Information on the identity and biological activity of an EAB contact pheromone would increase our understanding of the mating ecology of this and other buprestid species. We report here the identification and biological activity of a female-produced contact sex pheromone for EAB.
Materials and methods
Source of beetles
Trees infested with overwintering larvae of EAB were located in Windsor and Toronto, Ontario, felled, cut into bolts, transported in secure containers to the Great Lakes Forestry Centre in Sault Ste. Marie, Ontario, and placed into cold storage in a secure quarantine facility. As required, bolts were removed from cold storage and placed in rearing cages housed at 24°C and a 16:8 h light/dark (L/D) photoperiod. Upon emergence, adults were separated by sex (Rodriguez-Saona et al. 2007). Males and females (×6 of each sex) were used for chemical analysis. One set was frozen on the day of emergence, and the rest were reared for 10–14 days to produce sexually mature adults before being frozen. Three subsets were analyzed in total. Previous studies have demonstrated that mating only occurs in EAB once females are a minimum of 5–7 days from eclosion (Bauer et al. 2003; Cappaert et al. 2005; Rodriguez-Saona et al. 2007). Adults were maintained for 10–14 days at 21°C, 50% relative humidity (RH), and a 16:8 h L/D cycle and were supplied with water and fresh foliage of evergreen ash, Fraxinus uhdei (Wenzig) Linglesh, or green ash, Fraxinus pennsylvanica Marsh.
Identification of cuticular hydrocarbons
The relative abundances of cuticular hydrocarbons were determined using a manual solid-phase microextraction (SPME) assembly with a red 100-µm polydimethylsiloxane fiber (Supelco, PA, USA). Insects were held with forceps, and the fiber was wiped across the elytra for about 30 s while rotating but not bending it. For each sex and maturity class (i.e., 1-day-old males, 1-day-old females, 10- to 14-day-old males, 10- to 14-day-old females), six individuals were sampled and analyzed as composites.
The SPME samples and n-hexane rinses of individual males and females were analyzed by gas chromatography/mass spectrometry (GC/MS) on a Hewlett-Packard 5890 GC and a 5971 mass selective detector in the electron impact (EI, 70 eV) mode. The column used for analysis was a Supelco SPB-5 capillary (30 m × 0.32 mm × 0.25 µm film) in the splitless mode with helium as the carrier gas. The injection port was set at 280°C for solvent extracts and 250°C for SPME fibers. The oven temperature was programmed from 70°C, held for 1 min, and then increased at 10°C/min to 240°C and held for 30 min. Hydrocarbons were identified by comparing mass spectra and retention times with those of synthetic standards with reference to the parent M+ and molecular formulae. Diagnostic mass spectral fragments unequivocally demonstrated the position of methyl branches (Nelson and Blomquist 1995).
Synthesis of racemic 9-methyl-pentacosane
9-Methyl-pentacosane (9-methyl-C25) was prepared from base-catalyzed Wittig coupling of 2-decanone with (n-hexadecyl) triphenylphosphonium bromide and concomitant Pd/C hydrogenation of the resultant alkene (Ginzel et al. 2003a). All other methyl-branched compounds were synthesized in our laboratory in a similar manner and purified (>98%). N-Alkanes were obtained from Sigma-Aldrich (Oakville, ON, Canada) or Alltech (Deerfield, IL, USA) and used as received (>98% pure by GC/MS).
Field bioassays
Preparation of female treatments
A total of 78 EAB females that emerged from 10 to 15 July 2008 were reared for the field bioassay. Female EAB were reared in the quarantine facilities, as described above, until the age of 10–14 days, when they were frozen. Prior to the field bioassay, females were removed from the freezer and thawed for 2–3 h. For 52 of the females, each female was individually rinsed in 1 ml of hexane for about 2 min. Females were then allowed to dry for at least 30 min before being rinsed again. Females were rinsed a total of three times for 2 min each time and left to dry overnight after the final rinsing. All 78 females were then individually pinned through the right elytra at its base; the heads of the pins were then snipped off such that the end of the pin was only about 1 mm above the surface of the female's body and thus would not interfere with approaches by feral male EAB. Twenty-six of the rinsed females were then individually treated with 1 µl of a 5 mg/ml concentration of 9-methyl-C25 in hexane (one female equivalent, see “Results”) and allowed to dry. This divided the 78 dead females (hereafter referred to as “dummy” females) into three treatment groups: (1) unwashed, (2) washed, and (3) washed then treated (hereafter referred to as “treated”). The treatment groups were indicated by pinning a small paper insect “point” underneath the female that was not visible from above. The points were colored white (unwashed), yellow (washed), or blue (washed then treated). The paper points were shorter and narrower than the females' abdomen, were not visible from above, and did not appear to influence the behavior of feral males (pers. obs. of K L Ryall). Dummy females were kept at 3–4°C in a fridge for 2 days until used in the bioassay and were transported to the field in a cooler.
Behavioral field bioassay
The field bioassay was conducted from 7 to 11 July 2008 at a site of moderate EAB infestation about 1.8 km northeast of Port Lambton, ON (42° 40.16′ N, 82° 29.56′ W) within the generally infested area. Observations of feral males were made between 1,000 and 1,830 EDT, based on reports of EAB's most active time of day (Lelito et al. 2007; Rodriguez-Saona et al. 2007). About 40 h was spent observing feral males and collecting data on mating behavior. Trees were chosen based on the presence of both epicormic shoots and feral EAB males on the foliage. Dummy females were pinned to foliage on epicormic shoots of infested ash trees, growing from the base of the tree up to about 1.5 m above the ground. Females were placed and removed from foliage by grasping the pin underneath the female with tweezers and were never touched directly to avoid cross-contamination. All females were pinned on south-facing foliage so that they would be in direct sunlight; females were pinned through the mid-vein of the center of each leaf, oriented parallel with the vein with the head facing towards the tree. Treatments were laid out in groups of three with dummy females spaced 20–30 cm apart and randomly assigned to position in a triangle shape and with 30–40 cm spacing between groups. Three to seven groups of three females were set up at different times on different trees, based on the amount of foliage available.
Observations of dummy females began once all females were established on the foliage, and lasted for about 1 h per observation session with breaks of 5–10 min in between observation sessions. We recorded the time of arrival of each male observed to land on a female and then the time of departure for that same male to obtain its total time spent in contact with a given female. The locations of the various treatments were not recorded, so the observations on time spent in contact by males was collected by observers who were unaware of the treatment. The treatment group (unwashed, treated, washed) was then recorded by checking for the presence of the colored insect point under the female's abdomen after the male had flown away. For a subset of males that spent several minutes or more in contact with a given female (n = 7, 7, and 10 for unwashed, treated, and washed females, respectively), we recorded approximately every minute whether the male was attempting to mate with the female or whether he was merely in contact with her; from this, we calculated the percentage of time spent attempting to mate vs. time spent simply in contact with the females.
Data on the amount of time individual males spent in contact and the percentage of that time spent attempting to mate with females of the three different treatments were analyzed by one-way ANOVA after logarithmic transformation of data to meet the assumptions of normality of data and equality of variance; means were compared using Holm–Sidak post hoc tests. All analyses were conducted using SigmaStat (Vers. 3.5).
Results
GC/MS analyses
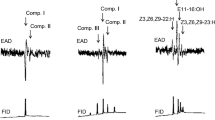
Analysis of the composite SPME samples from the cuticle of EAB revealed no differences in the hydrocarbon profiles of males and females that were 1 day old (i.e., sexually immature) or between 1- and 10- to 14-day-old males, but one compound was present exclusively in 10- to 14-day-old (sexually mature) females (Fig. 1a). This compound was 9-methyl-pentacosane (9-Me-C25) located by specific ion scanning and identified by EI mass spectrometry (Fig. 1c). The chromatograms appear identical at first sight because, under the conditions used, the 13-, 11-, and 9-Me-C25 isomers do not separate (Fig. 1a, peak no. 10), and the latter was, therefore, cryptically hidden (cf. Fig. 1b). We synthesized racemic 9-Me-C25 by the Wittig reaction of 2-decanone with (n-hexadecyl)-triphenylphosphonium bromide followed by catalytic reduction, and it matched the natural compound by GC/MS (retention time and EI mass spectrum). The compound was observed exclusively in sexually mature females in SPME samples obtained from three cohorts of immature and mature male and female EAB and was therefore considered a potential contact sex pheromone for EAB. It was estimated to be present on the elytra at ca. 5–10 µg/female. (This estimate was obtained by reapplying 1.0 μl of solutions of 9-Me-C25 in duplicate in hexane at concentrations of 0.1, 1.0, and 10.0 μg/μl onto freeze-killed rinsed females and determining and comparing peak areas of selected ions by resampling with SPME and GC/MS analysis as before.)
SPME analysis of the cuticular hydrocarbons from the elytra of 10 – to 14-day-old female (a) and male (b) emerald ash borer. c The EI mass spectrum of natural 9-Me-C25 found on 14-day-old females. Analysis conditions are described in the text. Numbers on peaks refer to compounds in Table 1; x = fiber impurity; y-axis is relative ion intensity
Observations and field bioassays
Over 100 males were observed to land and make contact with dummy females during the field bioassay. Most males were observed to arrive in the “paratrooper” manner (Lelito et al. 2007), flying in suddenly and landing in precise orientation on the pinned female. However, several males landed on adjacent leaves or on a different location on the same leaf as the female and spent several minutes walking around the foliage before they aligned themselves along the length of the pinned female and attempted copulation. When departing, some males immediately flew away from the female, whereas others walked onto the leaf and proceeded to feed along the edge of the leaf for several minutes before flying away. Some males were individually recognizable due to characteristic marks on their elytra, and we observed the same male make contact with several different dummy females at different times during the observation periods.
Similar numbers of feral males landed on females of the three different treatments, with 36 landing on unwashed females, 27 on treated females, and 43 on washed females (χ 2 = 1.809, df = 2, P = 0.405). Feral males spent the most time in contact with unwashed females and treated females and the least time on washed females (F = 11.7, df = 2, 103, P < 0.001; Fig. 2a). The time that males spent on females treated with 9-Me-C25 was significantly longer than that spent on washed females but no different from the time spent on unwashed females (Holm–Sidak test, P = 0.05; Fig. 2a). In addition, males spent a significantly greater proportion of their contact time attempting to mate with unwashed and 9-Me-C25-treated females (60%–70%) than they spent attempting to mate with washed females (20%; F = 29.349, df = 2,21, P < 0.001; ANOVA and Holm–Sidak test, P = 0.05; Fig. 2b).
Bars denote a the mean (± SE) number of minutes that feral EAB males spent in contact with dead EAB females of three different treatments and b the mean (± SE) percentage of total contact time that a subset of feral males spent attempting to copulate with dead EAB females of three different treatments. The three treatments were unwashed females with cuticular hydrocarbons intact, treated females with cuticular hydrocarbons rinsed off with hexane then treated with putative synthetic contact pheromone (9-Me-C25), and washed females that had been rinsed in hexane to remove cuticular hydrocarbons. Means with different letters differed significantly (one-way ANOVA and Holm–Sidak multiple comparison test; P < 0.05)
Discussion
The use of cuticular hydrocarbons for mate recognition has been shown in many insect genera (Howard and Blomquist 2005) but little is known about the chemical ecology and reproductive behavior of buprestids. Here, we provide evidence for contact pheromones on the cuticle of sexually mature female emerald ash borers that facilitate mate recognition and elicit copulatory behavior in feral males, and we identify 9-methyl-pentacosane (9-Me-C25) as a contact pheromone—the first contact pheromone identified in the Buprestidae family. This discovery improves our understanding of the chemical ecology of the EAB and other buprestids and may have potential applications for management of this invasive species.
Our observations that males remained in contact much longer with unwashed than hexane-washed dead female EAB indicate the presence of contact pheromones on the female cuticle and support earlier observations by Lelito et al. (2007). Analysis of EAB cuticular hydrocarbons revealed nearly identical chemical profiles in males and females, except for one compound, 9-Me-C25, that was present only in females that were sexually mature. Reapplication of synthetic 9-Me-C25 to hexane-rinsed females restored almost all contact and copulatory activity in field bioassays, i.e., the time feral males spent in contact with treated females and the percentage of that time spent in attempted copulation was significantly greater than that observed with hexane-washed females and not significantly different from that observed with unwashed females. These data support the hypothesis that 9-Me-C25 is a contact pheromone that triggers mate recognition and copulatory behavior in EAB. 9-Me-C25 is also one of the cuticular hydrocarbons that make up the contact pheromone on females of the cerambycid Xylotrechus colonus F. (Ginzel et al. 2003b).
Our results also confirm previous reports on the importance of visual cues in mate location (Otis et al. 2005; Lelito et al. 2007, 2008). We observed feral EAB males landing in similar numbers on dead females from the three different treatments, and similarly, Lelito et al. (2007) reported the same numbers of males landing on dead, washed, and unwashed male and female EAB. We only tested male response to dummy females but Rodriguez-Saona et al. (2007) reported that males discriminated among males and females while in flight, with 77% of feral EAB males landing on females. Tactile cues may also be used by male EAB because they immediately aligned themselves with the female's body upon contact, and some males remained in contact for a number of minutes even with washed females.
The paratrooper copulation behavior of male EAB (Lelito et al. 2007) was the most frequently observed mode of contact, but we also observed males landing either on the same leaf as the pinned females or on adjacent leaves and walking around for several minutes before contacting the female and attempting to mate. This behavior suggests that the visual cue provided by a resting female to a male in flight is an important one, but perhaps is not used exclusively by males to detect females. In laboratory bioassays, Pureswaran and Poland (2008) provide evidence that at close range (≤5 cm), volatile cues from female EAB, detected by EAB males using their antennae, may be involved in mate finding.
One major difference between our results and those of Lelito et al. (2007) is the length of time males spent in contact with females. Lelito et al. (2007) reported that males spent about 4 min on unwashed females and <30 s on average with washed females, whereas we recorded contacts at least five times as long for the same treatments. The reason for this is unclear. It is possible that our hexane washes did not remove 100% of the cuticular hydrocarbons; we rinsed each female three times in n-hexane for about 2 min, whereas Lelito et al. (2007) rinsed their EAB twice in dichloromethane for 10 min each. Despite the shorter washing time used in our study, it is known that hexane readily dissolves hydrocarbons of this nature, thus effectively removing them from the female's cuticle. This technique has also been successfully used in a number of studies on cerambycid contact pheromones (Ginzel and Hanks 2003; Ginzel et al. 2003a, b). Regardless, the washing in hexane definitely removed an important chemical cue, as clearly evidenced by the shorter periods of time males spent in contact with such females.
The fact that feral male EAB spent a considerable amount of time in contact with the unwashed and treated females supports the use of freeze-killed EAB females as an effective technique for observing EAB mate location and mating behavior. Numerous previous studies with cerambycids and buprestids have successfully used a similar method to investigate male mating behavior (Ginzel and Hanks 2003; Ginzel et al. 2003a, b; Lelito et al. 2007). Our observations of several natural EAB mating pairs indicated that mating lasted for at least 30 min, compared with about 17 and 2.5 min of attempted copulation with unwashed and washed dead females, respectively. Previous reports on duration of copulation in natural pairs of Agrilus sp. ranged from 20 to 90 min (Chinese Academy of Science 1986; Yu 1992; Rodriguez-Saona et al. 2007). Live females may provide additional behavioral cues (movement, auditory) that sustain copulation. It is also possible that feral females may have provided stronger olfactory cues than our unwashed freeze-killed females because cuticular hydrocarbons have some volatility, and concentrations on the cuticle may decline over time. Live females were not used in this study because of quarantine restrictions against the movement of living specimens; in addition, the use of feral females would not be possible because their age and mating status would be unknown. Regardless, using freeze-killed females removes any such additional behavioral or olfactory cues that may be produced by living females, reducing confounding influences. Hence, any changes in male response in our experiment were strictly due to the washing and reapplication treatments.
In insects, hydrocarbons are synthesized in oenocytes associated with either the fat body or epidermal tissue (Howard and Blomquist 2005) and are carried through the hemolymph by high-density lipoproteins such as lipophorin (Schal et al. 2001 and references therein). The appearance of 9-Me-C25 on only the cuticle of 10- to 14-day-old females suggests that its presence may be associated with sexual maturation or, more specifically, vitellogenesis (egg maturation) in the EAB. In the house fly, Musca domestica L., the amount of its sex pheromone ((Z)-9-tricosene) on the female's cuticle increased significantly from 1 to 6 days following eclosion, coinciding with the production of ovarian ecdysteroids and the progression of vitellogenesis (Dillwith et al. 1983; Schal et al. 2001). Differences in composition of cuticular hydrocarbons among adults within the same species have also been observed in a number of social insects, often in association with reproductive status (Howard and Blomquist 2005). For example, in the ponerine ant, Dinoponera quadriceps Kempf, the dominant reproductive female has much greater amounts of the cuticular hydrocarbon, 9-hentriacontene, than her sterile nestmates (Monnin et al. 1998).
Biosynthesis of methyl-branched hydrocarbons such as 9-Me-C25 likely occurs through elongation of fatty acid CoAs, with the methyl branch arising from methyl malonyl CoA in place of malonyl CoA at a specific point during chain elongation (Nelson and Blomquist 1995). Little is known, however, about the stereochemistry of methyl branches, and this requires investigation because contact chemoreception may have important chiral specificity. 9-Me-C25 has a chiral carbon, but current technology does not allow separation of the two possible enantiomers. The EAB males responded to the racemic synthetic 9-Me-C25 compound in our field trials, but it is possible that only one enantiomer is present on the unwashed EAB female cuticle and that males respond only to that enantiomer. If so, that may explain the slightly lower contact time and copulatory response to females treated with one female equivalent of racemic 9-Me-C25; each enantiomer would be present at only half the concentration of that on an unwashed female if only one enantiomer is naturally present. Synthesis of 9R- and 9S-enantiomers of 9-Me-C25 is underway, and their activity will be reported elsewhere. Further work should include optimization of application rate of the contact pheromone and testing of each enantiomer vs. the racemic blend. Other orientation mechanisms that need further exploration include potential long-range pheromones that may attract beetles (Bartelt et al. 2007), attractive host volatiles (Rodriguez-Saona et al. 2006; Crook et al. 2008; de Groot et al. 2008), and other visual and chemical cues (Pureswaran and Poland 2008) used in mate location. All of these mechanisms have potential for developing improved detection survey and management tools for this damaging invasive species.
References
Barbour JD, Lacey ES, Hanks LM (2007) Cuticular hydrocarbons mediate mate recognition in a species of longhorned beetle (Coleoptera: Cerambycidae) of the primitive subfamily Prioninae. Ann Entomol Soc Am 100:333–338
Bartelt R, Cossé AA, Zilkowski BW, Fraser I (2007) Antennally active macrolide from the emerald ash borer Agrilus planipennis emitted predominantly by females. J Chem Ecol 33:1299–1302
Bauer LS, Haack RA, Miller DL, Liu H, Petrice T (2003) Laboratory rearing of emerald ash borer. In: Mastro V, Reardon R (comps) Emerald Ash Borer Research and Technology Development Meeting, Port Huron, MI, 30 Sept–1 Oct 2003 (proceedings). pp 36–37 (online) URL: http://www.invasive.org/eab/eab2003.pdf
Cappaert D, McCullough DG, Poland TM, Siegert NW (2005) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51:152–165
Carlson RW, Knight FB (1969) Biology, taxonomy, and evolution of four sympatric Agrilus beetles (Coleoptera: Buprestidae). Contrib Am Entomol Inst 4:1–15
Chinese Academy of Science, Institute of Zoology (1986) Agrilus marcopoli Obenberger. In: Agricultural Insects of China (part I). p 445 China Agricultural Press, Beijing, China
Crook DJ, Krimian A, Francese J, Fraser I, Poland TM, Sawyer AJ, Mastro V (2008) Development of a host-based semiochemical lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Environ Entomol 37:356–365
de Groot P, Grant GG, Poland TM, Scharbach R, Buchan L, Nott RW, Macdonald L, Pitt D (2008) Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. J Chem Ecol 34:1170–1179
Dillwith JW, Adams TS, Blomquist GJ (1983) Correlation of housefly sex pheromone production with ovarian development. J Insect Physiol 29:377–386
Dunn JP, Potter DA (1988) Evidence for sexual attraction by the twolined chestnut borer, Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Can Entomol 120:1037–1039
Francese JA, Mastro VC, Oliver JB, Lance DR, Youseff N, Lavalee SG (2005) Evaluation of colors for trapping Agrilus planipennis (Coleoptera: Buprestidae). J Entomol Sci 40:93–95
Fukaya M, Yasuda T, Wakamura S, Honda H (1996) Reproductive biology of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). III. Identification of contact sex pheromone on female body surface. J Chem Ecol 22:259–270
Fukaya M, Wakamura S, Yasuda T, Senda S, Omata T, Fukusaki E (1997) Sex pheromone activity of geometric and optical isomers of synthetic contact pheromone to males of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl Entomol Zool 32:654–656
Fukaya M, Akino T, Yasuda T, Wakamura S, Satoda S, Senda S (2000) Hydrocarbon components in contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae), and pheromonal activity of synthetic hydrocarbons. Entomol Sci 3:211–218
Gibbs AG (1998) Water proofing properties of cuticular lipids. Am Zool. 38:471–482
Ginzel MD, Hanks LM (2003) Contact pheromones as mate recognition cues of four species of longhorned beetles (Coleoptera: Cerambycidae). J Insect Behav 16:181–187
Ginzel MD, Millar JG, Hanks LM (2003a) Z9-pentacosene-contact sex pheromone of the locust borer Megacyllene robinae. Chemoecology 13:135–141
Ginzel MD, Blomquist GJ, Millar JG, Hanks LM (2003b) Role of contact pheromones in mate recognition in Xylotrechus colonus. J Chem Ecol 29:533–545
Gullan PJ, Cranston PS (2005) The insects: an outline of entomology, 3rd edn. Blackwell, Malden, MA
Gwynne DT, Rentz DCF (1983) Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera). J Aust Entomol Soc 23:79–80
Haack RA, Jendek E, Liu K, Marchant TR, Petrice TR, Poland TM, Ye H (2002) The emerald ash borer: a new exotic pest in North America. Newslett Mich Entomol Soc 47:1–5
Howard RW, Blomquist GJ (2005) Ecological, behavioural and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Kim GH, Takabayashii J, Takahashi S, Tabata K (1993) Function of contact pheromone in the mating behavior of the cryptomeria bark borer, Semanotus japonicus Lacordaire (Coleoptera: Cerambycidae). Appl Entomol Zool 28:525–535
Lelito JP, Frazer I, Mastro V, Tumlinson JH, Böröczky K, Baker TC (2007) Visually mediated ‘paratrooper copulations’ in the mating behaviour of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J Insect Behav 20:537–552
Lelito JP, Frazer I, Mastro V, Tumlinson JH, Baker TC (2008) Novel visual-cue-based sticky traps for monitoring of emerald ash borers, Agrilus planipennis. J Appl Entomol 132:668–674
Marchant KR (2006) Managing the emerald ash borer in Canada-2005. In: Mastro VC, Reardon R, Parra G (eds.) Emerald ash borer research and technology development meeting, US Department of Agriculture Forest Health Technology Enterprise Team, 26–27 Sept 2005 Pittsburgh PA
Monnin T, Malosse C, Peeters C (1998) Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. J Chem Ecol 24:473–490
Nelson DR, Blomquist GJ (1995) Insect waxes. In: Hamilton RJ (ed) Waxes: chemistry, molecular biology and functions. Oily, Dundee, Scotland, pp 1–90
Otis GW, Youngs ME, Umphrey G (2005) Effects of colored objects and purple background on emerald ash borer trapping. In: Mastro V, Reardon R (eds) Emerald Ash Borer Research and Technology Development Meeting. USDA, Forest Health Technology Enterprise Team, Morgantown, WV, pp 31–32
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America's ash resource. J For 104:118–124
Pureswaran DS, Poland TM (2008) The role of olfactory cues in short-range mate finding by the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) J Insect Behav doi:10.1007/s10905-008-9166-8
Rodriguez-Saona C, Poland TM, Miller JR, Stelinski LL, Grant GG, de Groot P, Buchan L, MacDonald L (2006) Behavioural and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian Ash, Fraxinus mandshurica. Chemoecology 16:75–86
Rodriguez-Saona CR, Miller JR, Poland TM, Kuhn TM, Otis GW, Turk T, Ward DL (2007) Behaviors of adult Agrilus planipennis (Coleoptera: Buprestidae). Great Lakes Entomol 40:1–16
Schal C, Sevala V, Capurro M, Snyder TE, Blomquist GJ, Bagnères G (2001) Tissue distribution and lipophorin transport of hydrocarbons and sex pheromones in the house fly, Musca domestica. J Insect Sci 1:12 (online) URL: http://www.insectscience.org/papers/2001/ (accessed 24 July 2008)
Wang Q (1998) Evidence for a contact female sex pheromone in Anoplophora chinensis (Förster) (Coleoptera: Cerambycidae: Lamiinae). Coleopt Bull 52:363–368
Wei X, Reardon D, Wu Y, Sun JH (2004) Emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in China: a review and distribution survey. Acta Entomol Sin 47:679–685
Yu CM (1992) Agrilus marcopoli Obenberger (Coleoptera: Buprestidae). In: Xiao G (ed) Forest insects of China, 2nd edn. China Forestry, Beijing, China, pp 400–401
Acknowledgments
Funding was received from the Canadian Forest Service, the Ontario, New Brunswick, Saskatchewan, and Manitoba Departments of Natural Resources, and the US Forest Service, through SERG-International. We thank Hugh Evans, Jake St. Amour, Niall O'Brien, Ashton Kent, Gary Grant, and Gene Jones for assistance in the lab and field; Peter de Groot, Deepa Pureswaran, and Alex Mosseler for reviewing an earlier version of this manuscript and four anonymous reviewers; and we acknowledge the support of Ed Hurley, Ed Kettela, and Tony Hopkin. All experiments reported here comply with the laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silk, P.J., Ryall, K., Barry Lyons, D. et al. A contact sex pheromone component of the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Naturwissenschaften 96, 601–608 (2009). https://doi.org/10.1007/s00114-009-0513-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0513-1