Abstract
A number of arthropod taxa contain metals in their mandibles (jaws), such as zinc, manganese, iron, and calcium. The occurrence of zinc and its co-located halogen chlorine have been studied in relation to the mechanical properties and shown to be linked in a direct fashion with increasing concentration. Hardness along with elastic modulus (stiffness) has also been linked to zinc and halogen concentration in some marine polychaete worms. The metal appears to be incorporated within the biological matrix, possibly bonding with proteins. However, the comparative advantage of metal inclusion has not been tested. It is possible that without metals, alternative mechanisms are used to achieve hardness of equal value in similar ‘tools’ such as mandibles. This question has direct bearing on the significance of metal hardening. In the present article, we compare across mandibles from six termite species, including samples with major zinc concentration, minor manganese, and no metals. Nanoindentation, electron microscopy, and electron microanalysis are used to assess metal concentration, form, and mechanical properties. The data demonstrate that termite mandibles lacking metals when fully developed have lower values for hardness and elastic modulus. Zinc is linked to a relative 20% increase in hardness when compared with mandibles devoid of metals. The similar transition metal, manganese, found in minor concentrations, is not linked to any significant increase in these mechanical properties. This raises the question of the function of manganese, which is as commonly found in insect mandibles as zinc and often located in the same mandibles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In insects, as well as a variety of other organisms, a diversity of metals, including zinc, manganese, iron, and calcium are found concentrated within the cuticle (exoskeleton/ biological polymer) of mandibles, mouth hooks, claws, and ovipositors (Hillerton and Vincent 1982; Hillerton et al. 1984; Schofield and Lefevre 1989; Quicke et al. 1998; Fontaine et al. 1991; Schofield 2001; Lichtenegger et al. 2003; Morgan et al. 2003; Birkedal et al. 2006). While the characteristics of the biological polymer matrix are significant in terms of generating changes in physical properties (Vincent and Wegst 2004), the presence or absence of metals also appears to have an affect, which is not yet fully understood. Metal accumulation is referred to as biomineralization when it involves deposition of mineral phases; however, a number of these metals do not show mineral formation, for example, zinc (Zn). The biochemical form is yet to be understood but may involve binding with proteins (Schofield 2001; Lichtenegger et al. 2003; Birkedal et al. 2006). Incorporation occurs in the darkened (tanned) extremities, for example, at the cutting edges of mandibles that are prone to wear. Evidence in insects of enhanced mechanical properties associated with such metal incorporation comes from the grasshoppers, Schistocerca gregaria and Locusta migratoria (Orthoptera), and the leaf-cutter ant, Atta sexdens rubipilosa (Hymenoptera) where enhanced levels of Zn have been linked to greater hardness of cuticle (Hillerton et al. 1982; Edwards et al. 1993; Schofield et al. 2002). In the marine polychaete worm Nereis limbata, Zn in the jaws has been linked with increased local hardness and elastic modulus (Lichtenegger et al. 2003). While a true measure of the increase in hardness associated with metal incorporation has been achieved in the leaf-cutter ant, where hardness was correlated with metal increase over a time course (Schofield et al. 2002), no comparison has been made between similar mandibles with different metal profiles. Therefore, the comparative advantage of metal inclusion has not been tested. For example, it is possible that without metals, alternative mechanisms are used to achieve hardness of equal value in similar ‘tools’ such as mandibles. This question has direct bearing on the significance of metal hardening. In addition, measurements for manganese (Mn)-enriched cuticle are lacking. Mn is another transition metal commonly found in cuticle of insects, but its links with mechanical properties have not been established. Specific behaviors are displayed by insects that contain Mn and/or Zn, which logically benefit from hardened or wear-resistant structures, as evident within the Coleoptera (beetles) and Hymenoptera (wasps) (Quicke et al. 1998; Fontaine et al. 1991; Morgan et al. 2003). However, it has been suggested that the lower concentrations of Mn often found in insect mouthparts may be too low to support any significant level of hardening and therefore that this metal may play another, as yet an unclear role (Quicke et al. 1998). In the present article, we explore the role of Mn along side assessing the relative significance of metal hardening in arthropod cuticle. Understanding the function of metal incorporation has implications for interpreting insect behavior in terms of defining the environmental parameters that constrain pests and beneficial insects. It also promises information about biological polymers that may be applicable to production of novel fabricated polymers. We apply a number of state-of-the-art methods such as nanoindentation, electron microscopy and electron microanalysis to assess metal concentration, form and mechanical properties.
Materials and methods
Samples investigated represent six species from four families of the Isoptera: Cryptotermes primus and C. brevis (Kalotermitidae), Coptotermes acinaciformis and Reticulitermes hesperus (Rhinotermitidae), Nasutitermes magnus (Termitidae), and Mastotermes darwiniensis (Mastotermitidae). Mechanical measurements utilized nanoindentation following the Oliver–Pharr method (Oliver and Pharr 1992). Data were gathered using a Hysitron Triboindentor with a three-sided pyramidal Berkovich diamond indenter, with a total included angle of 142.3°, and the radius of curvature of the tip being 50 nm. The actual force resolution of the indenter was 100 nN and the maximum applicable load 9 mN. The preset maximum indentation load was kept to 200 μN, and the loading and unloading rates were both fixed at 40 μN per second. Loading was held at the maximum value for a period of 5 s to make sure that most of the plastic deformation was completed. Atomic force microscopy (AFM) was used to confirm that the indents were well-formed at this penetration depth. Three to five samples were measured for each species with three indentation sequences per sample at the transverse section of the apical tip. Indentations were spaced 3-μm apart, so that there was no intervention between the impressions. All tests were at room temperature, and the samples were firmly fixed to the holder. By testing on a transverse section through the tip of the mandible, measurements were taken normal to the direction of force applied during use of the mandible. After mechanical measurement, samples were assessed for elemental composition using standardless quantitative energy dispersive X-ray analysis: JEOL JSM 6460 LA low vacuum analytical scanning electron microscope equipped with an integrated JEOL Hyper mini-cup, 133 eV resolution, ultra thin window (UTW), SiLi crystal, Energy Dispersive X-ray spectrometer (EDS). Analysis was carried out in low vacuum mode to obviate a metal coating, with JEOL Analysis Station (V3.51) standardless quantitation software. Acquisition conditions on the scanning electron microscope (SEM) were 20 kV, 10 mm working distance, and 30 s live time acquisition at approximately 10–15% dead time. Because no suitable standard is currently available to match the material being assessed, standardless quantitation was used with a phi–rho–z correction. Comparison across samples is achievable but quantification must be interpreted with care (see Newbury 1991). Samples were prepared at two levels through the tissue: a flat section through the tip of the mandible ≈10 μm into the tissue and a flat section below the darkened edge region >150 μm into the sample. Mandibles were taken from samples stored in 70% ethanol. Because hydration levels can affect hardness and elastic modulus (Vincent and Wegst 2004; Schöberl and Jäger 2006), a comparison was made of samples available to us fresh (wet) and dry. Results showed no significant difference at the mandible tip (Mann–Whitney test: M. darwiniensis hardness: W = 15, P = 0.378, n 3,4; elastic modulus: W = 15, P = 0.378, n 3,4; C. primus hardness: W = 8, P = 0.2159, n 3,4; elastic modulus: W = 9, P = 0.3768, n 3,4); however, there was a trend for slightly harder and stiffer values in the dried samples by about 10 and 5%, respectively. Samples of C. Brevis and C. acinaciformis were also embedded in Epon resin, polymerized at 60°C for 24 h and sectioned using a diamond knife before being viewed unstained to assess the cuticle for microcrystalline or amorphous inclusions of the metals. Techniques used included contrast differentiation (at 80 kV on 60-nm thick unstained sections) and diffraction pattern analysis (camera length 60 cm) obtained via transmission electron microscopy (JEOL 1010).
Results
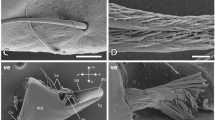
The mandible of a termite under SEM, when viewed in backscatter mode, highlights the Zn distribution (Fig. 1). The mandible is ≈0.6 mm in length. At the posterior end, a grinding plate is distinguishable from the more anterior cutting edges and teeth (Fig. 1). The reduced contrast (white areas) indicates a higher average atomic number (Goldstein et al. 2003) and signify the distribution of Zn, in this case, which is concentrated in the tips of the teeth, cutting edge and grinding plate. Termite mandibles are either enriched with Zn, Mn, or show no metal enrichment. Mandibles from six different species of termites were compared. At the tips, two species, Cryptotermes primus and C. brevis were enriched with Zn (5–20 wt%), three species, Coptotermes acinaciformis, R. hesperus, and N. magnus with Mn alone (<0.5–2 wt%), whereas M. darwiniensis contained no metals in any of the samples. Figure 2 shows representative spectra from energy dispersive X-ray analysis obtained using a SEM including the resin used to mount the samples indicating metals were not an artifact. Mn was found to be present at lower concentration than Zn (contrast Fig. 2a with b). There is a co-occurrence of chlorine (Cl) with Zn (Fig. 2a); the concentration of the former being lower than the latter. The two elements show a linear correlation for concentration. The relationship is apparent from the Pearson’s correlation coefficient reported in Table 1. The ratio of Cl/Zn (w/w) was determined as 0.12 ± 0.02, which equates to a molar ratio of 2:9. Both Zn and Cl also show good correlation with hardness and elastic modulus (Table 1). In contrast, the lower concentrations of Mn do not correlate well with either Cl or mechanical property (Table 1).
Scanning electron micrographs of the mandibles of the drywood termite, Cryptotermes primus, taken using backscattered electron imaging: metal enriched areas are lighter in contrast. a Whole mandible. Arrow indicates mandible tip region used to assess mechanical properties. T tip, C cutting edge, G grinding plate. b Section through edge of mandible. Bar 50 μm
Elemental composition as representative spectra from energy dispersive X-ray analysis. a–c Spectra from central region of termite mandible tips in transverse section: a Cryptotermes primus, b Coptotermes acinaciformis, c Mastotermes darwiniensis. Cryptotermes brevis: d 10 μm into tip region, e >150 μm below tip region. f Resin mounting medium (control)
Ultrastructural analysis using micrographs of mandibles enriched with Zn and Mn (Fig. 3) show relatively homogeneous contrast in the tissue. The unstained tissue in the images do not show clumps or clusters of black grains that would indicate regions of higher atomic number concomitant with small crystals or regions of dense amorphous metal. Large and small pore canals are evident in the tissue as white ovals, representing transverse sections through the tubules. The large canals are characteristic only of the areas of cuticle that undergo metal enrichment, whereas the small canals are found throughout the insect cuticle (images not shown). Some slight differences in contrast in the tissue surrounding the canals and throughout the tissue in Fig. 3a and b indicates variation in distribution of the dispersed metals (and halogens) binding within these areas. Diffraction patterns obtained from the tissue show two diffuse rings indicative of a lack of crystalline or microcrystalline structure (Fig. 3c,d).
Termite mandible tips in transverse section. Transmission electron micrographs of unstained tissue: a Zn-containing mandible from Cryptotermes brevis, b Mn-containing mandible from Coptotermes acinaciformis. Arrows indicate large and small pore canals in transverse section. Bar 200 nm. Electron diffraction images of termite mandible tips in transverse section: c Zn-containing mandible from Cryptotermes brevis, d Mn-containing mandible from Coptotermes acinaciformis. The central circle (contrast inverted to black) is the bleed from the electron beam with long exposure on film. Diffuse diffraction rings surround the central circle
Metal enrichment only occurs at the edge of the mandible. At >150 μm into the sample, no metals were found, using energy dispersive X-ray analysis, in samples that showed enrichment at the tip (Fig. 2d,e). Samples from six species of termites were tested for hardness and elastic modulus using nanoindentation. Differences are apparent in resultant graphs (Fig. 4). At the tip, those samples containing Zn appear harder and stiffer than those containing Mn or with no metal enrichment. Below the tip, the cuticle appears softer and less stiff.
Mechanical properties of termite mandibles. a Hardness H (GPa). b Reduced Young’s modulus Er (GPa). Box plots Median, first and third quartile, and range. Data are paired for each taxon: suffix a indicates mandible tip region, suffix b indicates cuticle below enriched tip region. K1, Cryptotermes primus; K2, Cryptotermes brevis; M, Mastotermes darwiniensis; R1, Coptotermes acinaciformis; R2, Reticulitermes hesperus; T, Nasutitermes magnus
Data were compared using nonparametric statistics. There was no significant difference between the data sets for Zn enriched samples (Kruskal–Wallis test for hardness: H = 1.05, df 1, P = 0.305; for elastic modulus: H = 0.64, df 1, P = 0.425). In addition, no significant difference was found between the species containing Mn and that with nil metal (Kruskal–Wallis test for hardness: H = 35.43, df 3, P = 0.284; for elastic modulus: H = 35.43, df 3, P = 0.054). But when these groups were compared (Zn vs Mn + Nil), the Zn group was found to have 20% harder mandible tips (Mann–Whitney test: W = 140, P = 0.001, n 7, 17), which also had appreciably higher elastic modulus (Mann–Whitney test: W = 147, P < 0.001, n 7, 17). When comparisons were made between the mandible tip and the region below the tip, cuticle differed significantly among samples for both hardness (Kruskal–Wallis test: H = 35.43, df 11, P < 0.001) and elastic modulus (Kruskal–Wallis test: H = 26.53, df 11, P = 0.005). The tip data set was significantly harder than underlying cuticle (Mann–Whitney test: W = 153, P < 0.001, n 17, 24) but did not differ significantly for elastic modulus (Mann–Whitney test: W = 300, P = 0.135, n 17, 24). Those mandibles enriched with Zn had slightly higher elastic modulus than mandibles of all other species in the unenriched region below the cuticle (Mann–Whitney test: W = 79, P = 0.014, n 11, 4).
Discussion
Previously, trace or minor levels of Mn have been demonstrated in the cutting edge of mandibles from two species of termites (Fawke et al. 1997; Yoshimura et al. 2002, 2005). The current study is the first record of termite mandibles containing Zn enrichment and also of termite mandibles without metal enrichment. This provides the opportunity to compare mechanical properties across similar mandible types that contain different metal profiles. It also enables analysis of the role of Mn in cuticle. Previous studies have addressed the role of transition metals such as Zn in mandibles (jaws), of arthropods, and also polychaete worms (Hillerton and Vincent 1982; Hillerton et al. 1984; Fontaine et al. 1991; Schofield and Lefevre 1989; Perry et al. 1988; Quicke et al. 1998; Schofield 2001; Lichtenegger et al. 2003; Morgan et al. 2003; Birkedal et al. 2006). To ascertain whether the termite system can justifiably be compared with these, it was necessary to subject samples to a number of characterization studies. Previous studies had shown a lack of crystalline metal for Zn and support for the theory that Zn binds to the polymer matrix, probably proteins (Schofield 2001; Lichtenegger et al. 2003; Birkedal et al. 2006). Similarly, in termites, there is no evidence from morphological examination or diffraction pattern analysis that either Zn or Mn are present in crystalline form or as amorphous clusters. Ca has been found to form amorphous calcium phosphate nano-clusters in one type of fly larva (Cribb et al. 2005). In this case, although the diffraction pattern showed an amorphous nature, the nano-clusters were readily visible in transmission electron micrographs as electron-dense granules (Fig. 3: Cribb et al. 2005). In contrast, nano-clusters are not seen in the termite mandibles (see Fig. 3a,b, this paper). The data for termites therefore support the theory that metals are present in termite mandibles in a form similar to Zn in other insects and in polychaete worms. This enables the termites to be used as a comparative model system to explore the advantage imparted by metal hardening in cuticle.
Previous studies on insects have reported a two- to threefold increase in hardness for metal-enriched cuticle when compared to regions of cuticle devoid of metals in the same insects (Hillerton et al. 1982; Edwards et al. 1993; Schofield et al. 2002). It is important to note that these studies run the risk of overestimating the role of metal hardening in arthropods because of the types of comparisons made. While such studies demonstrate a role for metal-linked hardening within certain cuticles, they do not address the question of whether tanned cuticle can achieve parity through alternative biochemical pathways, devoid of metals (and co-located halogens). A comparison across a variety of similar mandibles with different metal profiles allows for a more realistic assessment of the significance of metal-linked hardening. The current study undertakes such a comparison. The data show that major levels of metal, in the form of Zn, enhance hardness by 20% over similar mandibles tanned without incorporation of metals and undertaking similar tasks (e.g. feeding on sound wood). This increase is considerably smaller than the 200 to 300% (two- to threefold) but does indicate that arthropods are capable of achieving greater hardness with the metal model.
Mandibles with Mn at lower concentration do not show enhanced hardness: Many samples were similar in mechanical parameters to mandibles devoid of metal. From the disparity in concentrations present, it is not possible to conclude whether the difference in mandibles enriched with Zn vs Mn is a result of concentration or metal type; however, it is apparent that the low levels of Mn present are not linked to measured mechanical advantage. This is intriguing considering Mn is a metal commonly found in insect ‘tools’, for instance in Coleoptera (beetles) and Hymenoptera (wasps) (Quicke et al. 1998; Fontaine et al. 1991; Morgan et al. 2003). The low concentrations of Mn found in insect mouthparts are too low to support any significant level of hardening and presumably play another role. This position has been suggested previously (Schofield and Lefevre 1989; Quicke et al. 1998), but evidence was lacking in terms of measurement of physical properties. The suggestion has been made that cuticle enriched with Mn may show an increase in cuticle density and/or resistance to fracture (Morgan et al. 2003). These properties could be tested in future studies.
It is possible that Mn is sequestered into the tissue either using the same biochemical pathway as Zn, in other words in competition, or as a necessary part of the Zn accumulation. However, Schofield et al. 2003 found uptake of Mn followed Zn in ant and scorpion models. Further to this observation, using data from scorpion stings, Schofield 2001 suggested that Zn accumulation may actually interfere with Mn accumulation. Our data suggest that Mn in termite mandibles is not strictly linked to Zn accumulation, as samples were found where Mn was present while Zn was absent. The examples explored for termites, ants, and scorpions refer to relatively low concentrations of Mn. It would be worthwhile exploring the ovipositors of hymenopterans that show significantly higher levels of Mn to determine whether Mn plays a different role or behaves differently at higher concentration.
The additional element, Si, was seen in the spectra from a number of termite mandibles (see Fig. 2). Perry et al. 1988 found large concentrations of Si in addition to small concentrations of Zn present in the mandibles of a species of copepod (an arthropod), indicating that Si deposits are likely to be important in relation to the physical characteristics of some mandibles. In the current study, however, Si was not limited to the mandible edge and did not appear to be linked to physical properties of the cuticle in the way the metal or halogen were.
Higher elastic modulus has been reported for Zn-enriched cuticle in Nereis (Lichtenegger et al. 2003; Birkedal et al. 2006). Initial results from termites suggest similar links for Zn but not Mn. However, since underlying cuticle devoid of Zn also showed a higher elastic modulus in termites that used Zn enrichments at the mandible tips, it is possible that these kalotermitid mandibles have an inherently stiffer matrix throughout and that differences at the edge are unrelated to metal presence. The data caution against accepting that increased stiffness is linked directly to the presence of metals. There is also evidence that the chemistry of metal incorporation differs between biological models. Halogens have been reported as co-located with metals in arthropods and polychaetes (Schofield 2001; Lichtenegger et al. 2003; Schofield et al. 2003; Birkedal et al. 2006). The ratio of halogen to metal is reported for the annelid, Nereis (Lichtenegger et al. 2003), but has not been provided for arthropods previously. The current study shows that termites appear to have a different molar ratio of Cl/Zn than found for the marine polychaete (2:9 vs ≈2:5 (Lichtenegger et al. 2003)). Interestingly termites with Zn enrichment also show a greater hardness than Nereis [max H of jaws, 0.75 GPa; E, 11.8 GPa (Lichtenegger et al. 2003)], although states of hydration in measured tissue may have varied and affected outcomes, prompting caution in comparison. As in termites, there is less halogen present for a set quantity of Zn, the biological systems do not exploit identical chemistry. Comparisons of the termite data with that for Nereis indicate that a change in the Cl/Zn ratio does not disrupt the correlation of both elements with hardness. Presumably, the molecular incorporation is similar with increasing concentration, as the ratio is constant over a variety of concentrations: The Zn is not simply loading into the system at higher concentrations without concomitant Cl. Therefore, both halogen and metal appear integral to the biochemistry and mechanical effects; however, it is now evident that this ratio may vary in different biological systems.
References
Birkedal H, Khan RK, Slack N, Broomell C, Lichtenegger HC, Zok F, Stucky GD, Waite JH (2006) Halogenated veneers: protein cross-linking and halogenation in the jaws of Nereis, a marine polychaete worm. ChemBioChem 7:1392–1399
Cribb BW, Rasch R, Barry J, Palmer CM (2005) Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae). Arthropod Struct Dev 34:41–48
Edwards AJ, Fawke JD, McClements JG, Smith SA, Wyeth P (1993) Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting ant Atta sexdens rubropilosa. Cell Biol Int 17:697–698
Fawke JD, McClements JG, Wyeth P (1997) Cuticular metals: quantification and mapping by complementary techniques. Cell Biol Int 21:675–678
Fontaine AR, Olsen N, Ring RA, Singla CL (1991) Cuticular metal hardening of mouthparts and claws of some forest insects of British Columbia. J Entomol Soc Brit Columbia 88:45–55
Goldstein J, Newbury DE, David C, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer LC, Michael JR (2003) Scanning electron microscopy and x-ray micronalysis, 3rd edn. Kluwer, New York
Hillerton JE, Vincent JFV (1982) The specific location of zinc in insect mandibles. J Exp Biol 101:333–336
Hillerton JE, Reynolds SE, Vincent JFV (1982) On the indentation hardness of insect cuticle. J Exp Biol 96:45–52
Hillerton JE, Robertson B, Vincent JFV (1984) The presence of zinc or manganese as the predominant metal in the mandibles of adult, stored-product beetles. J Stored Prod Res 20:133–137
Lichtenegger HC, Schoberl T, Ruokolainen JT, Cross JO, Heald SM, Birkedal H, Waite JH, Stucky GD (2003) Zinc and mechanical prowess in jaws of Nereis, a marine worm. Proc Natl Acad Sci U S A 100:9144–9149
Morgan TD, Baker P, Kramer KJ, Basibuyuk HH, Quicke DLJ (2003) Metals in mandibles of stored products insects: do zinc and manganese enhance the ability of larvae to infest seeds. J Stored Prod Res 39:65–75
Newbury DE (1991) Standardless quantitative electron-excited x-ray microanalysis by energy-dispersive spectrometry: what is its proper role. Microsc Microanal 4:585–597
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7:1564–1583
Perry CC, Grime GW, Watt F (1988) An X-ray analytical study of mandibles from Calanus pacificus, an herbivorous copepod. Nucl Instrum Methods Phys Res, Sect B 30:367–371
Quicke DLJ, Wyeth P, Fawke JD, Basibuyuk HH, Vincent JFV (1998) Manganese and zinc in the ovipositors and mandibles of hymenopterous insects. Zool J Linn Soc 124:387–396
Schöberl T, Jäger IL (2006) Wet or dry-hardness, stiffness and wear resistance of biological materials on the micron scale. Adv Eng Mater 8:1164–1169
Schofield RMS (2001) Metals in cuticular structures. In: Brownell P, Polis G (eds) Scorpion biology and research. Oxford University Press, Oxford, pp 234–256
Schofield R, Lefevre H (1989) High concentrations of zinc in the fangs and manganese in the teeth of spiders. J Exp Biol 144:577–581
Schofield RMS, Nesson MH, Richardson KA (2002) Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 89:579–583
Schofield RMS, Nesson MH, Richardson KA, Wyeth P (2003) Zinc is incorporated into cuticular “tools” after ecdysis: the time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion. J Insect Phys 49:31–44
Vincent JFV, Wegst UGK (2004) Design and mechanical properties of insect cuticle. Arthropod Struct Dev 33:187–199
Yoshimura T, Kagemori N, Kawai S, Sera K, Futatsugawa S (2002) Trace elements in termites by PIXE analysis. Nucl Instrum Methods Phys Res, Sect B 189:450–453
Yoshimura T, Kagemori N, Sugiyama J, Kawai S, Sera K, Futatsugawa S, Yukawa M, Imazeki H (2005) Elemental analysis of worker mandibles of Coptotermes formosanus (Isoptera: Rhinotermitidae). Sociobiology 45:255–259
Acknowledgements
We gratefully acknowledge supply of some termite samples from Dr Michael K. Rust, Department of Entomology, UCR, Riverside, USA; Dr B. Peters, Queensland Department of Primary Industries and Fisheries; and Mrs Rachel Hancock Narangbar, Queensland, Australia. HH is grateful for experimental assistance by Mr. B. L. Wang at the University of Queensland, Brisbane, Australia. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cribb, B.W., Stewart, A., Huang, H. et al. Insect mandibles—comparative mechanical properties and links with metal incorporation. Naturwissenschaften 95, 17–23 (2008). https://doi.org/10.1007/s00114-007-0288-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-007-0288-1