Abstract
To explore neuro-endocrinal changes in the brain of European honeybee (Apis mellifera) queens before and after mating, we measured the amount of several biogenic amines, including dopamine and its metabolite in the brain of 6- and 12-day-old virgins and 12-day-old mated queens. Twelve-day-old mated queens showed significantly lower amounts of dopamine and its metabolite (N-acetyldopamine) than both 6- and 12-day-old virgin queens, whereas significant differences in the amounts of these amines were not detected between 6- and 12-day-old virgin queens. These results are explained by down-regulation of both synthesis and secretion of brain dopamine after mating. It is speculated that higher amounts of brain dopamine in virgin queens might be involved in activation of ovarian follicles arrested in previtellogenic stages, as well as regulation of their characteristic behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apis mellifera queens usually reach sexual maturity and make mating flights 5–6 days after emergence. After mating, their ovaries develop intensely and they start laying eggs within 2–4 days. Once egg laying is started, they never fly to mate again (Crane 1990). Tanaka and Hartfelder (2004) showed that oogenesis are initiated but ovarian follicles are arrested in previtellogenic stages in early adult development of queens. It suggests that a stimulus may be necessary to activate follicles for ovary development in queens. The experience of mating flight and the presence of semen in the spermatheca have been suggested as important factors causing changes in brain structure (Fahrbach et al. 1995) and triggering oviposition (Kaftanoglu and Peng 1982). However, the neuro-endocrinal mechanisms mediating the intense ovary development after mating still remain unclear.
Biogenic amines are well-known neurotransmitters, neuromodulators, and neurohormones, regulating behaviors and physiological states in invertebrates as well as vertebrates (Evans 1980). One of the functional biogenic amines, dopamine, has been suggested as being involved in honeybee ovary development. The ovaries of honeybee workers in queenless colonies can develop and become functional. In this process, the levels of norepinephrine, dopamine and a metabolite of dopamine, N-acetyldopamine (NADA), in the brain are positively correlated with ovary development (Harris and Woodring 1995; Sasaki and Nagao 2001, 2002). The increase in dopamine metabolites may indicate up-regulation of both synthesis and secretion of brain dopamine. Dombroski et al. (2003) showed that oral application of dopamine to queenless workers enhanced ovary development. It is also reported that queens, which have larger ovaries than workers, show higher levels of both brain dopamine and norepinephrine than workers, although a queen’s brain is smaller than a worker’s (Brandes et al. 1990). This may suggest an identical effect of dopamine on ovary development in both queens and workers. Such effects have been reported in other insects, including the fruit fly, Drosophila melanogaster (Neckameyer 1996).
If brain dopamine influences ovary development and/or reproductive behavior in honeybee queens, levels of brain dopamine and its metabolites should change before and after mating. In this study, we measured the levels of several biogenic amines including dopamine and NADA in the brains of virgin and mated honeybee queens.
Materials and methods
Queen rearing and collection
Queen rearing was performed in the Tamagawa University apiary using crossbred European honeybees derived mainly from A. m. ligustica during the reproductive season in Japan (early May to early June). Queens were obtained using the Doolittle method (Laidlaw and Page 1997) with a populous queenless colony. Newly hatched larvae from a single colony were grafted to obtain properly developed queens with reduced possible genetic variation. The group of grafted larvae was a mix of full- and half-sisters because the mother queen had been mated naturally. When queens matured in the queen cells, they were transferred individually from the rearing colony to small nucleus colonies in special hives (15×14×15 cm) 2 days before expected emergence. The nucleus colonies containing 150–300 workers were given a frame of honeycomb and empty comb with a feeding box. After transferring to the mating yard, the hive entrances were left open to allow workers to forage freely. One-half of the virgin queens were prevented from flying from the hives by fitting queen excluders to the entrances. The other half were allowed to fly and mate. Queen oviposition was monitored twice at days 8 and 11 after emergence. We sampled seven 12-day-old (12 days after emergence) mated ovipositing queens from the colonies. Six virgin queens were also collected from the colonies with queen excluders on the same day. We sampled eleven 6-day-old virgin queens in another round of queen rearing from nucleus colonies with queen excluders. All collected queens were frozen in liquid nitrogen at 12:00 noon and stored at −80°C until HPLC-ECD analysis within 2 weeks.
Dissection and HPLC-ECD analysis
Queen brains were dissected in ice-cold sterilized honeybee saline (Bailey 1952) on a dissecting dish. The hypopharyngeal glands, subesophageal ganglion and retinal pigment of each bee were removed and discarded. Dissected brains were homogenized with a micro-glass homogenizer in 50 μl of ice-cold 0.1 M perchloric acid containing 12.5 ng/ml of 3, 4-dihydroxybenzylamine (DHBA) as the internal standard. Each sample was then transferred to a 1.5-ml Eppendorf tube, and centrifuged at 20,000×g for 30 min at 0°C. The supernatant was transferred to a microvial for analysis by HPLC-ECD. Each queen’s abdomen was also dissected to check ovary development and the presence of spermatozoa in the spermatheca.
An improved version of the method originally described by Sasaki and Nagao (2001, 2002) was used for simultaneous determination of biogenic amines. The HPLC-ECD system consisted of a solvent-delivery pump (EP-300, EICOM, Japan), refrigerated automatic injector (231XL-402, Gilson, USA) and C18 reversed-phase column (250 mm × 4.6 mm i.d., 5-μm average particle size, UG 120, Shiseido, Japan) maintained at 35°C in a column oven. An electrochemical detector (ECD-300, EICOM) with glassy carbon electrode (WE-GC, EICOM) was used. The detector potential was usually set at 0.88 V referenced to an Ag/AgCl electrode. The detector cell was held at a constant temperature of 35°C by placing it in the column oven. Signals from the electrochemical detector were recorded and integrated by using data analysis software (PowerChrom, ADInstruments, Japan). The mobile phase contained 0.18 M monochloroacetic acid and 40 μM of 2Na-EDTA adjusted to pH 3.6 with NaOH. Both 1.62 mM of sodium-1-octanesulfonate as the ion-pair reagent, and 7% CH3CN as the organic modifier were added to this solution. The mobile phase buffer was filtered through a 0.22-μm filter (Millipore, USA) and degassed. The flow rate was kept constant at 0.7 ml/min.
Samples were analyzed in groups of four or six. External standards were run before and after sample runs to identify and quantify dopamine, NADA and serotonin. Each peak of a biogenic amine was identified by comparing both the retention time and hydrodynamic voltamograms with the standards. Measurements were obtained by comparing peak area between sample and standard chromatograms. The significance of differences between groups was tested using analysis of variance (ANOVA) followed by post-hoc multiple range tests (Fisher’s PLSD) at α = 0.05 level.
Results
We found mature eggs in the ovaries of all 12-day-old mated queens, and immature eggs in the ovaries of two-thirds of the 12-day-old virgin queens. Six-day-old virgin queens had no eggs in their ovaries. Only mated queens had spermatozoa in their spermatheca and laid eggs.
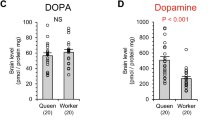
Mated 12-day-old queens showed significantly lower dopamine and NADA levels than both 6- and 12-day-old virgin queens (Fig. 1; dopamine: ANOVA, df=2, F=4.80, p=0.019, Fisher’s PLSD, p<0.05, NADA: ANOVA, df=2, F=8.29, p=0.002, Fisher’s PLSD, p<0.05). No significant differences in levels of these amines were detected between 6- and 12-day-old virgin queens (p>0.05). There were no significant differences among levels of serotonin detected in 6-, 12-day-old virgin and 12-day-old mated queens (6-day-old virgin, 3.29±1.31 (±SD) pmol/brain; 12-day-old virgin, 4.81±1.36 pmol/brain; 12-day-old mated, 4.24±1.90 pmol/brain; ANOVA, df=2, F=2.15, p=0.141).
Discussion
Previous studies have shown the accelerative effects of dopamine on ovary development in honeybee workers (Harris and Woodring 1995; Sasaki and Nagao 2001, 2002; Dombroski et al. 2003), suggesting its involvement in reproductive behavior. On the other hand, there is little information on brain dopamine in queens (Brandes et al. 1990; Harris et al. 1996). In our HPLC-ECD analysis, all queens have relatively larger amounts of brain dopamine (26–59 pmol/brain) in comparison to queenright workers (7–9 pmol/brain) and queenless workers (8–14 pmol/brain) reported by Sasaki and Nagao (2001). This is consistent with the results of Brandes et al. (1990). The quantitative differences in brain dopamine seem to reflect reproductive potential between queenless workers, which are temporal reproductives, and queens specialized for reproduction.
The present study showed lower levels of brain dopamine and its metabolite (NADA) in mated queens compared to virgin queens. These results can be explained by down-regulation of both synthesis and secretion of brain dopamine after mating. It is unlikely that the observed lower level of brain dopamine resulted from increased secretion and subsequent metabolism of brain dopamine in mated queens, because there was no sign of an increase in dopamine metabolite, NADA. Harris et al. (1996) reported that the level of brain dopamine was lower in virgin queens whose ovaries were led to develop by CO2-exposure than in untreated virgin queens. Although they did not measure the amounts of NADA, it is possible that the brain NADA level as well as dopamine level might also be lower in the CO2-exposed virgin queens.
Why does the down-regulation of brain dopamine occur in mated queens? One might think that the decline of brain dopamine level may trigger the ovary development of queens. However, this seems to be unlikely because dopamine shows an accelerative effect on ovary development in queenless workers (Harris and Woodring 1995; Sasaki and Nagao 2001, 2002; Dombroski et al. 2003) though we cannot exclude the possibility that dopamine works differently in each caste. At present, we do not have a satisfying answer to this question but several speculations may be useful to understand this phenomenon. Tanaka and Hartfelder (2004) observed ovarian follicles which were arrested in previtellogenic stages in virgin queens and queenright workers, suggesting that a stimulus could be required to activate follicles and continue the processes of oogenesis. The ovary development of queens is generally believed to be triggered by the mating experience. It does not contradict with the present observation that mated queens developed ovaries better than virgin queens. However, the mating stimulus is not enough to explain the ovary development in queenless workers, non-mated queens laying unfertilized eggs (Winston 1987) and 12-day-old virgin queens in this study. The slightly developed ovaries in 12-day-old virgin queens suggest that follicles have been activated at this age, irrespective of mating status. If the follicles are automatically activated in virgin queens, we could speculate that the higher level of brain dopamine in virgin queens might activate them and admit egg maturation as a primary step of ovary development. Mating stimulus might be involved in post-mating intensive ovary development in queens through enhancing the processes of maturation in the activated follicles.
Biogenic amines can simultaneously induce multiple behavioral and physiological changes. It is possible that brain dopamine in honeybees regulates not only ovary development but also several behaviors for reproduction. In workers, dopaminergic neurons project into a central complex that regulates innate motor programs and mushroom bodies implicated in higher integrative processes, including learning and memory (Schäfer and Rehder 1989; Schürmann et al. 1989). Menzel et al. (1999) showed that dopamine injection into the brain restored the proboscis extension reflex to sucrose in reserpinized bees and suggested that dopamine is involved in regulation of innate motor activity. Although functional roles of brain dopamine in the innate behaviors of queens have not been studied, some characteristic innate behaviors of virgin queens have been observed. They often fight with sister queens (Gilley 2001) and destroy queen cells containing sister queens (Harano and Obara 2004a, b) to win the reproductive status before the mating flight. Another behavioral trait of virgin queens is mating flight. Mated queens never fly out from the colony throughout the life except when swarming. Furthermore, it is known that virgin queens show higher locomotor activity than mated queens. Such behavioral changes may be mediated by several neuroactive substances, possibly including brain dopamine.
References
Bailey L (1952) The action of the proventriculus of the worker honeybee, Apis mellifera L. J Exp Biol 29:310–327
Brandes C, Sugawa M, Menzel R (1990) High-performance liquid chromatography (HPLC) measurement of catecholamines in single honeybee brains reveals caste-specific differences between worker bees and queens in Apis mellifera. Comp Biochem Physiol 97C:53–57
Crane E (1990) Bees and beekeeping. Cornel University Press, Ithaca, New York
Dombroski TCD, Simoes ZLP, Bitondi MMG (2003) Dietary dopamine causes ovary activation in queenless Apis mellifera workers. Apidologie 34:281–289
Evans PD (1980) Biogenic amines in the insect nervous system. Adv Insect Physiol 15:356–389
Fahrbach SE, Giray T, Robinson GE (1995) Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol Learn Memory 63:181–191
Gilley DC (2001) The behavior of honey bees (Apis mellifera ligustica) during queen duels. Ethology 107:601–622
Harano K, Obara Y (2004a) Virgin queens selectively destroy fully matured queen cells in the honeybee Apis mellifera L. Insectes Soc 51:253–258
Harano K, Obara Y (2004b) The role of chemical and acoustical stimuli in selective queen cell destruction by virgin queens of the honeybee Apis mellifera. Appl Entmol Zool 39:611–616
Harris JW, Woodring J (1995) Elevated brain dopamine levels associated with ovary development in queenless worker honey bees (Apis mellifera L.). Comp Biochem Physiol 111C:271–279
Harris JW, Woodring J, Harbo JR (1996) Effects of carbon dioxide on levels of biogenic amines in the brains of queenless worker and virgin queen honey bees (Apis mellifera). J Apic Res 35:69–78
Kaftanoglu O, Peng Y (1982) Effects of insemination on the initiation of oviposition in the queen honeybee. J Apic Res 21:3–6
Laidlaw HH, Page RE (1997) Queen rearing and bee breeding. Wicwas press, Cheshire, Connecticut, USA
Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A (1999) Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci 113:744–754
Neckameyer WS (1996) Multiple roles for dopamine in Drosophila development. Dev Biol 176:209–219
Sasaki K, Nagao T (2001) Distribution and levels of dopamine and its metabolites in brains of reproductive workers in honeybees. J Insect Physiol 47:1205–1216
Sasaki K, Nagao T (2002) Brain tyramine and reproductive states of workers in honeybees. J Insect Physiol 48:1075–1085
Schäfer S, Rehder V (1989) Dopamine-like immunoreactivity in the brain and suboesophageal ganglion of the honeybee. J Comp Neurol 280:43–58
Schürmann FW, Elekes K, Geffard M (1989) Dopamine-like immunoreactivity in the bee brain. Cell Tissue Res 256:399–410
Tanaka ED, Hartfelder K (2004) The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes. Arthropod Struct Dev 33:431–442
Winston ML (1987) The biology of the honeybee. Harvard University Press, Cambridge, Massachusetts
Acknowledgements
We thank the staff of the Laboratory of Entomology and the Honeybee Science Research Center at Tamagawa University for their valued comments, Mrs E. Nishida at the Kanazawa Institute of Technology for helping with maintenance of the HPLC and Dr Robert Hancock for improving English in the manuscript. We also would like to thank the anonymous reviewers for their advisable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harano, Ki., Sasaki, K. & Nagao, T. Depression of brain dopamine and its metabolite after mating in European honeybee (Apis mellifera) queens. Naturwissenschaften 92, 310–313 (2005). https://doi.org/10.1007/s00114-005-0631-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-005-0631-3