Abstract
Objective
This study determined growth and stool characteristics of infants fed a formula with reduced content of a partially hydrolyzed protein of improved quality and assessed the effect of the addition of a probiotic on infant growth and health.
Design, methods
The double-blind study involved three formulas: a reduced protein (1.90 g/100 kcal) formula (formula RP), the same formula with Bifidobacterium lactis (formula RP+P), and a higher-protein (2.39 g/100 kcal) control formula (C). Protein of the formulas consisted of partially hydrolyzed modified whey proteins. Normal term infants were randomly assigned to one of the formulas, which were fed from soon after birth to 4 months of age; 88 infants completed the study. Growth was measured and formula tolerance, stool characteristics, and illness incidence were recorded.
Results
All three formulas supported growth equally well. Plasma concentrations of urea and of threonine and proline were markedly (significantly) lower with RP and RP+P than with C. No differences in feeding-related behaviors were noted, but stools were softer with formula C than with the other formulas. Infants fed RP+P had significantly fewer episodes of diarrhea (0.21 episodes) than infants fed RP (0.41 episodes) or C (0.91 episodes).
Conclusion
A reduced-protein formula with and without added probiotics (B. lactis) supports normal growth of infants during the first 4 months. In addition, it leads to lower plasma levels of urea, threonine, and proline. Supplementation of the formula with Bifidobacterium lactis offers protection against diarrheal illness.
Zusammenfassung
Ziel
Ziel der Untersuchung war nachzuweisen, dass eine Nahrung mit niedrigem Gehalt an hochwertigem hydrolysiertem Kuhmilcheiweiß bei gesunden Säuglingen normales Wachstum ermöglicht. Des Weiteren sollte untersucht werden, ob der Zusatz eines Probiotikums zu der Nahrung das Wachstum der Säuglinge bzw. den Gesundheitszustand beeinflusst.
Material und Methoden
Die Untersuchung wurde als prospektive, randomisierte Untersuchung im Doppelblindverfahren durchgeführt. Zwei Nahrungen mit reduziertem Eiweißgehalt (1,90 g/100 kcal) enthielten ein neuartiges Gemisch an teilhydrolysierten Molkenproteinen. Eine dieser Nahrungen (RP+P) enhielt zusätzlich Bifidobacterium lactis. Eine dritte Nahrung mit einem höheren Gehalt (2,39 g/100 kcal) an etwas anders zusammengesetzten Molkenproteinen diente als Kontrollnahrung (C). Gesunde, reifgeborene Säuglinge wurden per Zufallsreihe auf die 3 Nahrungen aufgeteilt, welche vom 8. Lebenstag bis zum Ende des 4. Lebensmonats verfüttert wurden. Gewicht und Länge wurden regelmäßig gemessen, und die Eltern registrierten Stuhleigenschaften, kindliches Verhalten sowie etwaige Erkrankungen des Kindes.
Ergebnisse
Die Säuglinge gediehen mit allen 3 Nahrungen gleich gut. Die Plasmaspiegel von Harnstoff, Threonin und Prolin waren signifikant niedriger bei den Säuglingen, welche die Nahrung RP bzw. RP+P erhielten, als bei den mit der Nahrung C ernährten Säuglingen. Letztere zeigten signifikant weichere Stühle als Säuglinge, die mit RP bzw. RP+P ernährt wurden. Im Verhalten der Säuglinge wurden keine Unterschiede festgestellt. Säuglinge, denen Nahrung RP+P verfüttert wurde, hatten signifikant seltener Durchfall (0,21 Episoden pro Kind) als diejenigen, welchen Nahrung RP (0,41 Episoden) bzw. Nahrung C (0,91 Episoden) verfüttert wurde.
Schlussfolgerung
Nahrung mit reduziertem Gehalt an hochwertigem Eiweiß führt bei Säuglingen in den ersten 4 Monaten zu normalem Gedeihen bei gleichzeitig niedrigeren Plasmaspiegeln von Harnstoff, Threonin und Prolin. Der Zusatz eines Probiotikums (Bifidobacterium lactis) schützt gegen Durchfallerkrankungen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protein of infant formulas must meet the protein needs of infants, yet should exceed protein needs by the smallest possible margin. To meet these dual requirements, the protein needs of infants must be known and the efficiency with which formula protein is converted to body protein (i.e., protein quality) must be high. The higher the quality of the protein, the narrower the margin by which intake must exceed the requirement for protein.
Throughout the modern era of infant formula development, formula protein concentration has been lowered progressively. This was made possible to some extent by a better understanding of the actual protein requirements of infants, and to a larger part by advances in the use of the various proteins of cow milk. Recent advances in dairy technology have led to the availability of blends of cow milk proteins with superior quality for infants, thus enabling the reduction of the margin by which intake must exceed requirement.
As discussed elsewhere [17], protein requirements of infants can be estimated by theoretical approaches. Whether estimated from the protein intake of the breastfed infant or by the factorial method based on body composition [2], protein requirements are about 2.1 g/kg per day during the 1st month of life, decreasing by more than 40% during the first 6 months of life. Because infants regulate food intake according to their energy needs, it is useful to state protein requirements in relation to energy needs, i.e., as protein-energy ratio (g/100 kcal). Assuming an energy intake of 120 kcal/kg per day, the theoretical estimate of requirement for protein is 1.75 g/100 kcal during the 1st month of life. At an efficiency of conversion of dietary protein to body protein (protein quality) of 90%, the required protein-energy ratio is 1.94 g/100 kcal. However, at an efficiency greater than 90%, the required protein-energy ratio would be less than 1.94 g/100 kcal.
Relevant experimental evidence is available from feeding studies in normal infants. A study in which the protein-energy ratio was 1.56 g/100 kcal during the 1st month of life produced unequivocal evidence for the inadequacy of this protein level [6]. A subsequent study provided suggestive evidence that a protein-energy ratio of 1.70 g/100 kcal was marginally inadequate [5]. Thus, theoretical and experimental data suggest that the protein-energy ratio of formulas needs to be at least 1.75 g/100 kcal. How much greater than 1.75 g/100 kcal it needs to be is determined by the quality of the protein.
While it is necessary to meet the protein needs of infants at all times, it is also desirable to keep protein intakes as low as possible because excess protein increases the potential renal solute load and thus obligates water for renal excretion of urea [4]. Excessive protein intake should be avoided also because of the possibility that a high protein intake in early life may predispose to obesity later in life [12].
The present study asked the question whether a formula with a protein-energy ratio of 1.90 g/100 kcal meets the protein requirements of normal infants when the protein, partially hydrolyzed sweet whey with added amino acids, may be presumed to be of high quality (i.e., >90%).
Ingestion of certain live microbial agents (probiotics) can modify the native fecal flora and can be associated with a variety of health effects [15]. In the case of infants, the protection offered against diarrheal disease and other gastrointestinal disturbances is of particular interest [9, 11, 16]. Although formulas with added probiotics have been used for some time, there is little documentation of growth and health of normal infants fed such formulas [8]. The various microorganisms used as probiotics are likely to differ in their effects on the host organism. The present study examined whether the addition of Bifidobacterium lactis, a widely used probiotic, to an infant formula has effects on growth as well as health of normal infants.
Materials and methods
Study design
The study was designed as a prospective, randomized, double-blind trial involving normal term infants. At trial entry between 6 and 10 days of age, subjects were randomly assigned to one of three study formulas. The assigned formula was then fed during the entire trial through 112 days of age. Infants visited the study center within 2 days of ages 14, 28, 42, and 56 days and within 4 days of ages 84 and 112 days. At each visit weight and length were measured, completed behavior and stool questionnaires were collected, and an interval health history was obtained. A new supply of study formula was provided. Samples of capillary blood were obtained during visits at 28, 56, 84, and 112 days of age. The study hypothesis was that growth (gain in weight and length) and frequency of acute disease would be similar in the three study groups, but that outcomes that reflected the level of protein intake, such as plasma concentrations of albumin, urea nitrogen, and certain amino acids, would be lower with the experimental formula than with the control formula. The study protocol was reviewed by the Institutional Review Board of the University of Iowa and parental informed written consent was obtained.
Sample size
The main endpoints were gains in weight and length. To be clinically relevant, a difference in weight gain would have to be greater than 3.5 g/day, equal to 364 g over the entire study period. A sample size of 28 infants per group would permit detection of a difference of 3.5 g/day in weight gain and 0.07 mm/day in length gain between 8 and 112 days of age at α=0.05 and β=0.2, using as population variance the pooled standard deviation (5.25 g/day and 0.10 mm/day, respectively) of our published reference data for formula-fed infants [13]. The study protocol called for enrollment of infants and random allocation to study groups until it was likely that at least 28 infants in each group would complete the study as planned
Subjects
Normal male and female infants (gestational age 37 weeks or more) were eligible if their birth weight was between 2500 and 4500 g, had no major congenital malformations, and had no significant perinatal morbidity. Only parents who had decided to feed formula from birth were approached about enrollment. Infants were enrolled between 6 and 10 days of age. Although there was no restriction regarding race or color, the population of Iowa City is primarily Caucasian and all study infants were Caucasian.
Formulas
Composition of the two experimental formulas (formula RP and formula RP+P) and the control formula (formula C) is summarized in Table 1. All formulas were provided in powder form in metal cans. The formulas differed mainly with regard to the amount and type of protein. Formulas RP and RP+P, referred to collectively as reduced-protein formulas, provided less protein (1.92 and 1.89 g/100 kcal, respectively) than formula C (2.39 g/100 kcal). In all formulas the protein consisted of partially hydrolyzed whey proteins. The proteins of formulas RP and RP+P were derived from sweet whey and thus included less glycomacropeptide and more lactalbumin than the proteins of the control formula, which were derived from acid whey. These differences in whey proteins explain the relatively higher tryptophan and disproportionately low threonine concentrations of formulas RP and RP+P (Table 2). The addition of small amounts of arginine, histidine, and tyrosine to formulas RP and RP+P explains the higher concentrations of these amino acids. Other differences in amino acids between formulas RP and RP+P and formula C reflect the difference in total protein concentration.
Formulas RP and RP+P contained somewhat more small peptides (molecular weight <1000 Da) and fewer large peptides (molecular weight >5000 Da) than formula C (Table 2). Lactose was the main source of carbohydrate in all formulas, with maltodextrin providing 28–30% of total carbohydrate. Fat was provided by palm olein oil, soy oil, coconut oil, and high-oleic safflower oil. Formula RP+P contained Bifidobacterium lactis (C. Hansen, Hoersholm, Denmark) in a dose of 3.6×107 CFU per gram of formula powder, equal to 4.8×109 CFU per liter of reconstituted formula.
Procedures
Infant weight and length were measured by standard procedures [3]. Measurements were made at 8 days (between 6 and 10) of age, within 2 days of ages 14, 28, 42, and 56 days, and within 4 days of ages 84 and 112 days. Measured values were "adjusted" to the exact nominal ages by parabolic interpolation or extrapolation using two adjacent values. Adjusted values were used in describing mean size and change in size (gain). For calculating intakes per kg body weight, the average weight of the respective interval was used.
Using a disposable spring-loaded device (Tenderfoot, International Technidyne Corporation, Edison, N.J., USA), samples of capillary blood were obtained at 28, 56, 84, and 112 days of age without regard to time of the prior feeding. Samples were collected in heparinized tubes, mixed, and centrifuged immediately. Plasma was separated from cells and stored at −20°C until analysis.
Behavior, stool characteristics, illness records
During 2 days preceding each study visit and also for 2 days preceding study days 70 and 98, parents kept a record of the infant's crying and feeding-related behaviors such as spitting up, cramps, or crying and recorded the occurrence of "flatulence/gas." In each case the parents were asked to record each occurrence of the behavior. Parents separately recorded the color, consistency, and odor of each stool during the 2-day period. Parents also were asked to record for every day of the study whether the baby had diarrhea, cough, or fever and whether the baby was hospitalized. Records were collected every 2 weeks. They were reviewed immediately upon receipt and any missing data and ambiguities were clarified.
Laboratory analyses
Plasma concentration of albumin was determined by the bromcresol green method [10] and urea nitrogen by the urease method [7], in both cases with the use of a spectrophotometer (Model DU 650, Beckman Instruments, Fullerton, Calif., USA). The blank value obtained before urease treatment was subtracted from the value obtained after urease treatment. Amino acids were analyzed at the Laboratoire Central de Chimie Clinique of the University of Lausanne, Switzerland, using methods described previously [1].
Data analysis
Gains in weight and length were calculated from adjusted values as change during the interval divided by the number of days in the interval. Illness incidence was stated as number of events as well as number of days with illness. Categorical data (e.g., stool color) were tabulated and summarized per interval. Data analysis included descriptive statistics and general linear models analysis blocking for gender (SAS release 6.12, SAS Institute Inc., Cary, N.C., USA). Repeated measures and cross-sectional analyses were performed. Results of repeated measures analyses (not presented) were consistent with results of the cross-sectional analyses. Tukey multiple comparison tests and lsd (least significant difference) t-tests were used for pairwise comparisons between groups. P values (two-tailed) are reported as per comparison error rates for the lsd tests. Analyses were performed on an intent-to-treat basis as well as on a per-protocol basis. Because results were similar, only the results of the per-protocol analysis are presented.
Results
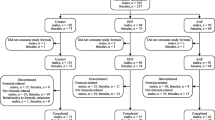
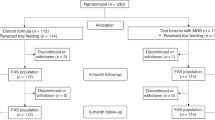
Of the 122 infants enrolled in the study, 40 were randomly assigned to formula RP, 40 to formula RP+P, and 42 to formula C. Three infants (one fed RP and two fed RP+P) were fed unduly dilute formula due to use of an inappropriate measuring scoop and their data were excluded. Three infants (two fed RP and one fed C) did not meet eligibility criteria and their data were also excluded. Twenty-eight infants (ten fed RP, ten fed RP+P, and eight fed C) failed to complete the study as planned. In 13 infants the reasons for discontinuation were extraneous circumstances or were not known. Fifteen infants (five fed RP, seven fed RP+P, and three fed C) discontinued participation because of minor health problems. Some of the health problems, for example constipation or flatulence, were potentially attributable to the study formulas, whereas other problems, such as upper respiratory infections, presumably were not. Up to the time of withdrawal, infants who dropped from the study did not differ in growth from infants who completed the study.
Data are presented for 27 infants fed RP, 28 fed RP+P, and 33 fed C who completed the study as planned. Although data are presented on a gender-specific basis, only overall statistical evaluations, taking into account gender, are presented. Data on gain in weight and length are presented in Table 3. For the interval 8–56 days there was a borderline formula effect (p=0.063), with pair-wise comparisons indicating a significant (p=0.023) difference in weight gain between formulas RP+P and C. There were no significant formula effects on weight gain for the intervals 56–112 days and 8–112 days. There were no significant formula effects on gain in length.
Data regarding plasma concentrations of albumin and urea nitrogen are presented in Table 4. Albumin did not show a statistically significant formula effect but showed the expected increase with age (p<0.001). Urea nitrogen was significantly (p<0.001) lower with formulas RP and RP+P than with formula C.
Data regarding plasma amino acid concentrations are presented in Table 5. A number of amino acids showed significantly lower concentrations with formulas RP and RP+P than with formula C. In most cases the differences were attributable to the lower protein concentration of the reduced-protein formulas. However, the concentration of threonine was disproportionately low with the reduced protein formulas, a reflection of the reduced threonine concentration of the protein of these formulas. Concentration of plasma arginine was significantly higher in infants fed formulas RP and RP+P than in infants fed formula C, a reflection of the supplementation of formulas RP and RP+P with arginine.
Table 6 indicates stool characteristics as recorded by the parents. There were no statistically significant effects of formula on stool color. Stool consistency, on the other hand, was significantly affected by formula. Infants fed formulas RP and RP+P had significantly more often hard or formed stool than infants fed formula C. Conversely, these infants had significantly less often soft or liquid stools. Stool odor was significantly less often perceived as foul in infants fed formulas RP and RP+P than in infants fed formula C. The small differences in consistency and in odor between formula RP and formula RP+P were not statistically significant. There was no difference between formulas in the number of episodes of flatulence. Neither were there significant formula effects on behaviors such as crying, colic, etc. (data not shown), with the exception of the frequency of spitting up which was significantly (p<0.04) lower among infants fed formula RP+P (1.38 events/day) than in infants fed formula RP (2.58 events/day).
Illness data are summarized in Table 7. There were no formula effects on frequency of hospitalization, cough, or fever. However, there were significant formula effects on diarrhea. The number of days with diarrhea was significantly (p=0.002) lower with formula RP+P than with formulas RP and C, and the number of episodes of diarrhea was significantly (p<0.001) less with formulas RP+P and RP than with formula C.
Discussion
The present study demonstrated that a formula with a protein-energy ratio of 1.90 g/100 kcal met the protein requirements of normal infants. This conclusion rests on the demonstration of normal growth, i.e., normal gain in weight and length, and on the absence of evidence of protein inadequacy (plasma urea and albumin). Plasma urea concentrations were markedly lower with the reduced-protein formulas than with the control formula, but were not as low as observed when protein intake is inadequate [6]. Plasma albumin concentrations likewise were in the range indicative of protein adequacy.
The estimated protein requirement of normal infants is 1.75 g/100 kcal. From the demonstration in the present study that a protein level of 1.90 g/100 kcal meets this requirement, it can be concluded that the efficiency of conversion to body protein (protein quality) of the protein used is at least 92%. The findings of the present study are in agreement with the results of Räihä et al. [14] with a formula containing a similar blend of unhydrolyzed whey proteins at a comparable protein-energy ratio of 1.80 g/100 kcal as in the present study.
The lower plasma urea concentrations seen with the reduced-protein formula in the present study provided evidence of reduced production of urea. This, in turn, meant reduced urinary excretion of urea and hence diminished renal solute load when infants were fed the lower protein formula. Diminished renal solute load indicates a greater margin of safety in dehydrating events [4].
Plasma concentrations of amino acids in the present study generally reflected the lower protein concentration of formulas RP and RP+P. However, the greatly diminished threonine concentrations reflected the reduced content of glycomacropeptide in the reduced-protein formulas, and increased tryptophan concentrations reflected the increased proportion of lactalbumin. These changes in plasma amino acid concentrations are in the desired direction, i.e., toward the pattern of the breastfed infant.
Infants fed the formula with added Bifidobacterium lactis experienced less diarrheal illness than infants fed the formulas without the added probiotic. Because the three formulas were studied concurrently, seasonal variation cannot explain the difference in diarrhea. Parents and investigators were blinded as to the identity of the formulas. Nevertheless, the number of subjects participating in the study was relatively limited and they lived in a narrowly defined geographical area. On the other hand, our results corroborate previous findings of a diarrhea-protective effect from regular ingestion of probiotics [9, 11, 16].
Infants fed the reduced-protein formulas had firmer stools that were less often foul smelling than stools of infants fed the control formula. This was probably not an effect of the reduced protein concentration per se, but rather may have been a consequence of the lesser degree of protein hydrolysis of the reduced-protein formulas. However, the possibility that other differences in formula composition may have been responsible for the difference in stool consistency cannot be excluded.
In summary, the present study has demonstrated that a formula with a protein-energy ratio of 1.90 g/100 kcal from modified, partially hydrolyzed whey proteins supports normal growth of term infants. The addition of the probiotic, Bifidobacterium lactis, provides protection against diarrhea.
References
Bachmann C, Haschke-Becher E (2002) Plasma amino acid concentrations in breast-fed and formula-fed infants and reference intervals. In: Räihä NCR, Rubaltelli FF (eds) Infant formula: closer to the reference. Lippincott Williams & Wilkins, Philadelphia, pp 121–137
Fomon SJ (1991) Requirements and recommended dietary intakes of protein during infancy. Pediatr Res 30:391–395
Fomon SJ, Nelson SE (1993) Size and growth. In: Fomon SJ (ed) Nutrition of normal infants. Mosby, St. Louis, pp 36–84
Fomon SJ, Ziegler EE (1999) Renal solute load and potential renal solute load in infancy. J Pediatr 134:11–14
Fomon SJ, Ziegler EE, Nelson SE, Rogers RR, Frantz JA (1999) Infant formula with protein-energy ratio of 1.7 g/100 kcal is adequate but may not be safe. J Pediatr Gastroenterol Nutr 28:495–501
Fomon SJ, Ziegler EE, Nelson SE, Frantz JA (1995) What is the safe protein-energy ratio for infant formulas? Am J Clin Nutr 62:358–363
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Ghisolfi J, Roberfroid M, Rigo J, Moro G, Polanco I (2002) Infant formula supplemented with probiotics or prebiotics: never, now, or someday? J Pediatr Gastroenterol Nutr 35:467–468
Guandalini S, Gupta P (2002) The role of probiotics in gastrointestinal disorders of infancy and childhood. In: Räihä NCR, Rubaltelli FF (eds) Infant formula: closer to the reference. Lippincott Williams & Wilkins, Philadelphia, pp 29–43
Lehane DP, Levy AL, Ergis AS (1978) Colorimetric quantification of albumin in microliter volumes of serum. Ann Clin Lab Sci 8:122–126
Marteau PR, de Verse M, Cellier CJ, Schrezenmeir J (2001) Protection from gastrointestinal diseases with use of probiotics. Am J Clin Nutr 73:430S-436S
Metges CC (2001) Does dietary protein in early life affect the development of adiposity in mammals? J Nutr 131:2062–2066
Nelson, SE, Rogers, RR, Ziegler, EE, Fomon, SJ (1989) Gain in weight and length during early infancy. Early Hum. Dev. 19:223–239
Räihä NCR, Fazzolari Nesci A, Cajozzo C, Puccio G, Monestier A, Moro GE, Minoli, Haschke-Becher E, Bachmann C, Van't Hof M, Carrié-Fässler A-L, Haschke F (2002) Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal is adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr 35:275–281
Saavedra JM (2002) Probiotic agents: clinical applications in infants and children. In: Räihä NCR, Rubaltelli FF (eds) Infant formula: closer to the reference. Lippincott Williams & Wilkins, Philadelphia, pp 15–24
Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH (1994) Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049
Ziegler EE (2002) Protein requirements in infancy. In: Räihä NCR, Rubaltelli FF (eds) Infant formula: closer to the reference. Lippincott Williams & Wilkins, Philadelphia, pp 97–110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziegler, E.E., Jeter, J.M., Drulis, J.M. et al. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: infant growth and health. Monatsschr Kinderheilkd 151 (Suppl 1), S65–S71 (2003). https://doi.org/10.1007/s00112-003-0804-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00112-003-0804-0