Abstract
Connexins (Cxs) are ubiquitous transmembrane proteins that possess both channel function (e.g., formations of gap junction and hemichannel) and non-channel properties (e.g., gene transcription and protein-protein interaction). Several factors have been identified to play a role in the regulation of Cxs, which include those acting intracellularly, as redox potential, pH, intramolecular interactions, and post-translational modifications (e.g., phosphorylation, S-nitrosylation) as well as those acting extracellularly, such as Ca2+ and Mg2+. The relationship between redox signaling and Cxs attracts considerable attention in recent years. There is ample evidence showing that redox signaling molecules (e.g., hydrogen peroxide (H2O2), nitric oxide (NO)) affect Cxs-based channel function while the opening of Cx channels also triggers the transfer of various redox-related metabolites (e.g., reactive oxygen species, glutathione, nicotinamide adenine dinucleotide, and NO). On the basis of these evidences, we propose the existence of redox-Cxs crosstalk. In this review, we briefly discuss the interaction between redox signaling and Cxs and the implications of the intersection in disease pathology and future therapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Connexins (Cxs) are ubiquitous transmembrane proteins present in multiple cell types (e.g., endothelial cells, smooth muscle cells) in almost all living organisms [1]. In humans, there are hitherto 21 genes encoding different Cx protein isoforms while 20 members have been identified in mice (Table 1). These proteins are named according to their predicted molecular weight, which ranges from 23 to 62 KDa (e.g., Cx23, Cx62) [2]. Based on sequence homology, Cxs are divided into five subfamilies (α, β, γ, δ, and ε or GJA, GJB, GJC, GJD, and GJE) [2, 3] (Table 1). Structurally, each Cx protein is formed by four transmembrane domains (TM1-TM4), two extracellular loops (EL1 and EL2), one intracellular loop (IL), and both the N- and C-terminus being oriented toward the cytoplasm [4]. Generally, TMs and ELs are highly conserved among the members of Cxs family. In contrast, the N- and C-terminals, as well as the IL, have marked differences among distinct Cx isoforms in the aspect of the length and composition [5, 6].
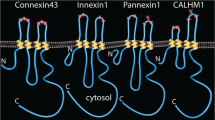
The members of Cxs family initially form high-conductance channels in plasma membrane, mediating the intercellular communication via gap junctions (GJs) which are composed of two homomeric or heteromeric hexamers (called connexons or hemichannels (HCs)) (Fig. 1). Cx-forming GJ channels allow direct passage of second messengers (e.g., cAMP, inositol 1, 4, 5-trisphosphate (IP3), cGMP), metabolites, ions, and electrical signals between adjacent cells [7, 8], indispensable for functional coordination in organisms. For instance, stimulation of irradiation by X-rays leads to the opening of Cx43-HCs in brain microvascular endothelial cells and subsequent release of IP3/Ca2+, ATP, reactive oxygen species (ROS) and nitric oxide (NO), which serves as crucial propagators of bystander DNA damage [9]. Delivery of glutathione (GSH) via Cx46-GJ can also occur in the lens nucleus, which is of vital importance for the protection against oxidative stress (OS) and lens transparency [10]. The research on the role of Cxs in the formations of GJs and HCs has been the topic of much discussion over the past 30 years. However, emerging evidence has delineated that, apart from the role in intercellular communication, Cxs also possess other biological functions including gene transcription [11] and protein-protein interactions [12,13,14,15] (Fig. 1). For example, recent evidence illustrates that basic transcription factor-3 (BTF3) binds to the Cx43 carboxy tail and translocates it into the nucleus [11]. Nuclear Cx43 then forms a complex with PolII, which regulates N-cadherin transcription via binding to its promoter, finally facilitating neural crest migration, pointing to the biological role of Cxs in transcriptional regulation. Aside from the role in gene transcription, the Cx43 carboxyl terminal domain has also been shown to interact with multiple proteins including zonula occludens-1 (ZO-1) [12], caveolin-1 (Cav-1) [15], c-Src [12], β-catenin [13], and tubulin [14]. Protein-protein interactions mediated by Cxs are likely to have regulatory effects.
The channel-based and non-channel functions of Cxs. Cxs are ubiquitous transmembrane proteins which possess both channel-dependent (e.g., gap junction and hemichannel) and channel-independent functions (e.g., gene transcription and protein-protein interactions). In terms of channel-dependent functions, gap junction is formed by two hemichannels (which is often composed of six connexin protein isoforms). With respect to channel-independent functions, Cxs can bind to various transcription factors (e.g., BTF3, Nrf2) to regulate gene expression. Besides, diverse proteins have been demonstrated to interact with Cx family members notably Cx43, which includes Cav-1, c-Src, p-catenin, ZO-1, and tubulin. Cxs, connexins; BTF3, basic transcription factor-3; Nrf2, nuclear factor erythroid 2-related factor 2; Cav-1, caveolin-1; ZO-1, zonula occludens-1
The regulatory mechanism underlying the biological roles of Cxs is complex. Several factors have been extensively studied, which generally include those acting intracellularly, as redox potential [16,17,18], pH [19], intramolecular interactions, and post-translational modifications [20] (e.g., phosphorylation, ubiquitination, SUMOylation, S-nitrosylation (SNO) and hydroxylation), as well as those acting extracellularly, such as Ca2+ [21] and Mg2+ [22]. Among these factors, the relationship between redox signaling and Cxs attracts great attention in recent years. Disrupted redox homeostasis often triggers the opening of HC or GJ channels. The direct evidence supporting redox regulation of Cxs arises from the fact that the decrease of intracellular redox potential augments the opening of Cx43-composed HCs [16]. Induction of oxidative injury after thioredoxin deficiency via incubation of a thioredoxin inhibitor, PX-12, in the renal tubular cell has also been demonstrated to cause overproduction of ROS and subsequently increase Cx43 expression [23]. In contrast, suppression of ROS generation with antioxidants (e.g., GSH, apocynin) or genetic silence of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase 2 (NOX2) can block Cx43 elevation and inflammasome activation in peritoneal macrophages [24]. In this respect, it is plausible that changes in redox potential have a direct effect on the expression of Cx protein subunits. The importance of the redox signaling molecule in the regulation of Cxs-based channel function also arises from the study showing that NO donor S-nitrosoglutathione (GSNO) promotes SNO of Cx46 at cysteine (Cys)212 residue in the fourth TM and triggers the opening of HCs in human epithelial lens cell line HLE-B3 [25]. These data raise the possibility that intracellular oxidative status has the regulatory role on the biological processes of Cxs [25]. However, there are also investigations showing that dysregulation of Cxs is able to influence the redox signaling. For example, suppression of Cx43 is found to decrease the expression of NOX2 [24]. Cx43 HCs are also reported to mediate transmembrane nicotinamide adenine dinucleotide (NAD+) fluxes in intact cells [26]. It suggests that redox signaling and Cxs interact with each other within living organisms. Until now, the molecular mechanism underlying this interaction is not well characterized. In this review, we aim to make a comprehensive summarization of the current knowledge on the interaction between redox signaling and Cxs and discuss the implications of these functions in disease pathology and future therapeutic interventions.
Redox-mediated regulation of biological functions of Cxs
Effects of redox potential on Cx-based channel function
Generally, Cx-based channels comprise GJs and HCs. The study showing the modulation of changes in redox potential on the functional properties of GJs and HCs has been extensively discussed. In this section, we will discuss redox-mediated regulation on GJs and HCs, respectively (Table 2).
Redox potential on GJs
The modulation of redox on GJs activity is exerted by activation of protein kinases. The direct evidence arises from the prior work showing treatment with hydrogen peroxide (H2O2) in lens epithelial cells promotes PKCγ-dependent Cx43 phosphorylation at Ser368 residue, leading to decreased GJ plaques and activity [37]. In liver epithelial cell WB-F344, it has also been reported that redox-active GSH peroxidase mimetics and antioxidants [39] prevent the inhibitory effect of hyper-phosphorylation of Cx43 by 12-O-tetradecanoylphorbol-13-acetate on GJs-mediated coupling. However, exposure to H2O2 increases gap junctional communication in astrocytes and the reducing agents dithiothreitol (DTT) and N-acetyl-cystein (NAC) reverse this phenomenon [38]. These findings suggest that protein kinase activation is involved in the modulatory effect of agents that modify the redox equilibrium (e.g., H2O2) on the functional state of GJs. The redox signaling molecule NO has also been shown to reduce gap junctional channel gating by activating the cGMP-cGMP-dependent protein kinase G (PKG) pathway as application of NO donor sodium nitroprusside (SNP) remarkably inhibits electrical synapses of hybrid bass H2-type horizontal cells [32]. Treatment with a specific PKG inhibitor (RKRARKE) blocked NO-mediated inhibition of GJs activity and a phosphodiesterase inhibitor potentiated the reduction of macroscopic junctional conductance elicited by SNP [32]. Following the stimulation of inflammatory cytokines containing TNF-α, IL-1β, and IFN-γ, the islet releases NO and activates PKCδ, which ultimately decreases Cx36 GJs coupling [28]. In HeLa cells, NO causes the uncoupling via PKA-dependent Cx35 phosphorylation at Ser110 and Ser276 residues and these results are prevented via mutation of these sites [33]. Collectively, it implicates that NO modulates GJs activity in different cell types at least via PKG, PKCδ, and PKA. In addition, there are other mechanisms underlying redox regulation of GJs communication, which include SNO modification. The direct evidence stems from the study depicting that endothelial NO synthase (eNOS)/GSNO reductase-induced SNO/denitrosylation of the Cys271 in the C-terminal of Cx43 in endothelial cells modulates the permeability of GJs to InsP3 [36]. Redox signaling also directly affects GJs communication. For instance, NO reduces Cx37-mediated GJs coupling in HeLa cells [34] and microvascular endothelial cells [35]. These data indicate that NO reduces the functional activity of GJs via PTMs including phosphorylation and SNO of C-terminal residues and direct oxidation.
Redox potential on HCs
In addition to the effect on GJs, redox potential also modulates HCs permeability and/or open probability. The first evidence arises from the study that treatment with free radical scavenger Trolox potently reduces the opening of Cx43 HCs following metabolic inhibition in rat astrocytes [40]. It implicates that, in metabolic stress conditions, accumulation of free radicals causes the opening of Cx43-mediated HCs via an unknown mechanism. Meanwhile, despite Cx43 dephosphorylation, which is sufficient for permeability, Cx43-HCs are still closed after lowering intracellular redox potential with Trolox [40]. It proves again that oxidation of Cx43 is critical for the open state of HCs. Similarly, extracellular application of GSH-ethyl ester, which is a membrane permeant-reducing agent and able to produce GSH intracellularly via esterases [41], also reduces dye uptake in Cx43-HCs, while incubation with the impermeant GSH has no significant effect [27]. It suggests that intracellular cysteine residue is affected by redox potential. However, it is noted that pharmacological inhibition of redox potential with DTT or GSH in the recording pipette potently increases Cx43-HC open probability in HeLa cells [16]. The differential effects of oxidative status on the HCs activity may be due to different cell types. The study concerning the effects of redox signaling molecule NO on HCs has also been extensively discussed. In a mouse model of Duchenne muscular dystrophy, NO promotes SNO of Cx43 at Cys271 and increases the opening of HCs [29]. Similarly, activation of NO synthase (NOS) by nebivolol also triggers NO production and subsequently promotes Cx43-SNO, finally increasing HCs activity and blunting the contraction in the myometrium, which presents a novel approach for treating spontaneous preterm labor [30]. Cx46-based HCs are also susceptible to oxidation by NO since the donor GSNO has been shown to increase HCs voltage sensitivity [42]. However, mutation of one or more Cys residues located in the C-terminal domain of Cx46 evidently changes HCs properties. The relationship between Cx46-SNO and lens opacity has also been studied in an animal model of cataract [25]. The NO donor GSNO promotes Cx46-SNO and increases HC opening in human lens epithelial cell HLE-B3 [25]. Mutation of the Cys212 located in TM4 abrogates the effect of GSNO on HCs conductivity, suggesting C212 is essential to observe the NO donors’ effects. Altogether, NO acts as an important redox signaling molecule to affect the property of Cx46 HCs. The role of NOX2, responsible for OS, in Cx43-SNO and HCs permeability are also investigated in mice with Duchenne muscular dystrophy [31]. In this model, enzymatic activity of NOX2 is found to be elevated and pharmacological inhibition of NOX2 with apocynin reduces OS, decreases SNO, and blunts the opening of Cx43 HCs. It implicates that NOX2-derived OS serves as an important mechanism for Cx43-SNO and HCs conductivity. Taken together, SNO modification is likely to be of utmost importance for the functional state of Cx-HCs. There are other reports on the role of carbonylation for Cx-HCs. Treatment with 4-hydroxynonenal (4-HNE), a common lipid peroxide following OS, promotes carbonylation of Cys located in the EL and inhibits Cx46-HCs, which can be blocked by DTT [43]. These data raise the possibility that carbonylation modification is another important mechanism for the redox regulation of Cx-HCs.
With respect to other redox signaling molecules such as carbon monoxide and hydrogen sulfide, there is hitherto no direct evidence to support their modulation on the properties of Cx-based channel function. Further investigation is indispensable to explore the effect of these molecules on channels formed by Cxs in order to comprehensively understand the regulatory role of redox signaling on Cxs-based channel functions.
Effects of redox potential on Cx-based non-channel function
In recent years, it has been illustrated that Cxs have channel-independent functions involving gene transcription and protein-protein interactions [44, 45]. There is ample evidence demonstrating that the presence of Cx43 in the nucleus has been described in human gliomas, colorectal tumors, and lung tumors and normal cells like primary chondrocytes [46,47,48,49]. Notably, Cx43 binds to BTF3 and promotes the translocation of Cx43 tail to the nucleus in neural crest cells [11]. Ectopic expression of Cx43 C-terminus is also found to translocate to the nucleus and suppress cell proliferation [50]. In the aspect of protein-protein interactions, diverse proteins have been demonstrated to bind to Cx family members notably Cx43, which includes Cav-1, c-Src, β-catenin, ZO-1, and tubulin [12,13,14]. Although there is no direct evidence supporting redox signaling regulates the interactions of Cxs and other proteins, superoxide anion and H2O2 have been shown to downregulate Cav-1 expression and inhibit cell migration and invasion [51, 52]. It implicates that redox can regulate Cxs-associated proteins. Given that the non-channel characteristics are important for the role of Cxs in regulating biological processes, future investigation is essential to explore the effect of redox signaling on the interactions of Cxs and other proteins.
Effects of Cxs on redox signaling
Permeability to redox-related metabolites
ROS
Cx43 is of vital importance in ROS transfer between cells as it has been demonstrated that Cx43-deficient hematopoietic stem cells are unable to transfer ROS to the hematopoietic microenvironment (HM), leading to the accumulation of ROS within hematopoietic stem and progenitor cells (HSCs) [53]. Re-expression of Cx43 in HSC and progenitor (HSC/P) cells rescue ROS transfer. It indicates that Cx43 exerts a protective role and regulates the ROS level in the HSC/P ROS through ROS transfer to the HM, resulting in functional recovery of HSC during hematopoietic regeneration. However, there are some other contradictory investigations. For example, Cx43 inhibition is found to protect against acute kidney injury following liver transplantation through reducing ROS transmission between the neighboring cells [54]. In brain microvascular colorectal cells, it has also shown that blockade of Cx43-HC with the channel-targeting peptide Gap26 and Gap19 as well as small molecule inhibitor carbenoxolone (CBX) reduces ROS transfer between adjacent cells [9]. Cx32 deficiency decreases ROS generation and distribution between the neighboring cells, which attenuates ischemia-reperfusion-induced cell apoptosis and renal damage [55]. The discrepancy on the role of Cxs in ROS transport from cell to cell or from cell to extracellular microenvironment is lack of mechanistic bases. However, it indicates, to some extent, that the biological roles of Cxs significantly affect ROS transfer.
GSH
HC opening can facilitate the transport of the antioxidant molecule GSH into extracellular environment [56]. HCs-mediated GSH uptake can also protects lens fiber cells against OS and maintain the functional status of HCs [57]. In this facet, permeability to GSH via HCs has a beneficial effect on the maintenance of homeostasis. Knock out of Cx46 is reported to reduce nuclear GSH level while it is not influenced by Cx50 deficiency [10], suggesting GSH diffuses from fiber cells to the nucleus are specific for Cx46.
NAD+
It has been demonstrated that treatment with a HC inhibitor, CBX, significantly abrogates the elevation of cellular NAD+ level and rescues poly-ADP-ribose-polymerase-induced cell death [58], suggesting that HCs are permeable to NAD+. The NAD+ transport is also affected by Cx43 expression as previous investigations revealed that treatment with a Cx43-antisense oligonucleotide completely inhibits intercellular NAD+ trafficking in murine 3T3 fibroblasts [26].
NO
The opening of HCs is found to be permeable to NO in HeLa cells transfected with Cx43, Cx40, or Cx37 [59]. Accordingly, blockade of Cx-based channels abolishes NO transfer in the myoendothelial cells and acetylcholine-induced vasodilation [60]. These results indicate that Cx-based channels significantly control NO-dependent vascular function.
Mitochondria transfer and Cx43 in mitochondria
In 2006, Spees and his colleagues firstly revealed the process of intercellular mitochondrial transfer, which enables A549 cells with functional mitochondria from mesenchymal stem cells (MSCs) [61]. Since then, mitochondrial transfer has been extensively investigated. The evidence supporting the relationship of Cxs and intercellular mitochondrial transfer stems from the study that mitochondria transmission from astrocytes to neurons is promoted by the downregulation of Cx43 phosphorylation elicited via GJA1-20K [62], the most abundant small isoform of Cx43 which is essential for full-length Cx43 trafficking, in nitrogen-oxygen induced traumatic brain injury-like damage in vitro. Promotion of normal mitochondrial transfer from astrocytes to neurons appears to be a novel treatment approach for brain injury. Hematopoietic Cx43-deficient chimeric mice also show reduced mitochondrial transfer, which is rescued upon re-expression of Cx43 in HSCs or culture with isolated mitochondria from Cx43-deficient HSCs [63]. Although there is no evidence showing the alteration of redox signaling during mitochondria trafficking, the biological function of Cxs is likely to be affected by ROS as mitochondria are the major sources of ROS generation [64].
In the aspect of mitochondrial Cx43, it was for the first time demonstrated that the common Cx isoform Cx43 is located in the mitochondria of human umbilical vein endothelial cells in 2002 [65]. Since then, full-length Cx43 has also been described in the mitochondria of astrocytes and cardiomyocytes [24, 66, 67]. Mitochondrial Cx43 expression is associated with respiratory complex I activity, oxygen consumption [66] and potassium uptake [68]. In recent years, the relationship between mitochondrial Cx43 and ROS production has been discussed. For instance, in doxorubicin-induced cardiotoxicity, mitochondrial Cx43 exerts cardioprotective effects via reducing mitochondrial ROS production [69]. Inhibition of Cx43 translocation to mitochondria significantly increases mitochondrial superoxide formation and the consequent apoptosis induced by trastuzumab [70]. Although the exact mechanisms by which Cx43 regulates ROS generation remain mostly unknown, these data described above implicate that overexpression of Cx43 in mitochondria plays a role in decreasing ROS concentration, essential for the protective effect of various disease pathologies including ischemia pre- or post-conditioning.
Conclusions and future perspectives
The evidence described in the review supports that redox and Cxs have a mutual interplay, which is involved in disease pathology. On the one hand, molecules associated with redox potential affect the properties of Cx channels including GJs and HCs. Oxidation of Cxs may act as an important mechanism to regulate the functional activity of GJs and HCs especially in many pathological processes because in these conditions release of diverse free radicals such as H2O2 and NO appear, which has been demonstrated to modulate GJs and HCs. While the exact mechanism underlying redox regulation of channels formed by Cxs is not well characterized, several probable mechanistic bases are highlighted which include protein kinase activation and SNO modification at the Cys residues of Cxs as mentioned above. On the other hand, Cx-composed channels mediate the permeability to redox-related metabolites such as ROS, GSH, NAD+, and NO (Fig. 2). Although the potential molecular mechanism requires an extensive discussion, it indicates, to some extent, that redox potential is influenced by alteration of Cx channels. Altogether, these descriptions point to the existence of redox-Cxs crosstalk particularly in disease pathology and targeting this interaction brings hope for treating various human diseases.
Cxs mediate the transport of redox signaling molecules. Cxs can mediate the permeability of diverse redox-related metabolites, including ROS, GSH, NAD+, and NO. Cx43-mediated GJs and HCs as well as Cx32-composed HCs can mediate the ROS transfer. Cx46-formed GJs are permeable to intracellular GSH flux. Cx43 and Cx50-composed HCs can cause efflux of GSH and intracellular GSH flux, respectively. NAD+ transport is affected by Cx43-HCs. The functional states of HCs formed by Cx43, Cx40, or Cx37 are able to modulate NO level. Cx, connexin; GJ, gap junction; GSH, glutathione; HC, hemichannel; NAD+, nicotinamide adenine dinucleotide; NO, nitric oxide; ROS, reactive oxygen species
There are several considerations to be clarified. Firstly, since Cxs have been recently shown to possess non-channel functions such as gene transcription and protein interaction as we mentioned above, redox potential may modulate Cx-based non-channel activity, which requires further clarification in ongoing studies. Secondly, in terms of the effect of Cxs on redox signaling, certain Cx isoform, in particular Cx43, located in the mitochondria, causes mitochondria dysfunction and ROS generation. It is necessary to completely clarify the exact molecular mechanisms to comprehensively understand the interaction of redox and Cxs.
Abbreviations
- BTF3:
-
Basic transcription factor-3
- Cav-1:
-
Caveolin-1
- CBX:
-
Carbenoxolone
- Cx:
-
Connexin
- Cys:
-
Cysteine
- DTT:
-
Dithiothreitol
- EL:
-
Extracellular loop
- eNOS:
-
Endothelial nitric oxide synthase
- GJ:
-
Gap junction
- GSH:
-
Glutathione
- GSNO:
-
S-nitrosoglutathione
- H2O2 :
-
Hydrogen peroxide
- HC:
-
Hemichannel
- HM:
-
Hematopoietic microenvironment
- HSCs:
-
Hematopoietic stem cells
- HSC/P:
-
HSC and progenitor
- IL:
-
Intracellular loop
- IP3:
-
Inositol 1, 4, 5-trisphosphate
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- NO:
-
Nitric oxide
- NOX2:
-
NADPH oxidase 2
- OS:
-
Oxidative stress
- PKG:
-
Protein kinase G
- ROS:
-
Reactive oxygen species
- SNO:
-
S-nitrosylation
- TM:
-
Transmembrane domain
- ZO-1:
-
Zonula occludens-1
References
Bonacquisti EE, Nguyen J (2019) Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions. Cancer Lett 442:439–444
Abascal F, Zardoya R (2013) Evolutionary analyses of gap junction protein families. Biochim Biophys Acta 1828:4–14
Laird DW, Lampe PD (2018) Therapeutic strategies targeting connexins. Nat Rev Drug Discov 17:905–921
Söhl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232
Oshima A (2014) Structure and closure of connexin gap junction channels. FEBS Lett 588:1230–1237
Bai D, Yue B, Aoyama H (2018) Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim Biophys Acta Biomembr 1860:9–21
Alexander DB, Goldberg GS (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 10:2045–2058
Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A 105:18770–18775
Hoorelbeke D, Decrock E, De Smet M, De Bock M, Descamps B, Van Haver V, Delvaeye T, Krysko DV, Vanhove C, Bultynck G et al (2020) Cx43 channels and signaling via IP(3)/Ca(2+), ATP, and ROS/NO propagate radiation-induced DNA damage to non-irradiated brain microvascular endothelial cells. Cell Death Dis 11:194
Slavi N, Rubinos C, Li L, Sellitto C, White TW, Mathias R, Srinivas M (2014) Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J Biol Chem 289:32694–32702
Kotini M, Barriga EH, Leslie J, Gentzel M, Rauschenberger V, Schambony A, Mayor R (2018) Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun 9:3846
Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC (2004) Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem 279:54695–54701
Spagnol G, Trease AJ, Zheng L, Gutierrez M, Basu I, Sarmiento C, Moore G, Cervantes M, Sorgen PL (2018) Connexin43 carboxyl-terminal domain directly interacts with β-catenin. Int J Mol Sci 19. https://doi.org/10.3390/ijms19061562
Saidi Brikci-Nigassa A, Clement MJ, Ha-Duong T, Adjadj E, Ziani L, Pastre D, Curmi PA, Savarin P (2012) Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules. Biochemistry 51:4331–4342
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2008) Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19:912–928
Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC (2007) Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci U S A 104:8322–8327
Rozas-Villanueva MF, Casanello P, Retamal MA (2020) Role of ROS/RNS in preeclampsia: are connexins the missing piece? Int J Mol Sci 21. https://doi.org/10.3390/ijms21134698
Hua R, Zhang J, Riquelme MA, Jiang JX (2021) Connexin gap junctions and hemichannels link oxidative stress to skeletal physiology and pathology. Curr Osteoporos Rep 19:66–74
Skeberdis VA, Rimkute L, Skeberdyte A, Paulauskas N, Bukauskas FF (2011) pH-dependent modulation of connexin-based gap junctional uncouplers. J Physiol 589:3495–3506
Aasen T, Johnstone S, Vidal-Brime L, Lynn KS, Koval M (2018) Connexins: synthesis, post-translational modifications, and trafficking in health and disease. Int J Mol Sci 19. https://doi.org/10.3390/ijms19051296
Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa C, Magalhães-Cardoso MT, Ferreirinha F, Costa MA, Correia-de-Sá P (2013) Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun Signal 11:70
Tejada MG, Sudhakar S, Kim NK, Aoyama H, Shilton BH, Bai D (2018) Variants with increased negative electrostatic potential in the Cx50 gap junction pore increased unitary channel conductance and magnesium modulation. Biochem J 475:3315–3330
Zhou Y, Gao L, Xia P, Zhao J, Li W, Zhou Y, Wei Q, Wu Q, Wu Q, Sun D, Gao K (2021) Glycyrrhetinic acid protects renal tubular cells against oxidative injury via reciprocal regulation of JNK-connexin 43-thioredoxin 1 signaling. Front Pharmacol 12:619567
Huang Y, Mao Z, Zhang Z, Obata F, Yang X, Zhang X, Huang Y, Mitsui T, Fan J, Takeda M, Yao J (2019) Connexin43 contributes to inflammasome activation and lipopolysaccharide-initiated acute renal injury via modulation of intracellular oxidative status. Antioxid Redox Signal 31:1194–1212
Retamal MA, Orellana VP, Arévalo NJ, Rojas CG, Arjona RJ, Alcaíno CA, González W, Canan JG, Moraga-Amaro R, Stehberg J, Reuss L, Altenberg GA (2019) Cx46 hemichannel modulation by nitric oxide: role of the fourth transmembrane helix cysteine and its possible involvement in cataract formation. Nitric Oxide 86:54–62
Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A (2001) Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 15:10–12
Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC (2006) S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A 103:4475–4480
Farnsworth NL, Walter RL, Hemmati A, Westacott MJ, Benninger RK (2016) Low level pro-inflammatory cytokines decrease connexin36 gap junction coupling in mouse and human islets through nitric oxide-mediated protein kinase Cδ. J Biol Chem 291:3184–3196
Lillo MA, Himelman E, Shirokova N, Xie LH, Fraidenraich D, Contreras JE (2019) S-nitrosylation of connexin43 hemichannels elicits cardiac stress-induced arrhythmias in Duchenne muscular dystrophy mice. JCI Insight 4. https://doi.org/10.1172/jci.insight.130091
Barnett SD, Asif H, Anderson M, Buxton ILO (2021) Novel tocolytic strategy: modulating Cx43 activity by S-nitrosation. J Pharmacol Exp Ther 376:444–453
Vielma AZ, Boric MP, Gonzalez DR (2020) Apocynin treatment prevents cardiac connexin 43 hemichannels hyperactivity by reducing nitroso-redox stress in Mdx mice. Int J Mol Sci 21. https://doi.org/10.3390/ijms21155415
Lu C, McMahon DG (1997) Modulation of hybrid bass retinal gap junctional channel gating by nitric oxide. J Physiol 499(Pt 3):689–699
Patel LS, Mitchell CK, Dubinsky WP, O'Brien J (2006) Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes 13:41–54
Kameritsch P, Hoffmann A, Pohl U (2003) Opposing effects of nitric oxide on different connexins expressed in the vascular system. Cell Commun Adhes 10:305–309
McKinnon RL, Bolon ML, Wang HX, Swarbreck S, Kidder GM, Simon AM, Tyml K (2009) Reduction of electrical coupling between microvascular endothelial cells by NO depends on connexin37. Am J Physiol Heart Circ Physiol 297:H93–h101
Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE (2011) Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31:399–407
Lin D, Takemoto DJ (2005) Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions J Biol Chem 280:13682–13693
Rouach N, Calvo CF, Duquennoy H, Glowinski J, Giaume C (2004) Hydrogen peroxide increases gap junctional communication and induces astrocyte toxicity: regulation by brain macrophages. Glia 45:28–38
Hu J, Engman L, Cotgreave IA (1995) Redox-active chalcogen-containing glutathione peroxidase mimetics and antioxidants inhibit tumour promoter-induced downregulation of gap junctional intercellular communication between WB-F344 liver epithelial cells. Carcinogenesis 16:1815–1824
Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC (2002) Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A 99:495–500
Anderson ME, Powrie F, Puri RN, Meister A (1985) Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys 239:538–548
Retamal MA, Yin S, Altenberg GA, Reuss L (2009) Modulation of Cx46 hemichannels by nitric oxide. Am J Phys Cell Phys 296:C1356–C1363
Retamal MA, Fiori MC, Fernandez-Olivares A, Linsambarth S, Peña F, Quintana D, Stehberg J, Altenberg GA (1865) 4-Hydroxynonenal induces Cx46 hemichannel inhibition through its carbonylation. Biochim Biophys Acta Mol Cell Biol Lipids 2020:158705
Elias LA, Wang DD, Kriegstein AR (2007) Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448:901–907
Theis M, Söhl G, Eiberger J, Willecke K (2005) Emerging complexities in identity and function of glial connexins. Trends Neurosci 28:188–195
Crespin S, Fromont G, Wager M, Levillain P, Cronier L, Monvoisin A, Defamie N, Mesnil M (2016) Expression of a gap junction protein, connexin43, in a large panel of human gliomas: new insights. Cancer Med 5:1742–1752
Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E (2012) Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer 131:570–581
Aasen T, Sansano I, Montero M, Romagosa C, Temprana-Salvador J, Martínez-Marti A, Moliné T, Hernández-Losa J, Ramón y Cajal S (2019) Insight into the role and regulation of gap junction genes in lung cancer and identification of nuclear Cx43 as a putative biomarker of poor prognosis. Cancers (Basel) 11 https://doi.org/10.3390/cancers11030320
Gago-Fuentes R, Fernández-Puente P, Megias D, Carpintero-Fernández P, Mateos J, Acea B, Fonseca E, Blanco FJ, Mayan MD (2015) Proteomic analysis of connexin 43 reveals novel interactors related to osteoarthritis. Mol Cell Proteomics 14:1831–1845
Dang X, Doble BW, Kardami E (2003) The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 242:35–38
Kotova A, Timonina K, Zoidl GR (2020) Endocytosis of connexin 36 is mediated by interaction with caveolin-1. Int J Mol Sci 21. https://doi.org/10.3390/ijms21155401
Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P (2010) Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem 285:38832–38840
Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, Cancelas JA (2012) Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci U S A 109:9071–9076
Yuan D, Li X, Luo C, Li X, Cheng N, Ji H, Qiu R, Luo G, Chen C, Hei Z (2019) Inhibition of gap junction composed of Cx43 prevents against acute kidney injury following liver transplantation. Cell Death Dis 10:767
Gu Y, Huang F, Wang Y, Chen C, Wu S, Zhou S, Hei Z, Yuan D (2018) Connexin32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injury. J Transl Med 16:117
Chi Y, Zhang X, Zhang Z, Mitsui T, Kamiyama M, Takeda M, Yao J (2016) Connexin43 hemichannels contributes to the disassembly of cell junctions through modulation of intracellular oxidative status. Redox Biol 9:198–209
Shi W, Riquelme MA, Gu S, Jiang JX (2018) Connexin hemichannels mediate glutathione transport and protect lens fiber cells from oxidative stress. J Cell Sci 131. https://doi.org/10.1242/jcs.212506
Okuda H, Nishida K, Higashi Y, Nagasawa K (2013) NAD(+) influx through connexin hemichannels prevents poly(ADP-ribose) polymerase-mediated astrocyte death. Life Sci 92:808–814
Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC (2013) Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology 75:471–478
Hill CE, Hickey H, Sandow SL (2000) Role of gap junctions in acetylcholine-induced vasodilation of proximal and distal arteries of the rat mesentery. J Auton Nerv Syst 81:122–127
Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A 103:1283–1288
Ren D, Zheng P, Zou S, Gong Y, Wang Y, Duan J, Deng J, Chen H, Feng J, Zhong C, Chen W (2021) GJA1-20K enhances mitochondria transfer from astrocytes to neurons via Cx43-TnTs after traumatic brain injury. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-021-01070-x
Golan K, Singh AK, Kollet O, Bertagna M, Althoff MJ, Khatib-Massalha E, Petrovich-Kopitman E, Wellendorf AM, Massalha H, Levin-Zaidman S, Dadosh T, Bohan B, V. Gawali M, Dasgupta B, Lapidot T, Cancelas JA (2020) Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood 136:2607–2619
Zhang DC, Chen R, Cai YH, Wang JJ, Yin C, Zou K (2020) Hyperactive reactive oxygen species impair function of porcine Sertoli cells via suppression of surface protein ITGB1 and connexin-43. Zool Res 41:203–207
Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS (2002) Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart Circ Physiol 282:H2124–H2133
Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F et al (2005) Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res 67:234–244
Kozoriz MG, Church J, Ozog MA, Naus CC, Krebs C (2010) Temporary sequestration of potassium by mitochondria in astrocytes. J Biol Chem 285:31107–31119
Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodríguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K et al (2009) Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res 83:747–756
Pecoraro M, Pala B, Di Marcantonio MC, Muraro R, Marzocco S, Pinto A, Mincione G, Popolo A (2020) Doxorubicin-induced oxidative and nitrosative stress: mitochondrial connexin 43 is at the crossroads. Int J Mol Med 46:1197–1209
Pecoraro M, Pinto A, Popolo A (2020) Trastuzumab-induced cardiotoxicity and role of mitochondrial connexin43 in the adaptive response. Toxicol in Vitro 67:104926
Availability of data and material
Not applicable
Code availability
Not applicable
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 81974502, 81671293, and 81302750), Natural Science Foundation of Hunan Province (No. 2020JJ3061), and Hunan Provincial Department of Education Innovation Platform Open Fund Project (No. 17K100).
Author information
Authors and Affiliations
Contributions
XYM designed and revised the manuscript. KZ, QWG, and XYZ wrote the manuscript. QXX, XXY, and HHZ contributed to literature arrangement and the generation of figures and tables. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Guan, QW., Zhou, XY. et al. The mutual interplay of redox signaling and connexins. J Mol Med 99, 933–941 (2021). https://doi.org/10.1007/s00109-021-02084-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02084-0