Abstract
Gastric cancer (GC) is the third leading cause of cancer-related death worldwide. Very few therapeutic options are currently available in this neoplasia. The use of 5-Aza-2′-deoxycytidine (5-AZAdC) was approved for the treatment of myelodysplastic syndromes, and this drug can treat solid tumours at low doses. Epigenetic manipulation of GC cell lines is a useful tool to better understand gene expression regulatory mechanisms for clinical applications. Therefore, we compared the gene expression profile of 5-AZAdC-treated and untreated GC cell lines by a microarray assay. Among the genes identified in this analysis, we selected NRN1 and TNFAIP3 to be evaluated for gene expression by RT-qPCR and DNA methylation by bisulfite DNA next-generation sequencing in 43 and 52 pairs of GC and adjacent non-neoplastic tissue samples, respectively. We identified 83 candidate genes modulated by DNA methylation in GC cell lines. Increased expression of NRN1 and TNFAIP3 was associated with advanced tumours (P < 0.05). We showed that increased NRN1 and TNFAIP3 expression seems to be regulated by DNA demethylation in GC samples: inverse correlations between the mRNA and DNA methylation levels in the promoter of NRN1 (P < 0.05) and the intron of TNFAIP3 (P < 0.05) were detected. Reduced NRN1 promoter methylation was associated with III/IV TNM stage tumours (P = 0.03) and the presence of Helicobacter pylori infection (P = 0.02). The identification of demethylated activated genes in GC may be useful in clinical practice, stratifying patients who are less likely to benefit from 5-AZAdC-based therapies.
Key messages
-
Higher expression of NRN1 and TNFAIP3 is associated with advanced gastric cancer (GC).
-
NRN1 promoter hypomethylation contributes to gene upregulation in advanced GC.
-
TNFAIP3 intronic-specific CpG site demethylation contributes to gene upregulation in GC.
-
These findings may be useful to stratify GC patients who are less likely to benefit from DNA demethylating-based therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the third leading cause of cancer-related death worldwide. The absence of specific symptoms in the early tumour stages contributes to the diagnosis at advanced stages and the poor response to the currently available therapeutic options [1]. Even after curative resection and adjuvant therapy, approximately 35% of patients still develop recurrences [2].
Currently, no molecular biomarkers have been widely applied in clinical practice, which limits the successful management of patients [3]. The numerous GC histological and molecular classifications currently available reflect the complexity and heterogeneity of this cancer (see review [4]). Although recent high-throughput studies have classified gastric tumours into subgroups with clinical relevance [5], the underlying mechanism of gastric carcinogenesis should be further elucidated for the identification of novel biomarkers and the development of new therapeutic strategies.
Epigenetic alterations play an important role in the cellular transformation to cancer, and these alterations have strong application potential for cancer detection, diagnosis, and therapy. Two epigenetic drugs, 5-Azacytidine and 5-Aza-2′-deoxycytidine (5-AZAdC), have Food and Drug Administration (FDA) approval for the treatment of myelodysplastic syndromes and can treat solid tumours at low doses. Several other epigenetic agents are in clinical and preclinical studies [6].
The analysis of epigenetic markers may elucidate the regulatory mechanism of drivers of gastric carcinogenesis and contribute to the discovery of biomarkers that may help to identify patients who are more likely to benefit from the use of epigenetic drugs. Therefore, in this study, we first identified genes modulated by DNA methylation in GC by assessing the gene expression profile modified by treatment with the epigenetic 5-AZAdC drug in cell lines. To further explore epigenetically regulated genes involved in GC, we selected two differentially expressed genes (DEGs): neuritin 1 (NRN1, also referred to as CPG15) and tumour necrosis factor alpha induced protein 3 (TNFAIP3, also referred to as A20) for analysis of mRNA and methylation levels in GC and paired non-neoplastic tissue samples. We showed that the expression of these two genes is associated with advanced GC, and using next-generation sequencing of bisulfite-converted DNA, we observed DNA demethylation in CpG sites around the transcription start sites (TSSs) of genes in tumour samples.
Materials and methods
Cell lines and 5-AZAdC treatment
ACP02 and ACP03 cell lines were previously established by our research group from primary gastric adenocarcinomas [7]. A cell culture of non-neoplastic gastric mucosa cells pooled from 10 patients without GC (MNP01) was also used to evaluate NRN1 and TNFAIP3 gene expression. All cell lines were cultured in RPMI 1640 media (GIBCO, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (GIBCO) and 100 μg/mL kanamycin (GIBCO).
To evaluate the best dosage and the period of treatment with 5-AZAdC, ACP02, and ACP03 cells were seeded in duplicate on 96-well plates and then treated with 2 μM, 5 μM, or 10 μM of 5-AZAdC (Sigma-Aldrich, St. Louis, MO, USA) for 24, 72, or 120 h. Untreated cells were used as controls. Viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Invitrogen, Eugene, OR, USA) assay [8]. Both cell lines showed decreased cell viability of approximately 70% when treated with 5 μM of 5-AZAdC for 120 h (Supplementary Fig. 1). Therefore, this dose and this period of treatment were selected for further evaluation of the 5-AZAdC effect.
For the gene expression profile, cells were seeded in 75-cm2 cell culture flasks in triplicate and treated with 5 μM 5-AZAdC (Sigma-Aldrich) for 120 h. Untreated GC cells were used as controls.
Clinical samples
According to the studied genes, the sample size varied from 43 to 52 matched pairs of GC and the corresponding adjacent non-neoplastic tissues (control group). These samples were obtained from patients with gastric adenocarcinoma who underwent gastric resection in João de Barros Barreto University Hospital (HUJBB) and São Paulo Hospital (HSP), Brazil, from 2009 to 2018. None of the patients had a history of exposure to either chemotherapy or radiotherapy prior to surgery or the co-occurrence of diagnosed cancers. Written informed consent with the approval of the ethics committees of HUJBB and HSP was obtained from all patients before sample collection (Ethics Committee number 0511/09).
All of the samples were classified according to Lauren [9] and TNM staging criteria [10]. The presence of Helicobacter pylori, a class I carcinogen, and the cagA virulence factor in gastric samples were detected by PCR as previously described [8].
DNA and RNA extraction
Total RNA was isolated from cell lines using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Only samples with an RNA integrity number of ≥ 7 based on a Bioanalyzer (Agilent 2100 Bioanalyzer; Agilent Technologies, Waldbronn, Germany) and no DNA contamination were used in the microarray hybridization.
Total DNA and RNA were isolated from tissue samples using the AllPrep DNA/RNA/Protein Kit (Qiagen) according to the manufacturer’s instructions.
Microarray and bioinformatics analyses
The gene expression profile was assessed using the Affymetrix Human Gene 1.0 ST array (which covers 36,079 transcripts) following the manufacturer’s instructions. Data were deposited in the ArrayExpress database [11] at the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) (https://www.ebi.ac.uk/arrayexpress/) under accession number E-MTAB-7880. Gene expression values were obtained using the three-step robust multiarray average preprocessing method implemented in the Affy package from R/Bioconductor (http://www.bioconductor.org/). The RankProd method [4] was employed for the selection of DEGs (FDR < 5%) [12]. Pathway analysis was carried out using the Ingenuity® Pathway Analysis (IPA®, http://www.ingenuity.com).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
cDNA was synthesized using High-Capacity® cDNA Reverse Transcription (Thermo Fisher Scientific, Waltham, MA, USA). TaqMan gene expression assays (Thermo Fisher Scientific) were used to evaluate the expression of NRN1 (Hs00213192_m1) and TNFAIP3 (Hs00234713_m1) together with B2 M (Hs00984230_m1), ACTB (Hs03023943_g1), or GAPDH (Hs99999905_m1) in triplicate. ACTB + B2M and GAPDH + B2M were used for the normalization of target gene expression in GC cell lines and tissue samples, respectively, as determined previously by our research group [13]. The mRNA levels were analysed using the ∆Ct method according to the manufacturer’s instructions.
DNA methylation analysis
The methylation profiles of NRN1 and TNFAIP3 CpG islands were evaluated by next-generation sequencing. Bisulfite treatment was carried out using 500 ng of DNA and the EpiTect Fast DNA Bisulfite kit (Qiagen, Hilden, Germany) in duplicate, in which only unmethylated cytosines are converted into uracils. Primers were designed by using MethPrimer software [14] to amplify sequences spanning the CpG island region (Figs. 2f and 3f). Supplementary Table 1 shows the primer sequences and PCR reaction conditions.
Sequencing was performed in duplicate using IonTorrent technology. Libraries were prepared using the Ion XpressTM Plus Fragment Library Kit (Thermo Fisher Scientific) and sequenced on an Ion Torrent™ PGM sequencer (Thermo Fisher Scientific) by using the Ion PGMTM Hi-QTM Sequencing kit (Thermo Fisher Scientific) and the Ion 318 Chip kit v2 (Thermo Fisher Scientific), following the manufacturer’s instructions.
Data were processed using a custom version of TABSAT [15, 16]. In detail, Bismark version 0.16.3 [17] using the TMAP mapper (version 5.2.25) was used to align the reads to the human reference (hg19) restricted to the target regions. Only reads with a range of 50–400 bp were used, and a maximum of one mismatch was tolerated. Short reads and low-coverage region sets were filtered out to reduce background noise [18]. After alignment, the methylation information was extracted and aggregated for the analysed samples. A minimum of 1000 CpG measurements across samples was required for each region set.
The percentage of methylation in CpG sites was determined as the percentage of cytosine reads from the total of cytosine and thymine reads of each CpG site. To quantify the percentage of methylation in a functional genomic region, the median percentage of methylation across CpG sites for each region was calculated. The median of the medians for the entire studied CpG island was also obtained.
Commercial non-methylated gDNA (Thermo Fisher Scientific) was used as an internal control to estimate the inefficiency of bisulfite conversion. More than 1% methylation was detected in seven (for NRN1) and five (for TNFAIP3) CpG sites, which were excluded from further analysis.
Statistical analysis of gene expression and DNA methylation
The Shapiro-Wilk normality test was used to evaluate the distribution of all data. Gene expression data from cell lines were normally distributed and analysed using t tests and Pearson correlation tests. Gene expression and DNA methylation data from paired tissue samples were not normally distributed and were analysed by using the Wilcoxon test and Spearman test. Clinicopathological associations were assessed by the Mann-Whitney test. P-values were two-sided, and all confidence intervals were at the 95% level. Statistical tendencies (0.05 < P ≤ 0.07) were considered significant when at least a medium effect size was detected by partial eta square (ηp2 > 0.16) or based on Cohen’s r (r > 0.11).
Results
Identification and selection of candidate genes regulated by DNA methylation in gastric cancer
We identified 83 DEGs, 50 upregulated and 33 downregulated, when comparing 5-AZAdC treated and untreated GC cell lines (Fig. 1a and Supplementary Table 2). Most of the DEGs have functions that are associated with gastrointestinal disease (Fig. 1b), and four relevant functions were enriched: (a) cell death and survival, cancer, organismal injury and abnormalities, and cellular development; (b) behaviour, gastrointestinal disease, and hepatic system disease; (c) cell death and survival, cell-to-cell signalling and interaction, and cell-mediated immune response; (d) cancer, carbohydrate metabolism, and the cell cycle (Fig. 1c).
Identification of candidate genes regulated by DNA methylation in GC cell lines. a List of 83 DEGs by comparing 5-AZAdC-treated and non-treated GC cell lines. b Diseases and disorders associated with DEGs by IPA. c Functional categories of the DEGs by IPA. The calculated score for each functional category is derived from a p value and indicates that the probability of genes has been found in a functional category and not by chance. A score of 2 or more has at least 99% confidence to not be generated by chance. FC; fold change
For the selection of candidate DEGs to be evaluated in the next step of the study, we established the following criteria: (a) upregulated DEGs to ensure a direct effect of 5-AZAdC; (b) fold change> 1.5-fold; (c) association with the main IPA functional category; and (d) presence of the CpG island near the TSS. Based on these criteria, we selected NRN1 and TNFAIP3 to be validated in the same RNA samples assessed by the microarray assay and to be evaluated for gene expression and DNA methylation levels in gastric tissue samples.
NRN1 and TNFAIP3 gene expression validation in gastric cancer cell lines
NRN1 and TNFAIP3 were increased in 5-AZA-treated GC cell lines compared with non-treated GC cell lines by microarray assay (P < 0.01, Fig. 2a; and P < 0.01, Fig. 3a, respectively) and mRNA levels detected by RT-qPCR (P = 0.04, Fig. 2b; and P = 0.04, Fig. 3b). Validating the microarray results, NRN1 and TNFAIP3 gene expression levels assessed by these two methodologies were strongly correlated (ρ = 0.7, P = 0.01, Fig. 2c; and ρ = 0.9, P < 0.01, Fig. 3c, respectively).
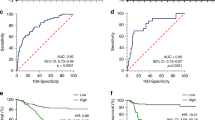
NRN1 mRNA and methylation levels in GC, a Significantly increased fluorescent signal of NRN1 in 5-AZAdC-treated GC cell lines compared with non-treated GC cells detected by a microarray assay. b Validation of microarray results showing increased NRN1 mRNA in 5-AZAdC-treated GC cell lines compared with non-treated GC cells by RT-qPCR. c Strong positive correlation between NRN1 gene expression assessed by a microarray assay and RT-qPCR. d Increased NRN1 mRNA in GC cell lines compared with non-neoplastic gastric mucosa cells, MNP01. e Absence of a significant difference in NRN1 mRNA between GC and the corresponding adjacent non-neoplastic tissue samples. f Schematic diagram of CpG dinucleotide density across the NRN1 locus and the location of the investigated CpG dinucleotides. g significant differences in NRN1 methylation levels at specific promoter CpG sites comparing GC and the corresponding adjacent non-neoplastic tissue samples. The purple squares represent the correlation between NRN1 mRNA and DNA methylation in all gastric tissue samples (tumour and control samples) and in tumour samples. h Significantly increased NRN1 mRNA in tumours of patients with distant metastasis. i Significantly increased NRN1 mRNA in tumours of patients with III/IV TNM stages. j Consistent hypomethylation throughout the 40 evaluated CpG sites of the NRN1 promoter region in tumours of patients with III/IV TNM stages. k Consistent hypomethylation throughout the 40 evaluated CpG sites of the NRN1 promoter region in tumours of patients positive for H. pylori infection. Cell line data are expressed as the mean ± standard deviation; tissue sample data are expressed as the median ± interquartile range. *Significant difference between groups by t test, Wilcoxon test or Mann-Whitney test. The arrow indicates the CpG binding site of the HIF-1 transcription factor TSS; transcription start site. SC; Spearman correlation coefficient
TNFAIP3 mRNA and methylation levels in GC. a Significantly increased fluorescent signal of TNFAIP3 in 5-AZAdC-treated GC cell lines compared with non-treated GC cells detected by a microarray assay. b Validation of microarray results showing increased TNFAIP3 mRNA in 5-AZAdC-treated GC cell lines compared with non-treated GC cells by RT-qPCR. c Very strong positive correlation between TNFAIP3 gene expression assessed by a microarray assay and RT-qPCR. d Reduced TNFAIP3 mRNA in GC cell lines compared with non-neoplastic gastric mucosa cells, MNP01. e Absence of a significant difference in TNFAIP3 mRNA between GC and the corresponding adjacent non-neoplastic tissue samples. f Schematic diagram of CpG dinucleotide density across the TNFAIP3 locus and the location of the investigated CpG dinucleotides. g Significant differences in overall methylation and at specific CpG sites of TNFAIP3 intronic region comparing GC and the corresponding adjacent non-neoplastic tissue samples. The colourful squares represent the correlation between TNFAIP3 mRNA and methylation levels in all gastric tissues (tumour and control samples) and in tumour samples. h Significantly increased TNFAIP3 mRNA in tumours of patients with poor cell differentiation. i Significantly increased TNFAIP3 mRNA in tumours of patients with T3/T4 invasion. j Significantly increased TNFAIP3 mRNA in tumours of patients with advanced stages. k Significantly increased TNFAIP3 mRNA in tumours of patients with III/IV TNM stages. l Significantly increased TNFAIP3 mRNA in tumours of patients with lymph node metastasis. Cell line data are expressed as the mean ± standard deviation; tissue sample data are expressed as the median ± interquartile range. *Significant difference between groups by t test, Wilcoxon test, or Mann-Whitney test. The arrow indicates the CpG binding site of the SMARCA4 transcription factor. TSS; transcription start site. SC; Spearman correlation coefficient
NRN1 and TNFAIP3 gene expression in gastric cancer
The expression of NRN1 was increased and TNFAIP3 was reduced in GC cell lines in relation to non-neoplastic gastric mucosa cells MNP01 (P = 0.06, ηp2 = 0.43, Fig. 2d; and P = 0.01, Fig. 3d).
As expected, the expression of these two genes was widely heterogeneous among GC tissue samples compared to the controls, with 58% of the tumours (25/43) showing reduced NRN1 levels (Fig. 2e) and 50% (26/52) showing increased TNFAIP3 levels (Fig. 3e).
NRN1 and TNFAIP3 methylation in gastric cancer
To better characterize the levels of DNA methylation and to understand its impact on gene expression regulation, the analysis of NRN1 and TNFAIP3 CpG island methylation was performed at CpG sites individually, as well as by grouping these CpG sites in functional gene regions (Figs. 2g and 3g).
Gastric tissue samples presented an NRN1 promoter methylation median of approximately 5% (Fig. 2g). Even so, GC tissue samples presented significantly increased methylation at the − 617 (P = 0.03), − 586 (P = 0.05, r = − 0.37), − 528 (P = 0.02), and − 493 (P = 0.03) CpG sites and reduced methylation at the − 218 (P = 0.07, r = − 0.34), and − 206 CpG sites (P = 0.03) compared with the controls (Fig. 2g).
Gastric tissue samples also presented a methylation median of the studied portion of the TNFAIP3 CpG island of approximately 0.3% (Fig. 3g). Even so, GC tissue samples presented significantly reduced methylation of the TNFAIP3 intron region (P = 0.04), as well as at the specific intron + 643 (P = 0.03) and + 741 (P = 0.04) CpG sites compared with controls (Fig. 3g).
Impact of NRN1 and TNFAIP3 gene expression and methylation on clinicopathological features
NRN1 gene expression was increased in tumours of patients with distant metastasis (P = 0.05, r = − 0.30; Fig. 2h and Supplementary Table 3) and III/IV TNM stages (P = 0.07, r = − 0.30; Fig. 2i and Supplementary Table 3).
NRN1 promoter methylation was reduced in tumours of patients at the III/IV TNM stage (P = 0.03; Fig. 2j and Supplementary Table 3). This reduced methylation was consistent throughout the 40 evaluated CpG sites of the promoter; 22 of them showed significantly reduced methylation levels (0.05 < P ≤ 0.07 and r > 0.11; Fig. 2j). In addition, NRN1 promoter methylation was reduced in tumours of patients positive for H. pylori infection (P = 0.02; Fig. 2k and Supplementary Table 3). This reduced methylation was consistent throughout the 40 evaluated CpG sites of the promoter; 26 of them showed significantly reduced methylation levels (0.05 < P ≤ 0.07 and r > 0.11; Fig. 2k).
TNFAIP3 gene expression was increased in tumours of patients with poor cell differentiation (P = 0.06, r = − 0.26; Fig. 3h and Supplementary Table 4), T3/T4 invasion (P = 0.02; Fig. 3i and Supplementary Table 4), advanced stages (P < 0.01; Fig. 3j and Supplementary Table 4), III/IV TNM stages (P = 0.02; Fig. 3k and Supplementary Table 4), and lymph node metastasis (P < 0.01; Fig. 3l and Supplementary Table 4). TNFAIP3 methylation showed no relevant clinicopathological associations (Supplementary Table 4).
Role of NRN1 and TNFAIP3 methylation in gene expression regulation
In general, considering GC and control samples, NRN1 expression and overall promoter methylation, as well as at specific CpG sites, were inversely correlated (P < 0.05 for each correlation; Fig. 2g and Supplementary Table 5). Some of these correlations were stronger in GC samples, even in sample size detriment (P < 0.05 for each correlation; Fig. 2g and Supplementary Table 5). In GC samples, the most significant inverse correlation was between NRN1 expression and methylation at the promoter − 145 CpG site (ρ = −0.68, P < 0.01; Fig. 2g and Supplementary Table 5). Using the Open Regulatory Annotation database (OregAnno) [19], we identified this CpG site as a HIF-1 transcription factor binding site.
Similarly, TNFAIP3 expression and methylation at specific CpG sites in the intronic region were significantly correlated considering GC and control samples (P < 0.05 for each correlation; Fig. 3g and Supplementary Table 6). Some of these correlations were strongly inversed in GC samples, even in sample size detriment (P < 0.05 for each correlation; Fig. 3g and Supplementary Table 6). In GC samples, the most significant inverse correlation was between TNFAIP3 expression and methylation at the intronic + 360 CpG site (ρ = −0.63, P < 0.01; Fig. 3g and Supplementary Table 6). Using OregAnno, we identified this CpG site as a SMARCA4 transcription factor binding site.
Discussion
In the present study, we initially focused on the screening of genes modulated by DNA methylation using GC cell lines. For this, we used cell line models established directly from primary GC [7] and treated them with a demethylation agent. This approach allowed us to identify 83 genes possibly regulated by DNA methylation. We then selected the NRN1 and TNFAIP3 genes to be further investigated in a panel of GC and matched controls to better elucidate the effect of DNA methylation in a heterogeneous population of tumours.
NRN1 has been associated with the development of tumours [20] through its role in hypoxia, angiogenesis, apoptosis, and proliferation [21]. However, very little is known about the function of NRN1 in GC. Yuan and collaborators [22] previously described increased NRN1 mRNA and protein levels in GC in relation to adjacent non-neoplastic samples. Although we observed that NRN1 is most highly expressed in GC cell lines in comparison to a cell culture of non-neoplastic gastric mucosa cells, our study revealed a very heterogeneous pattern of NRN1 gene expression in tumour samples in relation to controls. Further interrogating our population, we observed that tumours of patients with distant metastasis and III/IV TNM stages had the highest expression of NRN1. Therefore, our results suggest that the upregulation of NRN1 may play an important role in advanced GC.
To provide more evidence that NRN1 is regulated by epigenetic factors, we evaluated multiple CpG sites by next-generation sequencing. The NRN1 promoter was overall hypomethylated in gastric tissue samples; however, as expected, the DNA methylation status varied depending on the position of the CpG site. Most notably, four CpG sites more distant from TSS (approximately 550 bp upstream from TSS) were significantly less hypomethylated and two other CpG sites near TSS (approximately 200 bp upstream from TSS) were significantly more hypomethylated in GC samples in relation to controls. In addition, we identified an inverse correlation between NRN1 mRNA and overall promoter methylation levels and the 145 CpG site upstream of the TSS, which is a HIF-1 transcription factor binding site. HIF-1 constitutes an important mediator of cellular adaptation to hypoxia. HIF-1 transcriptionally upregulates several genes that play pivotal roles in the central features of cancer pathogenesis, such as angiogenesis, invasion, metastasis, and anti-apoptosis effects [23]. Supporting the relationship of NRN1 with poor GC prognosis, we also observed NRN1 promoter hypomethylation in tumours of patients at III/IV TNM stages. Taking together gene expression and promoter methylation results, as well as the inverse correlation between these parameters, our study shows for the first time that the loss of DNA methylation in the NRN1 promoter region may be a mechanism of gene activation in III/IV TNM stage GC.
It is known that H. pylori induces chronic inflammation that may lead to gene promoter hypermethylation [24] and gene type-specific methylation profiles involved in the multistep process of carcinogenesis [25].
Although the increased NRN1 mRNA levels in H. pylori positive tumours were not statistically significant in our results, the NRN1 promoter hypomethylation observed in these samples may be one of the mechanisms associated to increased NRN1 mRNA levels, allowing H. pylori to escape immune response. This hypothesis can be supported by a previous study that described the role of NRN1 in recruiting certain immune suppressive cells to escape immune surveillance [26].
Both TNFAIP3 overexpression [26,27,28,29,30,31,32] and downregulation [33, 34] were described in several tumour types. Our study reinforces this very heterogeneous pattern of TNFAIP3 expression in GC, probably related to its multiple roles in cancer development acting in apoptosis [35,36,37,38,39,40] and cell proliferation [36, 37, 41,42,43] pathways. Most notably, we observed that increased TNFAIP3 mRNA levels were associated with poor clinical patient outcomes, supporting a previous study in GC [43]. However, it is worth highlighting that CG cell lines showed lower expression of TNFAIP3 than normal cells. TNFAIP3 is a ubiquitin-editing enzyme originally identified as a nuclear factor-κB (NF-κβ) protein inhibitor, protecting cells from TNF-induced cytotoxicity [35] and preventing excessive inflammation via its deubiquitinase activity [36,37,38], but it also acts in TNF-independent inflammatory signalling [36] and acts outside the immune system [38]. This paradoxical role of TNFAIP3 may explain its heterogeneity in GC samples as well the discrepancy in relation to GC cell lines, because its expression must be modulated by the tumour microenvironment. The microenvironment along with external stimuli likely contributes to gene expression regulation, as well as changes in epigenetic markers [44].
Different mechanisms of TNFAIP3 transcription regulation have been described [45], including its activation by two NF-κβ binding sites in the promoter region [46] and by regulators of cell-intrinsic energy homeostasis, such as oestrogen-related α (ERRα), linking energy homeostasis to cell activation [47]. However, no study has reported the methylation pattern of TNFAIP3 in tumours, including GC. Thus, our study is the first to observe an inverse correlation between mRNA and specific CpG sites located in the studied TNFAIP3 intronic region, indicating that this gene may also be regulated epigenetically. Despite overall TNFAIP3 CpG island demethylation in gastric tissue samples, GC presented differences in DNA methylation in relation to controls, which depended on the genomic positions. Only the studied TNFAIP3 intronic region presented a significantly marked demethylation in GC compared with controls. Interestingly, our data also demonstrated a significant inverse correlation between TNFAIP3 mRNA CpG methylation at the intronic 360 bp downstream of TSS. This CpG site is a binding site of the SMARCA4 transcription factor, which is an ATPase subunit of the SWI/SNF chromatin remodelling complex. A study demonstrated that SMARCA4 is mostly overexpressed in many types of tumours [48] and has been associated with activated genes [49]. Therefore, we suggest that intronic CpG site demethylation may play a role in TNFAIP3 gene expression activation in GC, underscoring the importance of evaluating epigenetic markers in non-promoter CpG sites.
Our study is the first to investigate the possible involvement of DNA demethylation in NRN1 and TNFAIP3 activation in GC; however, there are two limitations that could be addressed here. First, it is worth noting that the correction for multiple comparisons (multiple CpG sites being evaluated simultaneously) was not carried out in the analysis of DNA methylation data. Because no similar study has been published previously, we chose to reject the null hypothesis to prioritize the biological effect rather than reject the involvement of an epigenetic event in GC due to statistical rigour (type II error).
The second limitation concerns our sample size. Because we reported in this study NRN1 and TNFAIP3 gene expression heterogeneity (some GC tissues presented gene upregulation and others presented gene downregulation), it would be interesting compare DNA methylation between these subgroups of samples, as well as to associate DNA methylation with clinicopathological features within each of these two subgroups. To analyse DNA methylation in a subdivided tissue sample according gene expression, it would be necessary a larger sample size.
In summary, our study demonstrated that higher expression of NRN1 and TNFAIP3 was associated with advanced CG. Using the bisulfite DNA sequencing standard gold methodology for methylation analysis, we originally demonstrated that NRN1 promoter hypomethylation may contribute to gene upregulation in GC, especially in advanced GC. In addition, TNFAIP3 intronic-specific CpG site demethylation may contribute to gene upregulation in GC. The identification of demethylated activated genes in GC may be applicable in the future, stratifying patients who are less likely to benefit from 5-AZAdC-based therapies.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Ito S, Ohashi Y, Sasako M (2018) Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer 18(1):449
Jin Z, Jiang W, Wang L (2015) Biomarkers for gastric cancer: progression in early diagnosis and prognosis (review). Oncol Lett 9(4):1502–1508
Breitling R, Armengaud P, Amtmann A, Herzyk P (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573(1–3):83–92
(2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517): 202–9
Zhao L, Duan YT, Lu P, Zhang ZJ, Zheng XK, Wang JL, Feng WS (2018) Epigenetic targets and their inhibitors in Cancer therapy. Curr Top Med Chem 18(28):2395–2419
Leal MF, Martins do Nascimento JL, da Silva CE, Vita Lamarao MF, Calcagno DQ, Khayat AS, Assumpcao PP, Cabral IR, de Arruda Cardoso Smith M, Burbano RR (2009) Establishment and conventional cytogenetic characterization of three gastric cancer cell lines. Cancer Genet Cytogenet 195(1):85–91
Wisnieski F, Calcagno DQ, Leal MF, Chen ES, Gigek CO, Santos LC, Pontes TB, Rasmussen LT, Payao SL, Assumpcao PP, Lourenco LG, Demachki S, Artigiani R, Burbano RR, Smith MC (2014) Differential expression of histone deacetylase and acetyltransferase genes in gastric cancer and their modulation by trichostatin a. Tumour Biol 35(7):6373–6381
Lauren P (1965) The two histological Main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a Histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17(12):3077–3079
Kolesnikov N, Hastings E, Keays M, Melnichuk O, Tang YA, Williams E, Dylag M, Kurbatova N, Brandizi M, Burdett T, Megy K, Pilicheva E, Rustici G, Tikhonov A, Parkinson H, Petryszak R, Sarkans U, Brazma A (2015) ArrayExpress update--simplifying data submissions. Nucleic Acids Res 43(Database issue):D1113–D1116
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Wisnieski F, Calcagno DQ, Leal MF, dos Santos LC, Gigek Cde O, Chen ES, Pontes TB, Assumpcao PP, de Assumpcao MB, Demachki S, Burbano RR, Smith Mde A (2013) Reference genes for quantitative RT-PCR data in gastric tissues and cell lines. World J Gastroenterol 19(41):7121–7128
Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18(11):1427–1431
Pabinger S, Ernst K, Pulverer W, Kallmeyer R, Valdes AM, Metrustry S, Katic D, Nuzzo A, Kriegner A, Vierlinger K, Weinhaeusel A (2016) Analysis and visualization tool for targeted amplicon bisulfite sequencing on ion torrent sequencers. PLoS One 11(7):e0160227
Krainer J, Weinhausel A, Hanak K, Pulverer W, Ozen S, Vierlinger K, Pabinger S (2019) EPIC-TABSAT: analysis tool for targeted bisulfite sequencing experiments and array-based methylation studies. Nucleic Acids Res 47(W1):W166–WW70
Krueger F, Andrews SR (2011) Bismark: a flexible aligner and methylation caller for bisulfite-Seq applications. Bioinformatics 27(11):1571–1572
Farlik M, Sheffield NC, Nuzzo A, Datlinger P, Schonegger A, Klughammer J, Bock C (2015) Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep 10(8):1386–1397
Lesurf R, Cotto KC, Wang G, Griffith M, Kasaian K, Jones SJ, Montgomery SB, Griffith OL (2016) ORegAnno 3.0: a community-driven resource for curated regulatory annotation. Nucleic Acids Res 44(D1):D126–D132
Hongchang Dong XL, Niu Y, Yu N, Gao R, Wang H, Yang L, Huang J (2018) Neuritin 1 expression in human normal tissues and its association with various human cancers. Int J Clin Exp Pathol 11(4):1956–1964
Zhang L, Zhao Y, Wang CG, Fei Z, Wang Y, Li L, Zhen HN (2011) Neuritin expression and its relation with proliferation, apoptosis, and angiogenesis in human astrocytoma. Med Oncol 28(3):907–912
Yuan M, Li Y, Zhong C, Niu J, Gong J (2015) Overexpression of neuritin in gastric cancer. Oncol Lett 10(6):3832–3836
Kitajima Y, Miyazaki K (2013) The critical impact of HIF-1a on gastric Cancer biology. Cancers (Basel) 5(1):15–26
Nardone G, Compare D, De Colibus P, de Nucci G, Rocco A (2007) Helicobacter pylori and epigenetic mechanisms underlying gastric carcinogenesis. Dig Dis 25(3):225–229
Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T (2009) The presence of a methylation fingerprint of helicobacter pylori infection in human gastric mucosae. Int J Cancer 124(4):905–910
Codd JD, Salisbury JR, Packham G, Nicholson LJ (1999) A20 RNA expression is associated with undifferentiated nasopharyngeal carcinoma and poorly differentiated head and neck squamous cell carcinoma. J Pathol 187(5):549–555
Hjelmeland AB, Wu Q, Wickman S, Eyler C, Heddleston J, Shi Q, Lathia JD, Macswords J, Lee J, McLendon RE, Rich JN (2010) Targeting A20 decreases glioma stem cell survival and tumor growth. PLoS Biol 8(2):e1000319
Guo Q, Dong H, Liu X, Wang C, Liu N, Zhang J, Li B, Cao W, Ding T, Yang Z, Zhang X (2009) A20 is overexpressed in glioma cells and may serve as a potential therapeutic target. Expert Opin Ther Targets 13(7):733–741
Dong B, Lv G, Wang Q, Wei F, Bellail AC, Hao C, Wang G (2012) Targeting A20 enhances TRAIL-induced apoptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun 418(2):433–438
Wang Y, Wan M, Zhou Q, Wang H, Wang Z, Zhong X, Zhang L, Tai S, Cui Y (2015) The prognostic role of SOCS3 and A20 in human Cholangiocarcinoma. PLoS One 10(10):e0141165
Hadisaputri YE, Miyazaki T, Yokobori T, Sohda M, Sakai M, Ozawa D, Hara K, Honjo H, Kumakura Y, Kuwano H (2017) TNFAIP3 overexpression is an independent factor for poor survival in esophageal squamous cell carcinoma. Int J Oncol 50(3):1002–1010
Lee JH, Jung SM, Yang KM, Bae E, Ahn SG, Park JS, Seo D, Kim M, Ha J, Lee J, Kim JH, Kim JH, Ooshima A, Park J, Shin D, Lee YS, Lee S, van Loo G, Jeong J, Kim SJ, Park SH (2017) A20 promotes metastasis of aggressive basal-like breast cancers through multi-monoubiquitylation of Snail1. Nat Cell Biol 19(10):1260–1273
Honma K, Tsuzuki S, Nakagawa M, Tagawa H, Nakamura S, Morishima Y, Seto M (2009) TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114(12):2467–2475
Ungerback J, Belenki D, Jawad ul-Hassan A, Fredrikson M, Fransen K, Elander N, Verma D, Soderkvist P (2012) Genetic variation and alterations of genes involved in NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis 33(11):2126–2134
Opipari AW Jr, Hu HM, Yabkowitz R, Dixit VM (1992) The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem 267(18):12424–12427
Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of toll-like receptor responses. Nat Immunol 5(10):1052–1060
Vereecke L, Beyaert R, van Loo G (2009) The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol 30(8):383–391
Lee E, Ouzounova M, Piranlioglu R, Ma MT, Guzel M, Marasco D, Chadli A, Gestwicki JE, Cowell JK, Wicha MS, Hassan KA, Korkaya H (2019) The pleiotropic effects of TNFalpha in breast cancer subtypes is regulated by TNFAIP3/A20. Oncogene 38(4):469–482
Maeda S, Otsuka M, Hirata Y, Mitsuno Y, Yoshida H, Shiratori Y, Masuho Y, Muramatsu M, Seki N, Omata M (2001) cDNA microarray analysis of helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem Biophys Res Commun 284(2):443–449
Sun F, Ni Y, Zhu H, Fang J, Wang H, Xia J, Ding F, Shen H, Shao S (2018) microRNA-29a-3p, up-regulated in human gastric cells and tissues with H. pylori infection, promotes the migration of GES-1 cells via A20-mediated EMT pathway. Cell Physiol Biochem 51(3):1250–1263
Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430(7000):694–699
Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289(5488):2350–2354
West AC, Tang K, Tye H, Yu L, Deng N, Najdovska M, Lin SJ, Balic JJ, Okochi-Takada E, McGuirk P, Keogh B, McCormack W, Bhathal PS, Reilly M, Oshima M, Ushijima T, Tan P, Jenkins BJ (2017) Identification of a TLR2-regulated gene signature associated with tumor cell growth in gastric cancer. Oncogene 36(36):5134–5144
Goodspeed A, Heiser LM, Gray JW, Costello JC (2016) Tumor-derived cell lines as molecular models of Cancer pharmacogenomics. Mol Cancer Res 14(1):3–13
Das T, Chen Z, Hendriks RW, Kool M (2018) A20/tumor necrosis factor alpha-induced protein 3 in immune cells controls development of autoinflammation and autoimmunity: lessons from mouse models. Front Immunol 9:104
Krikos A, Laherty CD, Dixit VM (1992) Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem 267(25):17971–17976
Yuk JM, Kim TS, Kim SY, Lee HM, Han J, Dufour CR, Kim JK, Jin HS, Yang CS, Park KS, Lee CH, Kim JM, Kweon GR, Choi HS, Vanacker JM, Moore DD, Giguere V, Jo EK (2015) Orphan nuclear receptor ERRalpha controls macrophage metabolic signaling and A20 expression to negatively regulate TLR-induced inflammation. Immunity 43(1):80–91
Guerrero-Martinez JA, Reyes JC (2018) High expression of SMARCA4 or SMARCA2 is frequently associated with an opposite prognosis in cancer. Sci Rep 8(1):2043
Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, Minder JL, Mercan F, Wang E, Eckersley-Maslin MA, Campbell AE, Kawaoka S, Shareef S, Zhu Z, Kendall J, Muhar M, Haslinger C, Yu M, Roeder RG, Wigler MH, Blobel GA, Zuber J, Spector DL, Young RA, Vakoc CR (2013) Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev 27(24):2648–2662
Acknowledgments
We are grateful to Brunno dos Santos Pereira, Renata Sanches de Almeida, and Camila Albuquerque Pinto for the help in sample collection and to Brunno dos Santos Pereira for the help in generating the final figures.
Funding
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; to FW, DQC, JCG, MFL, and MACS), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; to FW, DQC, JCG, RRB, and MACS), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; to COG and ACA).
Author information
Authors and Affiliations
Contributions
Conception and design: FW, MFL, RRB, and MACS. In vitro experiments: FW and DQ. Sample collection: FW, LCS, DQC, JCG, COG, ACA, ESC, PPA, LGL, and CHA. Pathological analysis: RA and SD. H. pylori diagnosis: LTR and SLMP. Molecular experiments: FW, LCS, DQC, JCG, COG, ACA, ESC, and MFL. Data analysis: FW, LCS, JCG, SP, and JK. Writing, review, and/or revision of the manuscript: all authors. Administrative, technical, or material support: MACS and RRB.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The study was approved by medical ethics committee of each study center. Written informed consent was obtained from each patient.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
GC cell line viability after 5-AZAdC treatment, Each point represents the mean ± standard error of three independent experiments. (PNG 1376 kb)
Supplementary Table 1
Primer sequences for DNA methylation analysis, *For target amplification, 25 ng of bisulfite-treated DNA was used in PCR reactions with Platinum™ Taq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA) (XLSX 10 kb)
Supplementary Table 2
List of 83 DEGs by comparing 5-AZAdC-treated and non-treated GC cell lines using de RP analysis. FC: fold change, FDR: false discovery rate (XLSX 12 kb)
Supplementary Table 3
Clinicopathological associations of NRN1 mRNA and DNA methylation levels. aAccording to the Lauren classification [9], bAccording to AJCC [10], c, dDetected in tumour samples as described previously [8], *Significant difference between groups by the Mann-Whitney test, n: number of samples. IQR: interquartile range (XLSX 13 kb)
Supplementary Table 4
Clinicopathological associations of TNFAIP3 mRNA and DNA methylation levels, aAccording to the Lauren classification [9], bAccording to AJCC [10], c, dDetected in tumour samples as described previously [8], *Significant difference between groups by Mann-Whitney test, n: number of samples. IQR: interquartile range (XLSX 15 kb)
Supplementary Table 5
Correlation between NRN1 mRNA and methylation levels in gastric tissue samples. (XLSX 13 kb)
Supplementary Table 6
Correlation between TNFAIP3 mRNA and methylation levels in GC tissue samples. (XLSX 15 kb)
Rights and permissions
About this article
Cite this article
Wisnieski, F., Santos, L.C., Calcagno, D.Q. et al. The impact of DNA demethylation on the upregulation of the NRN1 and TNFAIP3 genes associated with advanced gastric cancer. J Mol Med 98, 707–717 (2020). https://doi.org/10.1007/s00109-020-01902-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01902-1