Abstract
NF-κB and TGFβ play critical roles in renal inflammation and fibrosis, and their regulation in the kidney is thus of great interest. Early growth response-1 (Egr-1), a transcription factor belonging to the immediate early gene family, has been found to regulate inflammation and fibrosis in non-kidney tissues, but its role in renal failure has not been clear. In this study, wild-type and Egr1 −/− mice were fed with an adenine-enriched diet to induce tubulointerstitial nephritis (TIN), and primary tubular epithelial cells (PTECs) were treated with pro-inflammatory and pro-fibrotic cytokines. Kidney tissues from patients with or without renal failure were stained for Egr-1. Our results showed that Egr-1 expression was upregulated in the kidney with TIN, and the tubular epithelial cell is the primary site for Egr-1 upregulation and nuclear translocation. Egr1 −/− mice were protected from renal failure, reflected by low levels of serum urea and creatinine. The protective effect was related to an attenuation of tubular injury, immune cell infiltration, NF-κB activity, and cytokine/chemokine expressions in the kidney. Renal fibrotic area and TGFβ signaling were also reduced in Egr1 −/− mice. In vitro study showed that Egr-1 deficiency attenuated the ordinary responses of PTECs to TNFα and TGFβ. Importantly, Egr-1 is of clinical significance since the activity of Egr-1 in renal tubular cells was upregulated in renal failure patients. Our study highlights the integrative role of Egr-1 in renal inflammation and fibrosis. Thus, Egr-1 may serve as a therapeutic target for human kidney diseases.
Key messages
-
Renal failure activates Egr-1 in human and mouse tubular cells.
-
Egr-1 deficiency attenuates NF-κB and TGFβ-mediated renal inflammation/fibrosis.
-
Egr1 −/− PTECs respond weakly to pro-inflammatory or pro-fibrotic stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease is a pathological process of renal inflammation and fibrosis. Kidney injury activates NF-κB pathway, which in turn leads to renal inflammatory responses including macrophage infiltration and release of pro-inflammatory cytokines and chemokines [1]. The nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is also a potential regulator for renal inflammation [2]. Knocking down the components of NLRP3 inflammasome or its downstream inflammation-related genes attenuates tubulointerstitial nephritis (TIN) induced by an adenine-enriched diet [3]. Chronic renal inflammation leads to fibrosis, and transforming growth factor β (TGFβ) is central to the whole fibrogenic processes, mediating epithelial-mesenchymal transition (EMT) of tubular epithelial cells, and excess deposition of extracellular matrix (e.g., collagen) [4, 5]. Given the critical roles of NF-κB and TGFβ in inflammation and fibrosis, identifying how they are regulated is of great importance for the treatment of human renal diseases.
Early growth response-1 (Egr-1), an 80-kDa zinc-finger transcription factor of the immediate early gene family, has been found to regulate inflammation and fibrosis in various tissues. Egr-1 promotes inflammation in the animal models of cholestatic liver injury [6], ischemic and reperfusion lung injury [7], and atherogenesis [8]. Egr-1 upregulation is also essential for TGFβ-induced fibrosis in skin and lung [9, 10]. Several potential mechanisms have been proposed for Egr-1 in regulation of inflammation and fibrosis. For example, Egr-1 may enhance the expression of tumor necrosis factor-α (TNFα), NF-κB, and TGFβ, since the promoters of these genes all contain Egr-1 binding site [11–13]. Egr-1 also modulates NF-κB activity post-transcriptionally by direct interaction with NF-κB subunit [14], or indirectly via inducing activator of NF-κB, such as TNFα [15]. On the other hand, some studies found Egr-1 is able to antagonize NF-κB and TGFβ-mediated responses. For example, Egr-1 counteracts lipopolysaccharide (LPS)-induced NF-κB activation in human enterocytes [16], and hepatic fibrosis induced by carbon tetrachloride is enhanced in Egr1 −/− mice [17]. Thus, the consequences of Egr-1 activation in NF-κB and TGFβ pathways vary according to the insults and organs.
The role of Egr-1 in kidney injury has only been addressed in a few studies. During renal ischemia and reperfusion injury, Egr-1 protein is rapidly expressed and accumulated in the nuclei of the thick ascending limbs and collecting ducts [18]. Transfecting DNA enzyme to suppress Egr-1 activity in renal fibroblasts inhibits interstitial fibrosis in rats via downregulation of TGFβ, α-smooth muscle actin (αSMA) and type I collagen [19]. Despite these studies, the role of Egr-1 in renal inflammation and fibrosis has not been firmly established and not been tested in the animal model with systemic renal failure. In addition, it would be interesting to identify the major cell type responsible for Egr-1 upregulation in the kidney.
The aim of this study is to comprehensively explore the role of Egr-1 in kidney injury. The effects of Egr-1 deficiency on NF-κB-mediated renal inflammation and TGFβ-mediated renal fibrosis were verified, for the first time, in the animal model of TIN. The responses of Egr-1-deficient primary renal tubuloepithelial cells (PTECs) to pathological insults were also examined. Finally, Egr-1 expression in human kidney samples was assessed to correlate the bench work findings with clinical conditions.
Materials and methods
Mice
Egr1 −/− mice, kindly provided by J. Milbrandt (Washington University, St. Louis, MO, USA) [20], were maintained on the C57BL/6 genetic background and housed in a specific pathogen-free barrier facility. For the control group, wild-type littermates were generated by intercrossing Egr1 +/− mice. At 8 weeks of age, the mice were fed ad libitum with a 0.25 % adenine-enriched diet (LabDiet, St. Louis, MO) to induce TIN or a regular chow diet (Purina laboratory Rodent Diet 5001; PMI Nutrition International, Richmond, IN) as controls. After feeding for 10–13 days, the mice were sacrificed to harvest the kidneys. All procedures for handling animals were done in accordance to ethical statements approved by the Institutional Animal Care and Use Committee of National Cheng Kung University.
Primary cell culture and treatment
Mouse PTECs were isolated for culture according to the method of Tang and Tannen [21]. Briefly, mouse kidneys were removed, decapsulated, and the cortical tissues were minced and digested with 0.5 mg/ml collagenase type II (Worthington, Lakewood, NJ). The proximal tubules were isolated after density gradient centrifugation using 42 % Percoll solution as suspension and cultured in DMEM-F12 medium (Sigma-Aldrich) supplemented with 1× insulin-transferrin-selenium, 1× MEM non-essential amino acids, 0.1 μM hydrocortisone (Invitrogen, Carlsbad, CA), 100 IU/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich), and 1 % FBS (Invitrogen). To prove that authentic proximal tubuloepithelial cells were obtained, the expression of proximal tubule differentiation markers, including sodium-glucose cotransporter type 2 (Sglt2) and sodium-hydrogen exchanger isoform 3 (Nhe3), was analyzed [22]. For the treatments, PTECs were incubated in serum-free medium for 24 h and then treated with TNFα (PeproTech, Rocky Hill, NJ) at 10 ng/ml or 100 ng/ml for 1 h or 24 h, or with TGFβ (PeproTech) at 10 ng/ml for 4 h to 72 h.
Human renal tissues
Human renal tissues were obtained from the Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital. Ten tissues were kidney biopsy samples with histological diagnosis of diabetic nephropathy, confirmed by light microscopic, immunofluorescent, and electron microscopic examinations. These samples were classified as the “renal failure” group since the creatinine levels of these patients were above the normal limit (Supplementary Table 1). The other 12 renal tissues were derived from non-diabetic patients with serum creatinine level within normal limits and underwent nephrectomy for urothelial malignancy. The renal tissues of these patients, which were adjacent to the tumor, were classified as the “normal” group because no histological abnormality could be identified under microscopy. The study was conducted according to the study protocol approved by the Institutional Review Board of National Cheng Kung University Hospital (permit number: B-ER-101-008).
RNA analysis
Tissues and cells were stored in RNAlater solution (Ambion Inc., Austin, TX) and total RNA was extracted using REzol RNA extraction reagent (Protech Technology, Taipei, Taiwan). Samples of messenger RNA (mRNA) were analyzed by SYBR green-based real-time quantitative real-time polymerase chain reaction (RT-PCR) (Applied Biosystem, Foster City, CA), with β-actin (Actb), glyceraldehyde-3-phosphate dehydrogenase (Gapdh), or cyclophilin A (Ppia) as the reference gene in each reaction. The sequences of the primers are given in Supplementary Table 2.
Western blotting
Total proteins of kidneys were subjected to electrophoresis; transferred to PVDF membranes and probed with antibodies against Egr-1, apoptosis-associated speck-like protein containing a CARD (ASC), caspase-1 (Santa Cruz Biotechnology, CA); IκB, phosphorylated IκB kinase-α/β (p-IKKα/β), Smad3, phosphorylated-Smad3 (Cell Signaling Technology, Beverly, MA); TGFβ (PeproTech), NLRP3 (Abcam, Cambridge, MA); Interleukin-1β (IL-1β) (R&D systems, Minneapolis, MN); and β-actin (Sigma-Aldrich). Immunoreactive proteins were detected using an enhanced chemiluminescence detection system (GE Healthcare, Pittsburgh, PA).
Renal morphology
The kidneys were fixed in 4 % para-formaldehyde and embedded in paraffin. Sections with a thickness of 5 μm were cut and stained with hematoxylin and eosin (H&E) or Masson’s trichrome. The H&E stained sections were used to determine the tubular injury score, which estimates the percentage of injured tubules (tubule flattening, necrosis, apoptosis, or presence of cast) in the cortex and outer medulla. The score is as follows: 0, none; 1+, <10 %; 2+, 10–25 %; 3+, 26–45 %; 4+, 46–75 %; and 5+, > 75 %. The average score was determined by 10 random, non-overlapping ×400 magnification fields per kidney section. To quantify interstitial fibrosis, kidney sections with Masson’s trichrome staining were evaluated using a computer-aided evaluation program (AxioVision LE, 40 × 64v, Zeiss, Germany). The percentage of fibrotic area (stained in blue) relative to the entire field without glomeruli, and large vessels was calculated. Ten randomly selected fields of renal cortex per section were examined at ×400 magnification.
Immunohistochemical and immunofluorescence staining
For the immunohistochemical staining, formalin-fixed paraffin-embedded tissue sections (5-μm thick) were deparaffinized, boiled, blocked, and incubated overnight with the primary antibody against Egr-1 (Santa Cruz Biotechnology). Secondary antibody staining (VECTASTAIN ABC kit; Vector Laboratories) was performed. Slides were developed using 3,3′-diaminobenzidine substrate-chromogen solution (Dako, Glostrup, Denmark) and counterstained with hematoxylin. The percentage of positive nuclear staining of Egr-1 in renal tubule was scored as follows: 0, none; 1+, < 25 %; 2+, 26–50 %; 3+, 51–75 %; and 4+, > 75 %. The intensity of cytoplasmic staining of Egr-1 in renal tubule was scored as follows: 0, absent; 1+, weak; 2+, moderate; and 3+, strong. The average score of each kidney section was determined by 10 randomly selected, non-overlapping ×400 magnification fields.
For the immunofluorescence staining, frozen sections (20-μm thick) or cells were blocked with blocking buffer (Thermo Scientific Pierce) for 30 min and incubated overnight with antibodies against Egr-1 or NF-κB RelA/p65 (Santa Cruz). The sections or cells were then probed by secondary antibodies conjugated with Alexa Fluor 555 (Invitrogen) or 5(6)-TAMRA (Leadgene, Taiwan), and examined using a fluorescence microscope (Olympus, Tokyo, Japan).
Blood urea nitrogen and serum creatinine
Serum samples of the mice were collected via orbital sinus blood sampling or at the time of sacrifice. Serum levels of blood urea nitrogen (BUN) and creatinine were measured by a colorimetric method using a dry chemistry analyzer (Fujifilm, Tokyo, Japan).
Data analysis
Values are reported as mean ± SEM unless otherwise specified. Statistical analyses were conducted using Student’s t test. Differences were considered statistically significant at p ≤ 0.05.
Results
Egr-1 is upregulated in the animal model of TIN
In mammals, adenine is converted by xanthine dehydrogenase (XDH) into 2,8-dihydroxyadenine, an insoluble crystal that precipitates in the tubules of kidney and causes TIN and renal failure. Wild-type mice fed with an adenine-enriched diet for 10 days showed typical features of human chronic kidney disease, including body weight loss, high serum creatinine, and high blood urea nitrogen (BUN) (Supplementary Figure 1A). TIN was successfully induced, revealed by massive immune cell infiltration, interstitial fibrosis (Supplementary Figure 1B), increased renal expression of pro-inflammatory factors (macrophage inflammatory protein-2 [MIP-2], monocyte chemotactic protein-1 [MCP-1], interleukin-6 [IL-6], and TNFα), pro-fibrotic factors (TGFβ and connective tissue growth factor [CTGF]), and markers for macrophage (F4/80), extracellular matrix (type I collagen) and EMT (α-SMA) (Fig. 1a). These TIN pathologies were not associated with changes of XDH expression but associated with a fivefold upregulation of Egr-1 mRNA (Fig. 1a). Interestingly, the increases of Egr-1 expression and its nuclear translocation were prominent in the tubules rather than in the glomeruli (Fig. 1b). Immunoblotting confirmed the nearly twofold upregulation of Egr-1, which is accompanied by the increases of TGFβ protein level, the downstream signaling (Smad3 phosphorylation), and the EMT effector (α-SMA) (Fig. 1c). These results suggest that Egr-1 activation in the renal tubule is likely involved in the pathogenesis of TIN through governing the transcription network for renal inflammation and fibrosis.
Renal expression of Egr-1, inflammatory, and fibrotic markers in tubulointerstitial nephritis. a RT-PCR for the expression of Egr-1, inflammatory markers, NLRP3 inflammasome components, and fibrotic markers of the adenine-fed group relative to the control group (n = 4 in each group). b Immunofluorescence staining for Egr-1 (red) in the kidney. The DAPI nuclear counterstain appears blue. Scale bars, 50 μm. G, glomerulus. Arrows indicate renal tubular cells with Egr-1 nuclear translocation. c Immunoblot analyses for Egr-1 and fibrosis-related proteins. d Immunoblot analyses for NLRP3 inflammasome components. The intensities of bands quantified densitometrically relative to the control are represented as the bar graph and expressed as mean ± SEM. n = 4 in the adenine-fed and n = 3 in the control group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
TIN associates with increased NLRP3 protein level but not the activation of caspase-1
Since NLRP3 inflammasome has been suggested in TIN development [3], we then determined its expression and activation. mRNA levels of NLRP3 inflammasome components, including NLRP3, ASC, and pro-IL-1β, were all upregulated (Fig. 1a). Immunoblot analyses revealed increases of NLRP3 and pro-caspase-1 but a decrease of ASC (Fig. 1d). This is associated with an indistinguishable change in active caspase-1, the major effector of NLRP3 inflammasome. Since pro-IL-1β and active IL-1β were both upregulated despite no change of caspase-1 (Fig. 1d), the involvement of NLRP3 inflammasome in adenine-induced TIN remains debated.
Egr-1 deficiency attenuates renal inflammation
To address the functional significance of Egr-1 upregulation in TIN, Egr1 −/− mice were fed with an adenine-enriched diet for 10–13 days. Although the body weight loss was similar between Egr1 −/− mice and wild-type littermates, Egr1 −/− mice had lower serum creatinine and BUN levels (Fig. 2a), decreased immune cell infiltration and tubular injury score (Fig. 2b, c), and reduced expression of the pro-inflammatory cytokine (TNFα and pro-IL-1β), chemokines (MIP-2 and MCP-1), and the macrophage marker (F4/80) (Fig. 2d). These results suggest that Egr-1 deficiency protects from adenine-induced renal injury. The attenuation of inflammation in Egr1 −/− mice was attributed to decreased NF-κB activity, evidenced by a 40 % reduction of phosphorylated IKKα/β level (Fig. 2e). Another evidence supporting the decrease of NF-κB activity in Egr1 −/− mice was the reduced protein level of NLRP3 (Fig. 2e), which is known to be regulated by NF-κB activation. Intriguingly, the attenuated expression of NLRP3 in Egr1 −/− mice did not lead to a significant decrease in active caspase-1 and IL-1β, the major effector and end product of NLRP3 inflammasome (Fig. 2e). These results suggest that Egr-1 deficiency attenuates NF-κB-related inflammatory responses but has a minor effect on activation of NLRP3 inflammasome.
The effects of Egr-1 deficiency on renal failure and inflammation. a Body weight change, serum creatinine, and BUN levels of adenine-fed Egr1 −/− (n = 10) and wild-type mice (n = 12). b Representative images of H&E stained kidney sections (original magnification ×400). c Tubular injury score of the H&E stained kidney section. d Renal expression of inflammatory markers and NLRP3 components of adenine-fed Egr1 −/− mice (n = 10) relative to wild-type mice (n = 12). e Immunoblot analyses and quantification of phosphorylated IKKα/β, IκB, and NLRP3 inflammasome in the kidney of adenine-fed Egr1 −/− and wild-type mice (n = 4 in each group). The intensities of bands quantified densitometrically relative to the wild-type are represented as the bar graph and expressed as mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Egr-1 deficiency attenuates renal fibrosis
After renal failure had been induced for 10–13 days, Egr1 −/− mice showed less fibrotic area in the kidney than the wild-type littermates (Fig. 3a). This is associated with lower mRNA levels of TGFβ and type I collagen (Fig. 3b) and a trend toward decrease of TGFβ protein (Fig. 3c). The attenuation of phosphorylated Smad3 and the EMT marker α-SMA in Egr1 −/− mice (Fig. 3c) suggests that the suppressed TGFβ signaling is responsible for the decrease of renal fibrosis in Egr-1 deficiency.
The effects of Egr-1 deficiency on renal fibrosis. a Representative images and fibrotic area quantification of Masson’s trichrome stained kidney sections (original magnification ×400). b Renal expression of fibrotic markers of adenine-fed Egr1 −/− mice (n = 10) relative to wild-type mice (n = 12). c Immunoblot analyses and quantification of TGFβ, phosphorylated Smad3, total Smad3, and α-SMA in the kidneys of adenine-fed Egr1 −/− and wild-type mice (n = 4 in each group). The intensities of bands quantified densitometrically relative to the wild-type are represented as the bar graph and expressed as mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Egr-1 deficiency attenuates pro-inflammatory and pro-fibrotic responses in renal tubular cells
Since Egr-1 is predominantly upregulated and activated in the tubular cells, we then used mouse PTECs for the subsequent studies. The highly expressed proximal tubule differentiation markers, Sglt2 and Nhe3, in the cultured PTECs compared to the non-kidney cell lines suggest that authentic epithelial cells were obtained (Supplementary Figure 2). PTECs from Egr1 −/− mice and wild-type mice were stimulated with TNFα or TGFβ to mimic an exposure of pro-inflammatory or pro-fibrotic stimulation. The expression of MCP-1, MIP-2, pro-IL-1β, and TNFα upon TNFα stimulation was lower in Egr1 −/− PTECs than in wild-type PTECs (Fig. 4a). Egr-1 deficiency also attenuated TGFβ-induced expression of type I collagen, CTGF, TGF-β1, and Snail (Fig. 4b). These results were consistent when being normalized to the expression of different reference genes (Supplementary Figure 3). We further analyzed NF-κB activity in PTECs by immunofluorescent staining of RelA/p65 in response to TNFα. Treatment of TNFα (100 ng/ml) for 1 h induced RelA/p65 nuclear translocation in both genotypes of PTECs (Fig. 4c). However, the number of cells with RelA/p65 nuclear translocation was lower in Egr1 −/− PTECs (81/185 = 43.8 %) than in wild-type PTECs (130/254 = 51.2 %). These results suggest that the attenuated inflammatory responses in Egr1 −/− PTECs might be attributed to the downregulation of NF-κB activity. Collectively, these results suggest that Egr-1 is essential for NF-κB-mediated inflammatory responses and the induction of fibrotic responses in renal tubular cells.
The responses of PTECs to cytokine stimulation in Egr-1 deficiency. a Expression of inflammatory markers in response to TNFα stimulation, n = 3 per experiment condition. b Expression of fibrosis markers in response to TGFβ stimulation, n = 4 per experiment condition. The reference genes were Gapdh for Col1a1 and Ctgf, and Actb for the others. mRNA levels are expressed relative to the wild-type PTECs of control treatment. # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001 compared with the control treatment; *p ≤ 0.05, **p ≤ 0.01 compared with the respective wild-type PTECs within the treatment group. c Immunofluorescence staining for NF-κB RelA/p65 (red) in PTECs. The DAPI nuclear counterstain appears blue. White arrows indicate nuclear translocation of RelA/p65 (original magnification ×400)
For the study of PTECs, it is essential to use equal number of viable cells from wild-type and Egr1 −/− mice. For this, several points need to be clarified. First, using the methodology developed by Tang and Tannen [21], we actually obtained segments of renal tubules instead of separated cells from the kidney. The cell-cell contact in these isolated tubules may slow down their transition to mesenchymal cells before the experimental treatment [23]. However, because the cells were clustered together, it is difficult to calculate the cell number directly. Nevertheless, in the analysis of gene expression, we normalized to the expression of several reference genes, including Actb, Gapdh, and Ppia, and found the consistent results (Fig. 4 and Supplementary Figure 3). These reference genes, thought to be expressed in a stable and non-regulated constant level, are involved in processes essential for the survival of cells [24]. Second, we trypsinized the separated cells after growing from primary tubules for 5 days, and seeded equal number (4 × 103 cells) of Egr1 −/− or wild-type cells for the assay of LDH activity as an indicator of cell death. After culturing for 24 h, the LDH activity did not differ between Egr1 −/− PTECs (8.5 %) and wild-type PTECs (7.3 %). Since the seeding numbers were equal and cytolysis was not different, it is reasonable to speculate that the numbers of viable cell were equal. Third, although, to our knowledge, primary culture for Egr1 −/− PTECs has never been performed, Bastianelli et al. showed that the cell proliferation rate of cardiac mesenchymal stem cells (cMSCs) derived from Egr1 −/− mice did not differ from that of cMSCs derived from wild-type mice [25]. Therefore, Egr-1 deficiency does not necessarily correspond to the proliferation rate of primary cells ex vivo.
Egr-1 activity increases in patients with renal failure
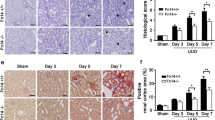
To study the clinical significance of Egr-1 in human kidney diseases, immunohistochemical staining for Egr-1 was applied to 10 renal biopsy tissues of diabetic patients with serum creatinine concentrations ranged from 1.63 mg/dl to 10.61 mg/dl, as well as to 12 kidney tissues obtained from patients with urothelial malignancy and normal serum creatinine levels. Patients with and without renal failure both expressed Egr-1 in the cytoplasm of renal tubular cells, but only those with renal failure had higher cytoplasmic staining of Egr-1 (Fig. 5a, b). In addition, a higher number of tubular cells with prominent Egr-1 stain within the nuclei was observed in the renal failure patients than in the controls. These results suggest that Egr-1 activity increases with kidney injury.
Immunohistochemical staining for Egr-1 in the kidneys of patients with/without renal failure. a Representative images. b Scoring for Egr-1 stain intensity in the cytoplasm of tubular cells (IHC score, cytoplasm) and scoring for the proportion of tubular cells with positive Egr-1 stain in the nucleus (IHC score, nucleus). n = 10 for renal failure patients, n = 12 for patients with normal renal function. Scale bars, 100 μm. ***p ≤ 0.001
Discussion
This study established the crucial role of Egr-1 in chronic kidney disease by showing the expression of Egr-1 in renal tubules during TIN, attenuation of renal inflammation and fibrosis in Egr1 −/− mice, and reduced inflammatory and fibrotic responses in PTECs with Egr-1 deficiency. To our knowledge, this is the first study using Egr1 −/− mice to verify the renal effects, which provides convincing evidence to support the importance of Egr-1 in the progression of renal failure. Importantly, Egr-1 also plays a role in human kidney disease, as the cytoplasmic expression and nuclear localization of Egr-1 were upregulated in patients with renal failure.
The primary site of Egr-1 activation in our study is the renal tubule, but the expression of Egr-1 has been reported in other cell types of kidney, depending on where the primary site of injury is in the animal model. For example, a rat model of mesangioproliferative glomerulonephritis induces Egr-1 expression in the mesangial cells [26], while the ischemic-reperfusion model or a model causing selective injury to the watershed area of kidney upregulates Egr-1 in the thick ascending limbs [18, 27]. In the model of tubulointerstitial fibrosis induced by unilateral ureteral ligation, Nakamura et al. showed the transfected DNA enzyme for Egr-1 is predominantly located in the interstitial fibroblasts, but they did not exclude the presence of Egr-1 in renal tubules [19]. In a rat model with 5/6 nephrectomy, Egr-1 is overexpressed in renal tubular epithelial cells [28]. In our study, we induced TIN in animals, and the increases of Egr-1 expression and nuclear translocation were mainly in, if not limited to, tubular epithelial cells. Being a transcription factor, Egr-1 in the nuclei is related to its transcriptional activity. The activity of Egr-1 in renal tubules may be clinically relevant because in our and other studies, immunohistochemistry analysis for patients with kidney diseases showed that increased staining of Egr-1 is mainly in the nuclei of renal tubular cells [28]. Although the concept that tubular cells undergo EMT in vivo has been challenged recently [29], the contribution of tubular cells to the progression of renal failure is still important [30]. We also confirmed Egr-1 deficiency in PTECs attenuated their responses to pro-inflammatory and pro-fibrotic stimuli ex vivo, but how Egr-1 works in tubules in vivo requires further investigations.
Our results suggest that Egr-1 aggravates renal failure via facilitating renal tubular cells in production of pro-inflammatory cytokines and chemokines. Egr-1, induced by TNFα, actively participates in the inflammatory process of non-kidney tissues and cell lines [6–8, 31–33]. Egr-1 usually acts with the transcription factor NF-κB in synergy for the transcription of pro-inflammatory mediators [34–36], but Egr-1 can itself modulate the transcriptional activity of NF-κB [12, 14, 16, 37]. With respect to renal inflammation, the role of Egr-1 has not been well defined in the past. Our study found that Egr1 −/− mice with TIN expressed less cytokines (TNFα), chemokines (MIP-2 and MCP-1), and had a lower NF-κB activity (phosphorylated IKKα/β) than wild-type mice. In addition, Egr-1 deficiency attenuated the response of ex vivo PTECs to TNFα stimulation possibly through the downregulation of NF-κB activity. Taken together, our results support the involvement of Egr-1 in NF-κB-mediated renal innate immunity.
In contrast, the present study suggests that the NLRP3 inflammasome pathway may not be involved in Egr-1-mediated renal inflammation. This is consistent with a recently proposed non-canonical NLRP3 effect, which suggests the influence of NLRP3 on kidney may not be dependent on IL-1β or IL-18 [2]. Andersen et al. showed attenuated kidney injury but intact IL-1β secretion in Nlrp3 −/− mice with immune complex glomerulonephritis [38]. Shigeoka et al. reported that NLRP3 deficiency protected mice from post-ischemic acute kidney injury, but IL-1 blockade or IL-1 receptor deficiency did not [39]. Our TIN model in wild-type mice did not find an increase in active caspase-1 despite the significant induction of NLRP3 and pro-IL-1β, suggesting the effect of NLRP3 inflammasome on Egr-1-mediated renal injury is marginal (Fig. 1d). No change of caspase-1 could be related to the downregulation of ASC protein, one key adaptor for NLRP3 inflammasome. We further demonstrated that Egr-1 deficiency downregulated NLRP3 but did not alter the levels of active caspase-1 and active IL-1β (Fig. 2e). These results suggest that Egr-1 may regulate NLRP3 expression in a NF-κB dependent manner, but NLRP3 inflammasome is unlikely to play a major role in renal inflammation during TIN.
Renal fibrosis is a common consequence of chronic kidney disease, and our study showed that Egr-1 participated in this pathological process. The role of Egr-1 in tissue fibrosis has drawn attentions in recent years. Egr-1 is a conserved factor in TGFβ-induced transcription responses across cell-type and species [40]. Given that the promoter of TGFβ1 gene has at least two Egr-1 binding sites, autonomous TGFβ activation may be mediated via Egr-1 [13]. Egr-1 expression driven by TGFβ or other fibrogenic stimuli is identified in animal models of scleroderma, lung fibrosis, and colitis [10, 41, 42], and Egr-1 deficiency ameliorates bleomycin-induced skin and lung fibrosis [43]. However, the profibrotic effect of Egr-1 has been challenged because one study shows carbon tetrachloride-induced hepatic fibrosis is enhanced in Egr1 −/− mice [17]. In the kidney, some studies showed that Egr-1 activated in the interstitial fibroblast and tubular epithelial cell is an aggravating factor for fibrosis [19, 28]. Our study also supports the pro-fibrotic role of Egr-1 since Egr-1 deficiency reduced the fibrotic area in the kidney and attenuated TGFβ-induced responses in PTECs. The molecular mechanism for the attenuation of TGFβ signaling in Egr1 −/− mice needs further investigations.
It is worth noting a discrepancy between the fivefold upregulation of Egr-1 mRNA (Fig. 1a) and the less than twofold upregulation of Egr-1 protein (Fig. 1c) in TIN. A similar discrepancy was found in our previous publication in pancreatic β cells [44]. The discrepancy may stem from the sensitivity of the detection system (quantitative RT-PCR versus Western blotting). In addition, being a member of the immediate early gene family, Egr-1 protein is both induced and degraded rapidly, possessing a half-life of less than 2 h [45]. The limited functional period of Egr-1 is also ensured by post-transcriptional regulations. For example, alternative polyadenylation produces two variants of Egr-1 mRNA in the retina with different stability [46]. The binding of HuR protein to the 3’-UTR of Egr-1 mRNA changes the translation rate [47]. Thus, we speculate that a rapid degradation of Egr-1 protein, as well as an altered stability and translation rate of Egr-1 mRNA, might change the Egr-1 mRNA to protein ratio in the injured kidney.
We finally translated our bench findings to the human study and found that Egr-1 was overexpressed and activated in the renal tubular cells of renal failure patients. Our human study has several limitations. First, since normal renal tissues were obtained from patients receiving nephrectomy for urothelial malignancy, some molecular changes may have occurred in these normal appearing tissues. Second, the renal tissues with renal failure were obtained from diabetic patients; thus, we cannot exclude the possibility that Egr-1 is upregulated due to diabetes mellitus rather than renal failure. Third, although Egr-1 upregulation was shown in the renal failure patients, whether Egr-1 plays a protective or detrimental role in these samples remains undefined. Nevertheless, our human data suggest that Egr-1 is involved in regulation of renal failure.
In conclusion, from animal models to human samples, the present study highlights a universal, integrative role of Egr-1 in renal inflammation and fibrosis. This early-responsive transcription factor activated in renal tubule is crucial for amplification of NF-κB and TGFβ-mediated detrimental effects, although its role in NLRP3 inflammasome remains unclear. The strength of our conclusion is derived from the Egr-1 knockout nature of the animal study and the corresponding human data. Thus, we believe our findings provide a solid foundation for future researches considering Egr-1 as a therapeutic target for human kidney diseases.
References
Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A (2010) NF-kappaB in renal inflammation. J Am Soc Nephrol 21:1254–1262
Lorenz G, Darisipudi MN, Anders HJ (2014) Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29:41–48
Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM, Barreto CR, Silva-Cunha C, Hyane MI, Goncalves GM et al (2011) Pivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One 6:e29004
Carew R, Wang B, Kantharidis P (2012) The role of EMT in renal fibrosis. Cell Tissue Res 347:103–116
Garcia-Sanchez O, Lopez-Hernandez FJ, Lopez-Novoa JM (2010) An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int 77:950–955
Allen K, Jaeschke H, Copple BL (2011) Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 178:175–186
Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM (2000) Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med 6:1355–1361
Harja E, Bucciarelli LG, Lu Y, Stern DM, Zou YS, Schmidt AM, Yan SF (2004) Early growth response-1 promotes atherogenesis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ Res 94:333–339
Chen S-J, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J (2006) The early-immediate gene EGR-1 is induced by transforming growth factor-β and mediates stimulation of collagen gene expression. J Biol Chem 281:21183–21197
Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J et al (2004) Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 200:377–389
Yao J, Mackman N, Edgington TS, Fan ST (1997) Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem 272:17795–17801
Cogswell PC, Mayo MW, Baldwin AS Jr (1997) Involvement of Egr-1/RelA synergy in distinguishing T cell activation from tumor necrosis factor-alpha-induced NF-kappa B1 transcription. J Exp Med 185:491–497
Liu C, Adamson E, Mercola D (1996) Transcription factor EGR-1 suppresses the growth and transformation of human HT-1080 fibrosarcoma cells by induction of transforming growth factor beta 1. Proc Natl Acad Sci 93:11831–11836
Chapman NR, Perkins ND (2000) Inhibition of the RelA(p65) NF-kappaB subunit by Egr-1. J Biol Chem 275:4719–4725
Chen G, Goeddel DV (2002) TNF-R1 signaling: a beautiful pathway. Science 296:1634–1635
Do KH, Choi HJ, Kim J, Park SH, Kim HH, Oh CG, Moon Y (2012) Ambivalent roles of early growth response 1 in inflammatory signaling following ribosomal insult in human enterocytes. Biochem Pharmacol 84:513–521
Pritchard MT, Nagy LE (2010) Hepatic fibrosis is enhanced and accompanied by robust oval cell activation after chronic carbon tetrachloride administration to Egr-1-deficient mice. Am J Pathol 176:2743–2752
Bonventre JV, Sukhatme VP, Bamberger M, Ouellette AJ, Brown D (1991) Localization of the protein product of the immediate early growth response gene, Egr-1, in the kidney after ischemia and reperfusion. Cell Regul 2:251–260
Nakamura H, Isaka Y, Tsujie M, Rupprecht HD, Akagi Y, Ueda N, Imai E, Hori M (2002) Introduction of DNA enzyme for Egr-1 into tubulointerstitial fibroblasts by electroporation reduced interstitial alpha-smooth muscle actin expression and fibrosis in unilateral ureteral obstruction (UUO) rats. Gene Ther 9:495–502
Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J (1995) Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem 270:9971–9977
Tang MJ, Tannen RL (1990) Relationship between proliferation and glucose metabolism in primary cultures of rabbit proximal tubules. Am J Physiol 259:C455–C461
Chen WC, Lin HH, Tang MJ (2014) Regulation of proximal tubular cell differentiation and proliferation in primary culture by matrix stiffness and ECM components. Am J Physiol Renal Physiol 307:F695–F707
Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A (2004) Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol 165:1955–1967
Kozera B, Rapacz M (2013) Reference genes in real-time PCR. J Appl Genet 54:391–406
Bastianelli D, Siciliano C, Puca R, Coccia A, Murdoch C, Bordin A, Mangino G, Pompilio G, Calogero A, De Falco E (2014) Influence of Egr-1 in cardiac tissue-derived mesenchymal stem cells in response to glucose variations. Biomed Res Int 2014:254793
Rupprecht HD, Hoffer G, de Heer E, Sterzel RB, Faller G, Schoecklmann HO (1997) Expression of the transcriptional regulator Egr-1 in experimental glomerulonephritis: requirement for mesangial cell proliferation. Kidney Int 51:694–702
Joannidis M, Cantley LG, Spokes K, Stuart-Tilley AK, Alper SL, Epstein FH (1997) Modulation of c-fos and egr-1 expression in the isolated perfused kidney by agents that alter tubular work. Kidney Int 52:130–139
Sun S, Ning X, Zhai Y, Du R, Lu Y, He L, Li R, Wu W, Sun W, Wang H (2014) Egr-1 mediates chronic hypoxia-induced renal interstitial fibrosis via the PKC/ERK pathway. Am J Nephrol 39:436–448
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97
Eddy AA (2014) Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl 4:2–8
Cao Y, Zhang J, Meng X, Wang D (2013) TNF-alpha induces early growth response gene-1 expression via ERK1/2 activation in endothelial cells. Acta Diabetol 50:27–31
Shin SY, Lee JM, Lim Y, Lee YH (2013) Transcriptional regulation of the growth-regulated oncogene alpha gene by early growth response protein-1 in response to tumor necrosis factor alpha stimulation. Biochim Biophy Acta 1829:1066–1074
Ramana CV, Cheng GS, Kumar A, Kwon HJ, Enelow RI (2009) Role of alveolar epithelial early growth response-1 (Egr-1) in CD8+ T cell-mediated lung injury. Mol Immunol 47:623–631
Lohoff M, Giaisi M, Kohler R, Casper B, Krammer PH, Li-Weber M (2010) Early growth response protein-1 (Egr-1) is preferentially expressed in T helper type 2 (Th2) cells and is involved in acute transcription of the Th2 cytokine interleukin-4. J Biol Chem 285:1643–1652
Singha B, Gatla HR, Manna S, Chang TP, Sanacora S, Poltoratsky V, Vancura A, Vancurova I (2014) Proteasome inhibition increases recruitment of IkappaB kinase beta (IKKbeta), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J Biol Chem 289:2687–2700
Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schonbeck U (2002) Induction of tissue factor expression in human endothelial cells by CD40 ligand is mediated via activator protein 1, nuclear factor kappa B, and Egr-1. J Biol Chem 277:25032–25039
Parra E, Ferreira J, Ortega A (2011) Overexpression of EGR-1 modulates the activity of NF-kappaB and AP-1 in prostate carcinoma PC-3 and LNCaP cell lines. Int J Oncol 39:345–352
Andersen K, Eltrich N, Lichtnekert J, Anders HJ, Vielhauer V (2014) The NLRP3/ASC inflammasome promotes T-cell-dependent immune complex glomerulonephritis by canonical and noncanonical mechanisms. Kidney Int 86:965–978
Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM, McKay DB (2010) An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185:6277–6285
Abnaof K, Mallela N, Walenda G, Meurer SK, Sere K, Lin Q, Smeets B, Hoffmann K, Wagner W, Zenke M et al (2014) TGF-beta stimulation in human and murine cells reveals commonly affected biological processes and pathways at transcription level. BMC Syst Biol 8:55
Bhattacharyya S, Chen SJ, Wu M, Warner-Blankenship M, Ning H, Lakos G, Mori Y, Chang E, Nihijima C, Takehara K et al (2008) Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol 173:1085–1099
Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W (2008) IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology 135:2003–2013
Wu M, Melichian DS, de la Garza M, Gruner K, Bhattacharyya S, Barr L, Nair A, Shahrara S, Sporn PH, Mustoe TA et al (2009) Essential roles for early growth response transcription factor Egr-1 in tissue fibrosis and wound healing. Am J Pathol 175:1041–1055
Cheong MW, Kuo LH, Cheng YN, Tsai PJ, Ho LC, Tai HC, Chiu WT, Chen SH, Lu PJ, Shan YS et al (2015) Loss of Egr-1 sensitizes pancreatic beta-cells to palmitate-induced ER stress and apoptosis. J Mol Med (Berl) 93:807–818
Waters CM, Hancock DC, Evan GI (1990) Identification and characterisation of the egr-1 gene product as an inducible, short-lived, nuclear phosphoprotein. Oncogene 5:669–674
Simon P, Schott K, Williams RW, Schaeffel F (2004) Posttranscriptional regulation of the immediate-early gene EGR1 by light in the mouse retina. Eur J Neurosci 20:3371–3377
Mou Z, You J, Xiao Q, Wei Y, Yuan J, Liu Y, Brewer G, Ma WJ (2012) HuR posttranscriptionally regulates early growth response-1 (Egr-1) expression at the early stage of T cell activation. FEBS Lett 586:4319–4325
Acknowledgments
We are grateful for the support from Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital. We thank Dr. Ming-Jer Tang and his laboratory for providing the protocol and instructions for the primary culture of mouse proximal tubular cells. This study is supported by the research grants of NCKUEDA10201 and NCKUEDA10301 from E-Da Hospital and National Cheng Kung University Hospital, and the research grants of NHRI-EX104-10231SI from National Health Research Institute. It is also a part of the National Cheng Kung University Top-Notch Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted according to the study protocol approved by the Institutional Review Board of National Cheng Kung University Hospital (permit number: B-ER-101-008). All Procedures for handling animals were done in accordance to ethical statements approved by the Institutional Animal Care and Use Committee of National Cheng Kung University.
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 733 kb)
Rights and permissions
About this article
Cite this article
Ho, LC., Sung, JM., Shen, YT. et al. Egr-1 deficiency protects from renal inflammation and fibrosis. J Mol Med 94, 933–942 (2016). https://doi.org/10.1007/s00109-016-1403-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1403-6