Abstract
Lymph node metastases occur frequently during the progression of many types of cancer, and their presence often reflects poor prognosis. The drainage of tumor-derived factors such as antigens, growth factors, cytokines, and exosomes through the lymphatic system to the regional lymph nodes plays an important role in the pre-metastatic conditioning of the microenvironment in lymph nodes, making them receptive and supportive metastatic niches for disseminating tumor cells. Modified immunological responses and remodeling of the vasculature are the most studied tumor-induced pre-metastatic changes in the lymph node microenvironment that promote metastasis, although other metastasis-relevant alterations are also starting to be studied. Here, I review our current understanding of the lymph node pre-metastatic niche, how tumors condition this niche, and the relevance of this conditioning for our understanding of the process of metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First formulated by David Lyden and colleagues [1], the concept of the pre-metastatic niche states that factors shed or secreted by tumor cells can pre-condition the microenvironment of organs where metastases will develop to make them receptive for disseminating tumor cells. Key events in the conditioning include the recruitment of particular types of cells that support metastatic growth, induction of the expression of pro-metastatic growth factors and cytokines, and remodeling of the extracellular matrix [2]. By extension, a microenvironment that supports the survival and outgrowth of disseminated tumor cells (DTCs) has been termed the metastatic niche. In addition to being formed by tumor-induced pre-metastatic conditioning, metastatic niches can be present endogenously in particular organ microenvironments. For example, the hematopoietic stem cell niche in the bone marrow can serve as a metastatic niche for disseminated prostate cancer cells [3]. Metastatic niches can presumably also be formed after the dissemination of tumor cells, for example, during the activation of dormant DTCs [2].
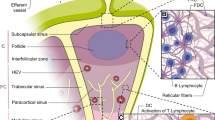
Lymph nodes are immune organs that respond to peripheral infections through their T cell-dependent paracortical areas in which antigen-presenting dendritic cells (DCs) prime naïve T lymphocytes and through their B cell-containing cortical germinal follicles where naïve B lymphocyte produce antibodies in response to antigen [4]. The afferent lymphatic fluid first enters the subcapsular sinus, then percolates through the node, and exits via the efferent lymphatics. It subsequently drains into further lymph nodes higher up the lymphatic drainage basin, before entering the blood stream via anastomoses such as the thoracic duct (Fig. 1). In the cancer context, lymphatic fluid from primary tumors almost invariably drains to a single so-called sentinel lymph node (SLN) in the lymphatic drainage basin [5].
Lymph node structure and function. Lymphatic fluid enters the lymph node through the afferent lymphatic vessels and enters the subcapsular sinus. It then percolates through the trabecular, cortical, and medullary sinuses before exiting via the efferent lymphatic vessel. High endothelial venues in the cortical region provide a portal for circulating lymphocytes. B cell follicles and the T cell zone are sites where immune responses develop as consequence of antigen presentation, for example, by interdigitating dendritic cells and follicular dendritic cells
Metastasis formation in lymph nodes occurs when tumor cells detach from the primary tumor, enter the lymphatics, and are subsequently transported to regional lymph nodes. Tumor cells initially accumulate in the lymph node subcapsular sinus. Within the lymph nodes, DTCs may be destroyed, they may pass through the lymph node and enter the efferent lymphatic fluid, or they may remain in the lymph node and form metastases [6].
During tumor progression, most types of human carcinoma metastasize frequently to regional lymph nodes. Indeed, in many cases, lymph nodes are the first organs in which metastases develop. Accordingly, cancer staging systems assess the presence or absence of lymph node metastases as an important means of evaluating patient prognosis [7]. It is becoming increasingly apparent that tumor-mediated pre-metastatic conditioning underlies the predominance of lymph node metastases and their prognostic relevance and that the unique conditioned microenvironment offered by the lymph node can be decisive for metastasis formation. Here, I review how the endogenous lymph node microenvironment, tumor-induced pre-metastatic changes in lymph nodes, and further microenvironmental changes that are induced in the lymph node once DTCs become established can act together to establish a mature metastatic niche.
The ability of the endogenous lymph node microenvironment to support metastasis formation
There is a long-standing debate in the literature as to whether as a seat of immunity, the lymph node microenvironment is intrinsically hostile to incoming tumor cells or whether the endogenous microenvironment can support metastatic growth [8]. The presence of a tumor draining to a lymph node has profound effects on the lymph node microenvironment (see below), making it difficult to assess the role of the underlying endogenous microenvironment on metastasis formation. Nevertheless, early experiments in which low numbers of tumor cells were injected directly into lymphatic vessels in non-tumor-bearing animals resulted in the formation of more lymph node metastases compared to the number of lung metastases that developed after intravenous injection of the same number of tumor cells [9]. While the ability of lymph nodes to retain higher numbers of tumor cells than the lungs makes these findings difficult to interpret quantitatively [8], the results do indicate that the endogenous lymph node microenvironment in the absence of conditioning by a tumor is in principle able to support the outgrowth of metastases, a finding confirmed in other studies [10]. However, in more recent experiments in which melanoma cells were injected directly into lymph nodes, tumors were rejected due to induction of a CD8+ cytotoxic T cell response [11]. Furthermore, breast cancer micrometastases can lie dormant in lymph nodes for many years [12], indicating that while the endogenous lymph node “soil” in the case of breast cancer can support DTC survival, it may not necessarily be fertile enough to support metastatic outgrowth. Moreover, lymph nodes draining carcinomas such as renal cell carcinoma [13] and various sarcomas [14] rarely develop metastases, suggesting that the endogenous lymph node microenvironment may not be supportive of DTCs from these tumor types. Together, these observations suggest that in many contexts, tumor-induced conditioning of the lymph node microenvironment may be necessary before lymph node metastases can successfully develop.
Pre-metastatic morphological and histological changes
Pre-metastatic changes in the lymph nodes that drain tumors and their significance for metastasis have been documented and discussed for well over a century, making the lymph node the oldest organ in which pre-metastatic conditioning has been investigated. Pre-metastatic swelling of tumor-draining lymph nodes was already described in the nineteenth century [15], and the significance of the associated hyperplasia for metastasis formation in lymph nodes has been widely discussed [16]. Tumor-reactive lymphadenopathy typified by paracortical hyperplasia and sinus histiocytosis prior to the presence of metastatic tumor cells can be detected in a significant proportion of tumor-draining lymph nodes taken from human cancer patients. This lymphadenopathy can take various forms, including increased lymphocyte numbers in paracortical areas, hyperplastic follicles, distended sinuses, and hyperplasia of the sinus histiocytes [17]. Sinus histiocytosis appears to be associated with better patient survival [18]. Conversely, pre-metastatic lymphocyte depletion and diminished lymph node size can also be observed and is associated with metastasis and poor prognosis [17].
Immunological alterations
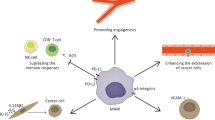
Conflicting points of view about whether the lymph node microenvironment represses metastasis formation or is permissive and stimulatory for metastatic outgrowth probably stem largely from dynamic changes in nodal immune responses that occur in response to the growth of tumors and which are reflected in the different forms of tumor-reactive lymphadenopathy described above. The picture that is emerging is that tumor antigens that drain to the lymph nodes can induce an anti-tumoral response that initially restricts metastasis formation, but as tumors progress, increasing concentrations of immunomodulatory factors draining from the tumor then pre-condition the lymph node and induce an immunosuppressive microenvironment that supports metastatic outgrowth [19–21] (Fig. 2). A full description is beyond the scope of this article and can only be covered superficially. An excellent recent review provides a detailed introduction to the role that immune cells play in regulating metastasis [22].
Pre-metastatic changes in tumor-draining lymph nodes. a Tumor cells, antigens, and activated dendritic cells draining from the tumor induce anti-tumor immune responses in the lymph node. b As tumors grow, they produce a number of factors that downregulate the anti-tumor response in the draining lymph nodes and condition the lymph node microenvironment in a number of ways that promotes the survival and outgrowth of tumor cells disseminating to the lymph node. For more details, see the main text
Anti-tumor responses in lymph nodes
Lymph nodes play a central role in orchestrating an immune response against tumors in their vicinity. A key event is the presentation of tumor antigens within the lymph node and the subsequent priming of effector cells such as antigen-specific T cells. A plethora of tumor antigens has been identified that can elicit such responses [23]. Several mechanisms for the presentation of tumor antigens have been described. DCs can be primed by antigen within the primary tumor and migrate to regional lymph nodes [24]. Antigen draining to the lymph nodes can be taken up and cross-presented by antigen-presenting cells within the lymph node by cells such as macrophages, follicular DCs, and B cells [25–27]. Interestingly, a recent study using intravital and whole-organ imaging found that melanoma antigens draining to lymph nodes are initially taken up by subcapsular macrophages and are then deposited on follicular DCs [28]. There is also evidence that tumor cells passing through lymph nodes may directly prime tumor-specific T cells [29].
Tumor antigen presentation in the lymph nodes can elicit a number of anti-tumor responses. Activation of naïve T cells can lead to the induction and clonal expansion of cytotoxic CD8+ T cells, key effector cells in anti-tumor immunity [30]. Priming and expansion of these CD8+ cells is weak in the absence of CD4+ TH1 cells, which are also activated through antigen presentation [31]. Consistently, identical T cell clonotypes were detected in the primary tumor and SLN from melanoma patients, even in the absence of tumor cells in the SLN [32], providing evidence that tumor antigens draining to the SLN are presented by antigen-presenting cells, leading to the priming and expansion of tumor-specific T cells. Antigen presentation to naïve B cells in lymph nodes is also important for the activation of a humoral response against tumor cells [18].
In response to the presence of a tumor, natural killer (NK) cells are recruited to the lymph nodes [33], where they are activated, likely by DCs [34]. In a study comparing tumor-draining lymph nodes from stage 1 melanoma (no metastases) with non-involved lymph nodes from stage 2 melanoma (with nodal metastasis), in both cases, increased numbers of CD56+ NK cells were found in lymph nodes closest to the primary tumor and were more numerous in stage 2 non-involved lymph nodes [35]. NK cells suppress metastasis formation in lymph nodes [33] and act through contact-dependent cytotoxicity and immunoregulatory cytokine production [36], as well as maintenance of TH1 polarization [37]. NK cells can also kill tumor-associated macrophages (TAMs) that promote tumor growth and metastasis [38], although whether they do so in tumor-draining lymph nodes remains to be investigated.
Although other immune cells undoubtedly take part in anti-tumor responses in the lymph node, they are less well investigated. For example, immunosuppressive myeloid-derived suppressor cells (MDSCs) accumulate in tumor-draining lymph nodes [39], but it remains to be demonstrated whether they do so prior to metastasis formation. Nevertheless, recent evidence suggests that CD8+ T cells constrain the accumulation of CD11b+ myeloid cells in pre-metastatic tumor-draining lymph nodes by inducing their apoptosis [40].
Tumor-induced immunosuppression in lymph nodes
Immune cells such as MDSCs, TAMs, and immature DCs play a central role in driving tumor growth and metastasis [22, 41]. A number of observations indicate that pre-metastatic conditioning of the lymph node microenvironment recruits such cells, inducing an immunosuppressive microenvironment typified by a chronic inflammatory milieu that supports the survival and outgrowth of DTCs. Gene expression profiling of non-involved lymph nodes from pNO and pN1 esophageal cancer patients showed that pre-metastatic lymph nodes draining more advanced tumors had a transcriptional profile associated with a suppressed immune response [42]. In melanoma patients, the induction of an immunosuppressive microenvironment precedes metastasis formation in SLN [43] and is associated with reduced numbers of interdigitating DCs [44], suggesting that pre-metastatic modulation of the immune response in SLN is likely to be a prerequisite for metastasis formation in this context. Similarly, prior to metastasis formation, the SLNs draining human breast cancers were found to have reduced cellular immune responses such as DC maturation and TH1 responses compared to lymph nodes higher up the drainage basin [45]. An immunosuppressive microenvironment associated with reduced numbers of dendritic cells is induced in lymph nodes by melanoma [21, 46, 47] and by breast cancers [48], in which context reduced numbers of CD4+ and CD8+ T cells were also observed [49]. In an animal model of melanoma, pre-metastatic induction of CD8+ T cell anergy in tumor-draining lymph nodes has been reported, which was sufficient to render the lymph node microenvironment conducive for tumor outgrowth [11]. Gastric cancer patients have increased numbers of tumor-promoting TAMs in pre-metastatic SLNs compared to lymph nodes further away from the primary tumor [50], which may reflect loss of NK restriction of these cells prior to metastasis formation.
Recent studies suggest that activation of STAT3 signaling in CD68+ myeloid cells allows these cells to pre-metastatically colonize tumor-draining lymph nodes from prostate and melanoma patients [51]. Mechanistically, STAT3 signaling appears to protect CD11b+ myeloid cells from CD8+ T cell-induced apoptosis in pre-metastatic lymph nodes [40]. Although these two studies have not characterized the myeloid cells involved, it is likely that they are tumor-promoting TAMs or MDSCs, and thus, induction of STAT3 signaling may contribute to the switch to an immunosuppressive microenvironment in the pre-metastatic lymph node. Accordingly, STAT3 activation in pre-metastatic lymph nodes taken from gastric cancer patients correlates with poor prognosis [52].
Although not formally proven, given that lymph nodes play an important role in regulating the anti-tumor immune response, it seems likely that during tumor progression, factors produced and secreted by primary tumors draining into SLNs can subvert an initial anti-tumor response into a metastasis-promoting one by creating an immunosuppressive microenvironment. IL-10, a potent anti-inflammatory cytokine that inhibits T cell proliferation, TH1 cytokine production, and antigen presentation and stimulates a TH2 response [53], is produced by many types of tumors and has been proposed to be one such factor [21]. Thus, IL-10 expression in melanomas correlates with the development of metastatic competence [54]. Nevertheless, IL-10 can also stimulate anti-tumor responses, for example, through stimulating NK activity [53], and thus, functional studies are needed to substantiate a role for IL-10 in creating an immunosuppressive microenvironment in tumor-draining lymph nodes. TGF-β production by non-small cell lung cancers has been shown to induce the apoptosis of DCs, resulting in fewer DCs in pre-metastatic SLNs compared to other lymph nodes higher up the lymphatic draining basin [55]. Increased systemic levels of VEGF-A result in recruitment of immature DCs from the bone marrow to the primary tumor [56]. After capturing tumor antigens and migrating to regional lymph nodes, these immature DCs induce anergy and peripheral tolerance due to their inability to present antigen, and suppress anti-tumor responses through the induction of Foxp3+ regulatory T cells [57, 58].
Although primary tumors may be the source of factors that induce a chronic inflammatory immunosuppressive microenvironment in tumor-draining lymph nodes, other observations suggest that factors such as IL-10, TGF-β, and GM-CSF can be produced within tumor-draining lymph nodes themselves [20]. While this can be a response to the presence of tumor cells within the lymph nodes, many studies show that SLNs contain increased levels of immunosuppressive cytokines prior to metastatic colonization by tumor cells [20, 21], indicating that upregulation of these cytokines contributes to the pre-metastatic conditioning of lymph nodes. Consistently, the prostanoid PGE2 is produced by DCs in lymph nodes prior to metastatic colonization and stimulates the accumulation of tumor-suppressive regulatory T cells [59].
Vascular remodeling
Extensive remodeling of lymphatic high endothelial venules (HEVs) and lymphatic vessels has been observed pre-metastatically in tumor-draining lymph nodes. VEGF-A, VEGF-C, and VEGF-D, members of the vascular endothelial growth factor family, are produced by primary tumors and drain to the regional lymph nodes where they play a key role in this remodeling (reviewed in [60]). The VEGFR cell surface receptor family member VEGFR-2 is activated by VEGF-A. VEGF-C and VEGF-D activate another VEGFR family member VEGFR-3 and to a lesser extent also VEGFR-2 when they are fully proteolytically processed [7]. Activation of VEGFR-2 in the main induces angiogenesis, while VEGFR-3 activation primarily stimulates lymphangiogenesis. However, VEGFR-2 activation can contribute to lymphangiogenesis, and VEGFR-3 activation to angiogenesis [5].
Pre-metastatic induction of lymphangiogenesis in lymph nodes is well documented for many tumor entities. Compared to chemically induced skin tumors in wild-type mice, similarly induced skin tumors in transgenic mice expressing VEGF-A or VEGF-C in the skin were found to induce more lymphangiogenesis in tumor-draining lymph nodes prior to entry of metastatic cells [61, 62]. Pre-metastatic lymphangiogenesis in sentinel lymph nodes has been reported in melanoma transplantation models and chemically induced skin tumors in the absence of local lymphangiogenesis induction around the primary tumor [63, 64]. In other animal models of nasopharyngeal, oral squamous cell, and breast carcinomas, pre-metastatic lymph node lymphangiogenesis was also observed [65–67]. These experimental observations are reflected in human cancers, as pre-metastatic lymph node lymphangiogenesis has been reported in tumor entities such as breast and tongue carcinomas [65–67]. The functional importance of pre-metastatic remodeling of the lymph node lymphatics was recently demonstrated in a rat model of breast cancer, where specific blocking of VEGF-C-driven pre-metastatic lymph node lymphangiogenesis using anti-VEGFR-3 antibodies suppressed fulminant outgrowth of lymph node metastases [68].
Evidence concerning pre-metastatic induction of angiogenesis in sentinel lymph nodes is incomplete and partially contradictory. In the transgenic mice expressing VEGF-A in the skin mentioned above, chemically induced skin tumors substantially induced pre-metastatic sentinel node lymphangiogenesis, but had no effect on CD31+ blood vessel numbers [61], despite the fact that VEGF-A is a major inducer of angiogenesis. In other animal models where substantial lymphangiogenesis is induced in tumor-draining lymph nodes prior to metastasis, a similar absence of pre-metastatic angiogenesis induction was observed [62–64, 69]. On the other hand, a pre-metastatic increase in CD31+ blood vessels in tumor-draining lymph nodes has been reported in oral squamous cell carcinoma xenografts, together with vasculogenesis mediated by incorporation of CD31+ bone marrow-derived cells into existing blood vessels [70]. Moreover, a recent study found that IL-6 draining from primary breast tumors upregulated VEGF-A expression pre-metastatically in lymph node lymphatic vessels [71]. This study provided indirect evidence using conditioned medium that pre-metastatic induction of angiogenesis in tumor-draining lymph nodes could in principle occur through such upregulated VEGF-A expression and indeed foster metastasis formation. However, no direct evidence was presented regarding whether tumor-derived IL-6 draining to lymph nodes is able to induce pre-metastatic lymph node angiogenesis through this mechanism under conditions of normal tumor growth in vivo. Further studies are required to determine to what degree pre-metastatic angiogenesis occurs in tumor-draining lymph nodes and what its significance is for subsequent metastasis.
Extensive pre-metastatic remodeling of HEVs in lymph nodes draining nasopharyngeal and breast tumors has been reported [65, 72]. HEV remodeling is characterized by vessel dilation, increased HEV proliferation, and thinning and flattening of the vessel walls [65, 72]. Similar pre-metastatic changes in HEVs as well as higher HEV numbers have been observed in lymph nodes draining squamous cell carcinomas of the tongue [67, 71]. However, reduced numbers of HEVs have been reported in pre-metastatic SLNs in melanoma patients [73], suggesting that not all tumor types have equivalent pre-metastatic effects on HEVs. Mechanistically, tumor-derived VEGF-D that drains to lymph nodes downregulates expression of BMP-4 in HEVs. This functionally causes the phenotypic and proliferative changes associated with HEV remodeling because the VEGF-D-induced remodeling of HEVs could be reversed by treatment with exogenous BMP-4 [72].
Other pre-metastatic niche components
VEGFR1+ clusters of hematopoietic progenitor cells accumulate in the pre-metastatic lymph nodes draining several types of human cancers [1]. These cells play an important role in stimulating metastasis formation as they promote tumor cell mobility, adherence and growth, and produce SDF-1, which attracts CXCR4+ tumor cells.
A number of microenvironmental changes such as extracellular matrix (ECM) remodeling that have been observed during pre-metastatic conditioning of other organs have been poorly investigated in the lymph node. Only indirect observations currently suggest that modification of the stromal architecture takes place during metastatic conditioning of the lymph node microenvironment. In normal lymph nodes, myofibroblasts are not found within the body of the lymph node, whereas in lymph nodes draining colorectal tumors in which only a single cancer cell could be detected, approximately 16 % of the internal lymph node area was comprised of myofibroblasts [74]. However, it is unclear whether this accumulation of myofibroblasts can result from pre-metastatic conditioning by the primary tumor. In breast cancer, changes in collagen I fiber density have been reported in tumor-draining lymph nodes which probably reflects the presence of DTCs [75]. Whether these changes can be induced pre-metastatically was not investigated.
Tumor-derived conditioning factors
Some of the tumor-derived factors that have been implicated in triggering an immunosuppressive microenvironment have been mentioned above. Indirect evidence suggests that gangliosides may also be involved [35]. More recently, PGE2 and SDF-1 have been functionally implicated in recruiting immunosuppressive regulatory T cells to the pre-metastatic lymph node [59]. Although chemokines such as CCL5/RANTES produced by lymphatic endothelial cells (LECs) in pre-metastatic lymph nodes in response to IL-6 draining from primary breast tumors could conceivably play a role in the orchestration of pre-metastatic modulation of the immune cell complement in tumor-draining lymph nodes, this proved not to be the case [71].
The role of VEGF family members and their receptors in inducing pre-metastatic remodeling of the lymph node vasculature has been described above. VEGF-A could in principle also play a role in creating an immunosuppressive microenvironment through its ability to suppress DC maturation [76] and induce TH2 polarization in CD4+ T cells [77], although this has not been specifically addressed in the context of the lymph node. In addition, recent work suggests that pre-metastatic conditioning of draining lymph nodes by experimental 3LL lung tumors upregulates COX-2 expression in lymph node DCs. In turn, COX-2-produced PGE2 acts via its receptor EP3 to upregulate VEGF-C and VEGF-D expression in lymph node macrophages and increases VEGFR-3 expression in LECs, leading to increased lymph node lymphangiogenesis [59].
Exosomes are microvesicles produced and shed by many cells. Less than 100 nm in diameter, they carry a variety of cargoes, including DNA, miRNAs, mRNAs, and proteins (reviewed in [78]). Upon fusing with target cells, exosomes can regulate cell properties and behavior through the cargoes they carry. A broad range of literature implicates exosomes in a variety of tumor-promoting activities [78]. Several studies have shown that tumor-derived exosomes that drain to lymph nodes can condition the microenvironment and contribute to metastatic niche formation. For example, exosomes from melanomas were found to home to regional lymph nodes, where they are thought to orchestrate a variety of pre-metastatic changes, including matrix remodeling, angiogenesis, and probably immune suppression, resulting in subsequent recruitment of disseminating melanoma cells to exosome-rich sites in the lymph node [79]. In a pancreatic cancer model, injection of exosomes from highly metastatic cells into the foot pad stimulated metastasis in the popliteal lymph node of poorly metastatic tumor cells that were subsequently injected into the foot pad [80]. Further experiments showed that stromal cells in the lymph node were the target for the exosomes, and that key exosome cargoes were miR-494 and miR-542-3p, which suppress cadherin-17 leading to upregulated matrix metalloproteinase expression [81].
How does pre-metastatic conditioning of the lymph node microenvironment promote metastasis?
The induction of an immunosuppressive microenvironment and the creation of a chronic inflammatory milieu support the metastatic outgrowth of DTCs in a number of ways. DTC survival is promoted due to suppression of the anti-tumoral immune response. In addition, immune cells within the lymph node pre-metastatic niche produce growth factors and cytokines that directly support the survival and proliferation of tumor cells, as exemplified by the production of TNFα by TAMs [82, 83]. Other factors such as chemokines produced by the immune cells can chemotactically attract disseminating tumor cells if they express the appropriate cognate receptor. For example, CD206+ tolerogenic immature DCs produce the chemokine CCL20, which chemotactically attracts head and neck squamous cell carcinoma cells that express CCR4 (the cognate receptor for CCL20), and thereby promotes lymph node metastasis [84, 85].
Several mechanisms seem to underlie the metastasis-promoting role of pre-metastatic lymph node lymphangiogenesis. First, lymph flow from tumors to lymph nodes increases as a consequence of lymph node lymphangiogenesis [63], which may foster transportation of disseminating tumor cells to the draining lymph nodes. Second, increased lymphatic vessel density in lymph nodes could stimulate the development of immune tolerance to tumor cells, due to the expression of high levels of the immune checkpoint inhibitory ligand PD-L1 in LECs [86] and the ability of LECs to contribute to antigen cross-presentation [87] which leads to CD8+ T cell tolerance. Third, pre-metastatically conditioned LECs produce factors that act as chemoattractants for incoming DTCs. For example, they produce SDF-1, which acts as a chemoattractant for CXCR4+/CD133+ melanoma cells, thereby stimulating metastasis formation [88]. Similarly, IL-6 draining from experimental human breast tumors stimulates pre-metastatic lymph node lymphatic vessels to produce CCL5 (RANTES) and CXCL7 [71]. In this model, CCL5 acted as a chemoattractant for CCR7+ breast cancer cells and functionally stimulated metastasis. Consistent with these observations, DTCs accumulate in close proximity to LECs in lymph nodes [68, 89]. Fourth, VEGF-C stimulation of LECs during lymphangiogenesis upregulates expression of α4β1 integrin on LECs, which promotes adhesion of VCAM1+ tumor cells to the lymphatic vessels and fosters metastasis formation [90]. Finally, DCs recruited to pre-metastatic lymph nodes also localize to lymphatic vessels, although the functional significance of this is not clear [59].
HEV remodeling serves to create flat-walled dilated vessels that have reduced lymphocyte transmigration rates, possibility leading to impaired immune function and improved survival of tumor cells [72]. Consistently, remodeled HEVs exhibit downregulation of MECA-79, otherwise known as peripheral node addressin (PNAd), an L-selectin ligand that plays an important role in lymphocyte trafficking [65]. Reduced HEV numbers observed in melanoma-draining lymph nodes [73] would similarly be expected to suppress anti-tumor immunity. In addition, vessel dilation may improve the supply of oxygen and nutrients to the lymph node. Importantly, remodeled HEVs are thought to make an important contribution to the vasculature of developing metastases once DTCs arrive in lymph nodes [65], supporting their outgrowth. Although poorly investigated, HEVs could conceivably act as a portal for entry of tumor cells into the blood stream from lymph nodes, and it is interesting to speculate that HEV remodeling might facilitate this route of dissemination.
Although not currently fully investigated, a pre-metastatic expansion of the blood vasculature would be expected to favor the survival and growth of DTCs once they arrive in the pre-metastatically conditioned lymph node. Additionally, increased blood and lymphatic vessel density could conceivably support metastasis through providing a perivascular niche that supports the survival and outgrowth of DTCs [91, 92], possibly by fostering cancer stem cell maintenance through the secretion of factors that enhance survival and self-renewal [93–95].
A recent paper provides a fascinating insight into how different components of the lymph node pre-metastatic niche interact to condition the lymph node [59]. As yet unidentified factors produced by LLC lung tumors induce the accumulation of COX-2-expressing DCs in the subcapsular regions of the tumor-draining lymph nodes. Inhibition of COX-2 activity or down-stream effectors (PGE2/EP3) blocked lymph node metastasis. Mechanistically, COX-2-produced PGE2 acts in positive feedback loop to recruit more DCs. It also signals in an autocrine manner via EP3 in DCs to induce expression of SDF-1, which in turn stimulates accumulation of CXCR4+ LLC cells in the lymph node. Together, PGE2/EP3 and SDF-1 recruit regulatory T cells to the pre-metastatic lymph node, contributing to an immunosuppressive microenvironment. Finally, PGE2/EP3 were also found to upregulate lymph node lymphangiogenesis through the VEGFR-3/VEGF-C/VEGF-D axis.
Maturation of the metastatic niche upon establishment of DTCs in the lymph nodes
After trafficking of cancer cells to the lymph node from the primary tumor, appropriate pre-metastatic conditioning of the lymph node microenvironment is clearly decisive for the survival and successful establishment of DTCs within the lymph node. Once established, the DTCs induce further changes in the lymph node microenvironment, resulting in maturation of the metastatic niche. For example, lymph node lymphangiogenesis is further induced [61, 62]. Increased expression of immunosuppressive cytokines is also observed [96], which is reflected in the types of immune cells found in lymph nodes with metastasis. For example, lymph nodes draining melanomas [97] and human colorectal cancers [98] have increased numbers of Foxp3+ regulatory T cells if they contain metastases. Similarly, SLNs from melanoma patients with lymph node metastases contain decreased numbers of DCs and increased numbers of Foxp3+ regulatory T cells compared to pre-metastatic SLNs [99]. Increased numbers of TAMs are also recruited [50].
Maturation of the lymph node metastatic niche supports fulminant outgrowth of lymph node metastases. The necessity for pre-metastatic conditioning and subsequent niche maturation reflects the dependency of DTCs on the tumor microenvironment that develops through stromal progression in the primary cancer and which needs to be established at distant organ sites if metastatic cells are to survive and grow [100].
Concluding comments
Pre-metastatic changes in the lymph nodes draining tumors have been recognized for more than a century, but only in recent years has the underlying cellular and molecular basis been elucidated. The induction of anti-tumor immune responses that then transition to a metastasis-promoting immunosuppressive microenvironment, as well as tumor-induced vascular remodeling in tumor-draining lymph nodes are the best understood pre-metastatic changes. However, there is clearly much still to be discovered. Nevertheless, it is clear that factors produced by growing tumors can be decisive in inducing the formation of a pre-metastatic niche in the lymph node, similar to the case in future metastatic sites in other organs. Thus, Virchow was not entirely wrong in surmising that “juices” produced by tumors cause metastasis formation [101, 102]—not because they induce transformation at sites of secondary tumor growth as he suggested, but rather because they prepare the soil and make it receptive and supportive for incoming DTCs.
For pre-metastatic conditioning of the lymph node microenvironment to occur, primary tumors need to produce sufficient pre-metastatic niche-inducing factors if conditioning processes are to be induced. This is reflected in several studies that have analyzed tumor-induced changes in the lymph node microenvironment as a function of the distance of the lymph node from the primary tumor and which have shown gradients of changes along the lymphatic chain, demonstrating concentration-dependent effects. Examples include the anti-tumor immune response [103], immune cell content [21], and the expression of immunomodulatory cytokines [104, 105]. This presumably also explains why tumor size often correlates with metastasis formation: larger tumors produce more factors that can condition the lymph node, resulting in higher concentrations of these factors reaching the lymph node. For example, in an autochthonous mouse melanoma model, levels of IL-10 and TGF-β correlated with tumor size [106]. Consistently, tumor size correlates with reduced immunoreactivity of lymphocytes taken from lymph nodes draining human breast cancers [48].
Factors produced by primary tumors can potentially pre-metastatically condition not only the lymph node microenvironment but also the microenvironment of other organs where metastases form. However, SLNs and, to a lesser extent, the other lymph nodes higher up the drainage basin are maximally exposed to the relatively high concentration of materials shed or secreted by primary tumors into the tumor-draining lymphatic fluid, compared to other organs that receive only diluted amounts of these materials in the blood. Pre-metastatic changes in lymph nodes therefore act as a sensitive gauge that primary tumors are developing the ability to pre-metastatically condition putative future sites of metastasis through the growth factors and cytokines that they produce. This helps to explain the predominance of lymph node metastases in prognostic evaluation and tumor grading schemes. In addition, it also means that pre-metastatic changes in lymph nodes could serve as discriminating indicators that tumors are beginning to develop metastatic potential before the onset of metastasis, not only in lymph nodes but also in other organs. First studies suggest that this might be the case [99]. Thus, the monitoring of pre-metastatic changes in tumor-draining lymph nodes may therefore potentially allow appropriate therapeutic intervention to be undertaken before overt metastases are formed.
Given the importance of the lymph node in the immune response to tumor antigens, modification of the microenvironment in tumor-draining lymph nodes for therapeutic purposes holds promise. For example, a recent study showed that nanoparticle targeting of DCs in tumor-draining lymph nodes stimulated DC maturation, thereby inducing a TH1 response and increasing the numbers of tumor antigen-specific CD8+ T cells, which resulted in slower tumor growth [107]. Thus, reversing the development of an immunosuppressive niche in tumor-draining lymph nodes has the potential not only to inhibit metastasis formation in the lymph nodes but also to stimulate an anti-tumor response.
References
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Sleeman JP (2012) The metastatic niche and stromal progression. Cancer Metastasis Rev 31:429–440
Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312
von Andrian UH, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878
Sleeman JP, Thiele W (2009) Tumor metastasis and the lymphatic vasculature. Int J Cancer 125:2747–2756
Sleeman JP, Nazarenko I, Thiele W (2011) Do all roads lead to Rome? Routes to metastasis development. Int J Cancer 128:2511–2526
Sleeman J, Schmid A, Thiele W (2009) Tumor lymphatics. Semin Cancer Biol 19:285–297
Weiss L (1980) The pathophysiology of metastasis within the lymphatic system. In: Weiss L, Gilbert HA, Ballon SC (eds) Lymphatic system metastasis. G.K. Hall & Co, Boston, pp 2–40
Wallace AC, Hollenberg NK (1965) The transplantability of tumours by intravenous and intralymphatic routes. Br J Cancer 19:338–342
Kurokawa Y (1970) Experiments on lymph node metastasis by intralymphatic inoculation of rat ascites tumor cells, with special reference to lodgement, passage, and growth of tumor cells in lymph nodes. Gann 61:461–471
Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B (2007) Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res 67:5009–5016
Cady B (2007) Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann Surg Oncol 14:1790–1800
Terrone C, Cracco C, Porpiglia F, Bollito E, Scoffone C, Poggio M, Berruti A, Ragni F, Cossu M, Scarpa RM et al (2006) Reassessing the current TNM lymph node staging for renal cell carcinoma. Eur Urol 49:324–331
Blazer DG 3rd, Sabel MS, Sondak VK (2003) Is there a role for sentinel lymph node biopsy in the management of sarcoma? Surg Oncol 12:201–206
FV B-H (1887) Lehrbuch der pathologischen Anatomie. Band 2 specielle pathologische Anatomie, 3rd Edn. FCW Vogel, Leipzig
Willis R (1973) The spread of tumors in the human body, 3rd edn. Butterworth, London
Ioachim HL, Medeiros LJ (2008) In: Ioachim HL, Medeiros LJ (eds) Tumor-reactive lymphadenopathy. Ioachim’s Lymph Node Pathology Lippincott Williams & Wilkins, Philadelphia, pp 243–247
Coronella-Wood JA, Hersh EM (2003) Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother CII 52:715–738
Tachibana T, Yoshida K (1986) Role of the regional lymph node in cancer metastasis. Cancer Metastasis Rev 5:55–66
Kim R, Emi M, Tanabe K, Arihiro K (2006) Immunobiology of the sentinel lymph node and its potential role for antitumour immunity. Lancet Oncol 7:1006–1016
Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R (2006) Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol 6:659–670
Kitamura T, Qian BZ, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15:73–86
Gjerstorff MF, Andersen MH, Ditzel HJ (2015) Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 6:15772–15787
Alvarez D, Vollmann EH, von Andrian UH (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29:325–342
Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M (2011) CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 34:85–95
Heesters BA, Myers RC, Carroll MC (2014) Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol 14:495–504
Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE (2009) In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol 183:3195–3203
Moalli F, Proulx ST, Schwendener R, Detmar M, Schlapbach C, Stein JV (2015) Intravital and whole-organ imaging reveals capture of melanoma-derived antigen by lymph node subcapsular macrophages leading to widespread deposition on follicular dendritic cells. Front Immunol 6:114
Wolkers MC, Stoetter G, Vyth-Dreese FA, Schumacher TN (2001) Redundancy of direct priming and cross-priming in tumor-specific CD8+ T cell responses. J Immunol 167:3577–3584
Lai YP, Jeng CJ, Chen SC (2011) The roles of CD4+ T cells in tumor immunity. ISRN Immunol. doi:10.5402/2011/497397, 6 pages
Kim HJ, Cantor H (2014) The path to reactivation of antitumor immunity and checkpoint immunotherapy. Cancer Immunol Res 2:926–936
Straten P, Dahl C, Schrama D, Pedersen LO, Andersen MH, Seremet T, Brocker EB, Guldberg P, Becker JC (2006) Identification of identical TCRs in primary melanoma lesions and tumor free corresponding sentinel lymph nodes. Cancer Immunol Immunother CII 55:495–502
Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M (2005) Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med 202:1679–1689
Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C (2004) Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A 101:16606–16611
Farzad Z, Cochran AJ, McBride WH, Gray JD, Wong V, Morton DL (1990) Lymphocyte subset alterations in nodes regional to human melanoma. Cancer Res 50:3585–3588
Yoon SR, Kim TD, Choi I (2015) Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med 47:e141
Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F (2004) Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 5:1260–1265
Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC et al (2009) Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest 119:1524–1536
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182:4499–4506
Zhang W, Zhang C, Li W, Deng J, Herrmann A, Priceman SJ, Liang W, Shen S, Pal SK, Hoon DS et al (2015) CD8+ T-cell immunosurveillance constrains lymphoid premetastatic myeloid cell accumulation. Eur J Immunol 45:71–81
Melief CJ (2008) Cancer immunotherapy by dendritic cells. Immunity 29:372–383
Otto B, Koenig AM, Tolstonog GV, Jeschke A, Klaetschke K, Vashist YK, Wicklein D, Wagener C, Izbicki JR, Streichert T (2014) Molecular changes in pre-metastatic lymph nodes of esophageal cancer patients. PLoS ONE 9:e102552
Mansfield AS, Holtan SG, Grotz TE, Allred JB, Jakub JW, Erickson LA, Markovic SN (2011) Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol Off J U S Can Acad Pathol Inc 24:487–494
Lana AM, Wen DR, Cochran AJ (2001) The morphology, immunophenotype and distribution of paracortical dendritic leucocytes in lymph nodes regional to cutaneous melanoma. Melanoma Res 11:401–410
Matsuura K, Yamaguchi Y, Ueno H, Osaki A, Arihiro K, Toge T (2006) Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 106:1227–1236
Cochran AJ, Wen DR, Farzad Z, Stene MA, McBride W, Lana AM, Hoon DS, Morton DL (1989) Immunosuppression by melanoma cells as a factor in the generation of metastatic disease. Anticancer Res 9:859–864
Huang RR, Wen DR, Guo J, Giuliano AE, Nguyen M, Offodile R, Stern S, Turner R, Cochran AJ (2000) Selective modulation of paracortical dendritic cells and T-lymphocytes in breast cancer sentinel lymph nodes. Breast J 6:225–232
Reiss CK, Volenec FJ, Humphrey M, Singla O, Humphrey LJ (1983) The role of the regional lymph node in breast cancer: a comparison between nodal and systemic reactivity. J Surg Oncol 22:249–253
Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP (2005) Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med 2:e284
Go Y, Tanaka H, Tokumoto M, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K, Ohira M, Hirakawa K (2015) Tumor-associated macrophages extend along lymphatic flow in the pre-metastatic lymph nodes of human gastric cancer. Ann Surg Oncol. doi:10.1245/s10434-015-4458-7
Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, Shen S, Priceman SJ, Kujawski M, Pal SK et al (2012) S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell 21:642–654
Wu LJ, Li HX, Luo XT, Lu RZ, Ma YF, Wang R, Zhang J, Yang DQ, Yu H, Liu J (2014) STAT3 activation in tumor cell-free lymph nodes predicts a poor prognosis for gastric cancer. Int J Clin Exp Pathol 7:1140–1146
Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. doi:10.1016/j.canlet.2015.07.009
Itakura E, Huang RR, Wen DR, Paul E, Wunsch PH, Cochran AJ (2011) IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod Pathol Off J U S Can Acad Pathol Inc 24:801–809
Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J (2006) Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol 176:5637–5643
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166
Kusmartsev S, Gabrilovich DI (2002) Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother CII 51:293–298
Munn DH (2011) Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr Med Chem 18:2240–2246
Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K, Kitasato H, Iyoda A, Iwabuchi K, Kumagai Y et al (2014) Prostanoid induces premetastatic niche in regional lymph nodes. J Clin Invest 124:4882–4894
Farnsworth RH, Lackmann M, Achen MG, Stacker SA (2014) Vascular remodeling in cancer. Oncogene 33:3496–3505
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M (2005) VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 201:1089–1099
Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M (2007) VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109:1010–1017
Harrell MI, Iritani BM, Ruddell A (2007) Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 170:774–786
Ruddell A, Kelly-Spratt KS, Furuya M, Parghi SS, Kemp CJ (2008) p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene 27:3145–3155
Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D et al (2006) Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res 66:10365–10376
Van den Eynden GG, Van der Auwera I, Van Laere SJ, Huygelen V, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA (2006) Induction of lymphangiogenesis in and around axillary lymph node metastases of patients with breast cancer. Br J Cancer 95:1362–1366
Chung MK, Do IG, Jung E, Son YI, Jeong HS, Baek CH (2012) Lymphatic vessels and high endothelial venules are increased in the sentinel lymph nodes of patients with oral squamous cell carcinoma before the arrival of tumor cells. Ann Surg Oncol 19:1595–1601
Quagliata L, Klusmeier S, Cremers N, Pytowski B, Harvey A, Pettis RJ, Thiele W, Sleeman JP (2014) Inhibition of VEGFR-3 activation in tumor-draining lymph nodes suppresses the outgrowth of lymph node metastases in the MT-450 syngeneic rat breast cancer model. Clin Exp Metastasis 31:351–365
Liersch R, Hirakawa S, Berdel WE, Mesters RM, Detmar M (2012) Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF-C as the earliest premetastatic indicator. Int J Oncol 41:2073–2078
Mayorca-Guiliani AE, Yano H, Nakashiro K, Hamakawa H, Tanaka J (2012) Premetastatic vasculogenesis in oral squamous cell carcinoma xenograft-draining lymph nodes. Oral Oncol 48:663–670
Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS (2014) Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun 5:4715
Farnsworth RH, Karnezis T, Shayan R, Matsumoto M, Nowell CJ, Achen MG, Stacker SA (2011) A role for bone morphogenetic protein-4 in lymph node vascular remodeling and primary tumor growth. Cancer Res 71:6547–6557
Cochran AJ, Huang RR, Su A, Itakura E, Wen DR (2015) Is sentinel node susceptibility to metastases related to nodal immune modulation? Cancer J 21:39–46
Yeung TM, Buskens C, Wang LM, Mortensen NJ, Bodmer WF (2013) Myofibroblast activation in colorectal cancer lymph node metastases. Br J Cancer 108:2106–2115
Kakkad SM, Solaiyappan M, Argani P, Sukumar S, Jacobs LK, Leibfritz D, Bhujwalla ZM, Glunde K (2012) Collagen I fiber density increases in lymph node positive breast cancers: pilot study. J Biomed Opt 17:116017
Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A et al (2011) Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30:83–95
Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN, Melanoma Study Group of the Mayo Clinic Cancer C (2009) Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res Off J Am Assoc Cancer Res 15:1931–1939
Azmi AS, Bao B, Sarkar FH (2013) Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 32:623–642
Hood JL, San RS, Wickline SA (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71:3792–3801
Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zoller M (2009) CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 11:1093–1105
Rana S, Malinowska K, Zoller M (2013) Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 15:281–295
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912
Schmid MC, Varner JA (2010) Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol2010: 201026. doi:10.1155/2010/201026
Layseca-Espinosa E, Korniotis S, Montandon R, Gras C, Bouillie M, Gonzalez-Amaro R, Dy M, Zavala F (2013) CCL22-producing CD8alpha- myeloid dendritic cells mediate regulatory T cell recruitment in response to G-CSF treatment. J Immunol 191:2266–2272
Tsujikawa T, Yaguchi T, Ohmura G, Ohta S, Kobayashi A, Kawamura N, Fujita T, Nakano H, Shimada T, Takahashi T et al (2013) Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int J Cancer 132:2755–2766
Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT et al (2012) Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120:4772–4782
Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA (2012) VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 1:191–199
Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY (2010) CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res 70:10411–10421
Hirakawa S (2009) From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci 100:983–989
Garmy-Susini B, Avraamides CJ, Desgrosellier JS, Schmid MC, Foubert P, Ellies LG, Lowy AM, Blair SL, Vandenberg SR, Datnow B et al (2013) PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proc Natl Acad Sci U S A 110:9042–9047
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122
Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M (2006) Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res 66:2650–2657
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82
Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A et al (2011) A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478:399–403
Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE (2010) Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res 70:9969–9978
Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, Morton DL, Essner R (2005) Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res Off J Am Assoc Cancer Res 11:107–112
Mohos A, Sebestyen T, Liszkay G, Plotar V, Horvath S, Gaudi I, Ladanyi A (2013) Immune cell profile of sentinel lymph nodes in patients with malignant melanoma—FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J Transl Med 11:43
Deng L, Zhang H, Luan Y, Zhang J, Xing Q, Dong S, Wu X, Liu M, Wang S (2010) Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res 16:4105–4112
Ma MW, Medicherla RC, Qian M, Vega-Saenz de Miera E, Friedman EB, Berman RS, Shapiro RL, Pavlick AC, Ott PA, Bhardwaj N et al (2012) Immune response in melanoma: an in-depth analysis of the primary tumor and corresponding sentinel lymph node. Mod Pathol Off J U S Can Acad Pathol Inc 25:1000–1010
Sleeman JP, Christofori G, Fodde R, Collard JG, Berx G, Decraene C, Ruegg C (2012) Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol 22:174–186
Virchow R (1863) Die krankhaften Geschwülste, 1st edn. August Hirschwald, Berlin
Virchow R (1858) Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre, 1st edn. August Hirschwald, Berlin
Farzad Z, McBride WH, Ogbechi H, Asnong-Holthoff C, Morton DL, Cochran AJ (1997) Lymphocytes from lymph nodes at different distances from human melanoma vary in their capacity to inhibit/enhance tumor cell growth in vitro. Melanoma Res 7(Suppl 2):S59–S65
Botella-Estrada R, Dasi F, Ramos D, Nagore E, Herrero MJ, Gimenez J, Fuster C, Sanmartin O, Guillen C, Alino S (2005) Cytokine expression and dendritic cell density in melanoma sentinel nodes. Melanoma Res 15:99–106
Leong SP, Peng M, Zhou YM, Vaquerano JE, Chang JW (2002) Cytokine profiles of sentinel lymph nodes draining the primary melanoma. Ann Surg Oncol 9:82–87
Alb M, Sie C, Adam C, Chen S, Becker JC, Schrama D (2012) Cellular and cytokine-dependent immunosuppressive mechanisms of grm1-transgenic murine melanoma. Cancer Immunol Immunother CII 61:2239–2249
Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA (2014) Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35:814–824
Acknowledgments
JPS is the “Franz-Volhard-Stipftungsprofessur für Mikrovaskuläre Biologie und Pathobiologie” funded by the Klinikum Mannheim gGmbH. This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft under the auspices of Research Training Group/Graduiertenkolleg 2099 “Hallmarks of Skin Cancer.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sleeman, J.P. The lymph node pre-metastatic niche. J Mol Med 93, 1173–1184 (2015). https://doi.org/10.1007/s00109-015-1351-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1351-6