Abstract
Isoliquiritigenin (ISL) is an important flavonoid component of licorice and has been reported to possess anti-inflammatory and antioxidant properties, but its exact mechanism of action remains poorly understood. Previously, we demonstrated that ISL could suppress IL-6 expression in multiple myeloma. Here, we further characterized the anti-inflammatory effects of ISL in several psoriasis models, including the keratin 14/vascular endothelial growth factor (VEGF) transgenic mouse, the imiquimod (IMQ)-induced psoriasis-like mouse, and the human keratinocytes HaCaT and NHEK in vitro. We found that ISL ameliorated the inflammatory process in psoriasis models but not in their respective controls. Moreover, the anti-inflammatory effects of ISL were attributed to the suppression of nuclear factor-κB (NF-κB) activity, which consequently resulted in the reduction of pro-inflammation cytokines IL-6 and IL-8 expression. In conclusion, ISL exhibited anti-inflammatory effects in psoriasis models, by downregulating IL-6 and IL-8 via suppression of NF-κB activity, suggesting that ISL might serve as a potential candidate for treatment of psoriasis and other autoimmune inflammatory diseases.
Key message
-
ISL could ameliorate the inflammatory process of psoriasis.
-
ISL could suppress NF-κB and subsequent production of a series of pro-inflammatory cytokines.
-
Dual-inhibitory activity against IL-6 and IL-8 of ISL is implemented via inhibiting NF-κB.
-
ISL exerts no inhibitory effects on normal human keratinocytes or wild-type Balb/c mice, implying its low toxicity and safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycyrrhiza uralensis Fisch, commonly known as “licorice,” has been used as a medicinal plant with detoxification, antiulcer, and anti-inflammatory effects for 4000 years [1–4]. In China, licorice is used in at least 75 % of Chinese medicine formulae. It is also a natural flavoring, sweetening agent for food additives, tobacco, and beer flavors [5–8]. Isoliquiritigenin (2′,4′,4-trihydroxychalcone, ISL) is one flavonoid component of licorice, which possesses various pharmacological activities, including antioxidant, antivirus, antitumor, and anti-inflammatory activities [9, 10]. We have shown that ISL could inhibit the growth of myeloma via reduction of the expression of inflammatory cytokine IL-6 [11], suggesting its potential use in other disorders such as psoriasis that involve inflammatory cytokines.
Psoriasis is a common chronic autoimmune disease, and it is characterized by hyperproliferative keratinocytes, parakeratosis, raised plaques, and massive infiltration of inflammatory cells [12, 13]. The prevalence of psoriasis is up to approximately 3 %, and the disease severely affects the quality of life [12, 14, 15]. Traditional treatments include psoralen plus UVA irradiation (PUVA), acitretin, methotrexate, and cyclosporine, but they have limited therapeutic effects [16]. Therefore, investigating the mechanism of pathogenesis and developing effective treatment of psoriasis are still needed.
Nuclear factor-κB (NF-κB) is thought to play a pivotal role in immune and inflammatory responses through the regulation of pro-inflammatory cytokines, chemokines, and growth factors [17, 18]. Increasing evidence has suggested the critical involvement of NF-κB and its regulatory cytokine in the pathogenesis of psoriasis [19]. The cytokine micromilieu in inflamed skin of psoriasis is composed of multiple pro-inflammatory mediators, including IL-6 and IL-8. Both of them are regulated by activation of transcription factor NF-κB and have been identified as key players in the pathogenesis and course of psoriasis [15, 20].
Here, to explore the therapeutic benefits of ISL and its possible mechanism of action, we investigated the effects of ISL in multiple psoriasis models and its potential interaction with NF-κB and downstream cytokines in the same systems. We found that ISL could prevent the progression of psoriasis-like symptoms in psoriasis models both in vivo and in vitro, suggesting that ISL might be a potential anti-psoriasis drug candidate.
Methods
Reagents and antibodies
Cell Counting Kit-8 (CCK-8) and Hoechst 33258 were purchased from Dojindo (Japan). Both iScript cDNA Synthesis Kit and SsoAdvanced SYBR Green Supermix were obtained from Bio-Rad (USA). All human and mouse IL-2, IL-4, IL-6, IL-8, IL-12p70, IL-23p40, and IL-17A ELISA kits were purchased from NeoBioscience Technology Co., Ltd (China). Recombinant human IL-1α, IL-17A, IL-22, TNF-α, OSM, and NF-κB were obtained from PeproTech (USA). Imiquimod (IMQ) was purchased from 3M Pharmaceuticals (UK). Nuclear and Cytoplasmic Extraction Kit was from CWBiotech. The primary antibodies against NF-κBp65, p-NF-κBp65, IκBα, and β-actin were acquired from Cell Signaling Technology (Beverly, MA, USA), CD4 and CD11b/c from Novus (UK), CD8 and F4/80 from Biorbyt (USA), vascular endothelial growth factor (VEGF) from Abcam (UK), and the NF-κB inhibitor Bay11-7082 from Selleck Chemicals (Houston, TX, USA). Anti-rabbit and anti-mouse IgG antibodies conjugated with horseradish peroxidase were purchased from Zhongshan Biological (China). Isoliquiritigenin (purity >98 %) provided by Chengdu Must Bio-Technology Co. Ltd (China) was prepared in DMSO at a concentration of 10 mg/ml until use.
Cell lines and culture
Human keratinocyte cell line HaCaT was cultured in RPMI 1640 (Gibco, USA) supplemented with 10 % fetal bovine serum (Biowest, France), 100 units/ml penicillin, and 100 units/ml streptomycin at 37 °C, in a humidified atmosphere containing 5 % CO2. In the research, in vitro psoriatic models were induced using human keratinocytes HaCaT and NHEK, respectively. Human keratinocyte cell NHEK (sixth passage) were cultured in keratinocyte basal medium (KBM-2; Lonza) supplemented with KGM-2 SingleQuots (Lonza, USA). The keratinocytes were stimulated with M5 to induce inflammation that recapitulates numerous features of psoriasis as described in [28], respectively. Briefly, HaCaT or NHEK (3 × 105/well) was seeded in six-well plates and cultured for 24 h according to the method described previously. Next, the supernatant fluids were replaced by serum-free medium supplemented with M5 which is a combination of recombinant human IL-1α, IL-17A, IL-22, TNF-α, and OSM (10 ng/ml of each cytokine), respectively, and then cultured in the same conditions for another 24 h.
Cell viability assay
Keratinocytes were stimulated with and without M5 for 24 h and then treated by ISL at different concentrations; next, cell viability was determined by the CCK-8 Kit. Briefly, cells (5 × 103/well) were seeded in 96-well plates with different concentrations of ISL (0, 2, 4, 8, and 16 μg/ml) treatment for 24, 48, or 72 h in triplicates, respectively. The supernatant was removed, and 10 μl of CCK-8 regent was added per well filled with 90 μl medium and incubated for 3 h at 37 °C away from light. The effect of ISL on cells viability were typified by absorbance values determined on the Microplate Reader (Thermo Scientific) at 450 nm wavelength. The effect of ISL on the proliferation of cells was represented by the percentage of cell growth inhibition, which is calculated using the following formula: Inhibitory rate = ([OD control group − OD experiment group]/OD control group) × 100 %.
Real-time quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription and qPCR were performed using iScript cDNA Synthesis Kit and SsoAdvanced SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions, respectively. Primers used in this study are listed below: human NF-κB p65, sense 5′-GCT CCT GTG CGT GTC TCC AT-3′ and antisense 5′-TTA CGT TTC TCC TCA ATC CGG T-3′ (119 bp) and human β-actin, sense 5′-CCA CGA AAC TAC CTT CAA CTC C-3′ and antisense 5′-GTG ATC TCC TTC TGC ATC CTG T-3′ (132 bp). PCR was performed using the following conditions: 95 °C for 3 min, 44 cycles of 10 s at 95 °C and 30 s at 58 °C, and melting curve analysis. All real-time PCR were performed in duplicates.
Western blotting
Sections of keratinocytes treated with and without M5 and psoriatic lesion tissues from animal models were prepared according to the method described above, and proteins were extracted using Nuclear and Cytoplasmic Extraction Kit according to the manufacturer’s instructions. The resulted proteins were subjected to 10 % SDS-PAGE and transferred to PVDF membranes (Millipore). After blocked with 5 % BSA at 37 °C for 2 h, the membrane was probed with the primary antibodies: rabbit anti-NF-κB, p-NF-κB, and IκBα (diluted 1:1000) at 4 °C overnight, respectively. After washing out non-specific binding with TBST, the blots were incubated with secondary antibody (diluted 1:10,000) conjugated with horseradish peroxidase at 37 °C for 1.5 h. Blots were visualized by enhanced chemiluminescence reagents (Amersham Biosciences). β-Actin was used as an internal control.
Animal studies
Herein, animals were treated in accordance with National Institutes of Health guidelines for animal care and use, and in vivo experimental procedures were approved by the Institutional Animal Care and Treatment Committee of Sichuan University. One model was human keratin 14 (K14)/VEGF transgenic mice (K14/VEGF transgenic mice), which were purchased from Jackson Laboratory, and the mouse spontaneously develops a chronic inflammatory skin disease with many characteristic features similar to human psoriasis [21–23]. The other was IMQ-induced (Aldara; 3M Pharmaceuticals) psoriasis mouse model which was established according to the method described in [27]. Briefly, some weeks before the formal experiments, several preliminary experiments were conducted. Then, 8–10-week-old female mice exhibiting psoriasis-like lesions were used, and treatments were given i.p. with NS, ISL (500 μg/kg/day), ISL (1 mg/kg/day), and ISL (2 mg/kg/day) for 30 days in K14/VEGF transgenic psoriasis models. While, in IMQ-induced psoriasis mouse model, treatments were given i.p. with NS (DMSO <1 ‰), ISL (1 mg/kg/day), and ISL (2 mg/kg/day) for 15 days. Then, mice were sacrificed, ears or skins were removed, cleaned from adjacent tissues, fixed in 4 % poly-formaldehyde, paraffin-embedded, and then sections (thickness 4 μm) were prepared. Some sections were stained with H&E and others for subsequent immunohistochemical analysis. Besides, at the end of the experiments, levels of cytokines of each group in the murine sera from retro-orbital sinus were measured using ELISA (NeoBioscience, China) following the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemistry was performed to detect the expressions of NF-κB, CD4, CD8, CD11b/c, F4/80, and VEGF in the ear or back skin tissue. Briefly, sections from the formalin-fixed, paraffin-embedded tissues were cut to 4 μm thick and deparaffinized in xylene followed by treatment with graded ethanol solutions and rehydration in PBS. Endogenous peroxidase was blocked with 3 % hydrogen peroxide for 15 min, followed by microwave antigen retrieval, and then, slides were incubated with rabbit monoclonal antibodies against NF-κB (at a dilution of 1:800), CD4 (1:100), CD8 (1:30), CD11b/c (1:150), F4/80 (1:250), and VEGF (1:200) at 4 °C overnight, followed by incubation with secondary biotinylated polyclonal antibody goat anti-rabbit (1:2000) at 37 °C for 1 h. Positive reaction was observed with 3,3-diaminobenzidine as chromagen (DAB Substrate Kit; Vector Laboratories), then sections were counterstained with hematoxylin. Positive reaction was analyzed on an OLYMPUS microscope (magnification ×400).
Statistical analysis
All values were reported as means + SEM. Statistical analysis was performed using GraphPad Prism 6 software. Two-tailed Student’s t test was used to compare differences. Significance level was set at P < 0.05.
Results
ISL attenuated the development of psoriasis-like lesions in mouse models
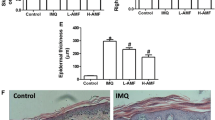
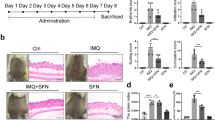
To investigate the therapeutic potential of ISL against psoriasis in vivo, two psoriasis animal models were used including the human K14/VEGF transgenic mouse model and the IMQ-induced psoriasis mouse model. On the one hand, the K14/VEGF transgenic mice spontaneously developed a chronic inflammatory skin disease with many characteristic features similar to human psoriasis, including increased density of tortuous cutaneous blood capillaries, psoriasis-like lesion on the ear of the mouse, dermal mast cell accumulation, and leukocyte recruitment to the sites of inflammation [21–24]. Here, 8–10-week-old K14 mice were randomly separated into four groups (n = 6), and psoriasis-like lesions on the ears were observed in 100 % of treated animals. After 30 days of treatment with different doses of ISL, the thickness of the ears were decreased and psoriasis-like plaque on the ears tended to be improved in the ISL-treated groups than those in the control group in a dose- and time-dependent manner (Fig.1a, also see Supplemental Figure 1a), especially in 2-mg/kg group. H&E staining of skin sections from the ear showed exciting decreased parakeratosis and hyperkeratosis and reduced severe lymphocyte infiltration into the dermis and epidermal layers in the ISL treatment group versus the control group (Fig. 1b). Pathological score by the Baker score system [25, 26] revealed that ISL treatment group exhibited lower score than that of control (Fig. 1d). On the other hand, the IMQ-induced psoriasis mouse was recently established experimental model of psoriasis-like dermatitis induced by daily application of IMQ, a TLR7/8 ligand and potent immune activator, on the back skin of the mouse. IMQ-induced skin lesions manifest psoriatic changes such as increased epidermal proliferation, abnormal differentiation, epidermal accumulation of neutrophils in microabcesses and infiltrates consisting of CD4 T cells and CD11b/c dendritic cells [27]. In this study, the IMQ-induced psoriasis model was established and treated with different doses of ISL. Consistent with the data above, similar protective effects in these mice with ISL treatment were obtained (Fig. 2a–c). Taken together, these results demonstrated that ISL possessed significant effects on slowing down psoriatic disease process in these mouse models.
ISL showed remarkable anti-psoriasis activity in K14/VEGF transgenic mice. a ISL attenuated development of psoriasis-like lesions in mouse models. Macroscopic characteristics of the psoriatic lesions after treatment with ISL. b Histopathological changes of ear lesions of each group. The right panel depicted higher magnifications of them. (Left panel, ×200, scale bars, 50 μm. Right panel, ×400, scale bars, 20 μm). c ISL treatment led to significant decrease in the serum levels of IL-6 and IL-8 in a dose-dependent manner in this model of psoriasis, data are presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). d Baker score of H&E staining of ears for each group, pathological score revealed that ISL treatment groups exhibited lower score than that of control, data were presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). e–f Immunohistochemical analysis of ears in K14/VEGF transgenic mice. ISL treatment could significantly downregulate expression of CD4 (Th cells), CD8 (CTLs), CD11b/c (DC), F4/80 (macrophages), VEGF, and NF-κB in the ear of psoriatic mice in a dose-dependent manner. The bottom panel depicted higher magnifications of them. (×400, scale bars, 20 μm). WT Balb/c, wild-type Balb/c mice
ISL repressed skin inflammation in the IMQ-induced psoriasis mouse model. a ISL attenuated development of psoriasis-like lesions in the back skin of mice. Macroscopic characteristics of the psoriasis skin lesions after treatment with ISL. b Histopathological changes of back skin lesions of each group. The right panel depicted higher magnifications of them. (Left panel, ×200, scale bars, 50 μm. Right panel, ×400, scale bars, 20 μm). c Baker score of H&E staining of ears for each group, pathological score revealed that ISL treatment groups exhibited lower score than that of control, data were presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). d ISL treatment led to significant decrease in the serum levels of IL-6 and IL-8 in a dose-dependent manner in this model of psoriasis, data are presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). e–f Immunohistochemical analysis of the back skin in IMQ-induced psoriasis mouse model. ISL treatment could effectively decrease expression of CD4 (Th cells), CD8 (CTLs), CD11b/c (DC), F4/80 (macrophages), VEGF, and NF-κB in the back skin of psoriatic mice in a dose-dependent manner. The bottom panel depicted higher magnifications of them. (×400, scale bars, 20 μm)
ISL inhibited levels of IL-6 and IL-8 in mouse models of psoriasis
In pathologic process of psoriasis, several keratinocyte-derived pro-inflammatory cytokines have major roles in continuing the recruitment of leukocytes invading the inflammatory sites [12]. Among these, IL-6 and IL-8 have been identified as key players in the pathogenesis and course of cutaneous inflammation in psoriasis. To determine the effects of ISL on these cytokines in two psoriasis animal models above, we treated K14 mice for 30 days, while 15 days for IMQ-induced mice with different doses of ISL, respectively. At the end of the experiments, the levels of several cytokines of each group in the murine sera from retro-orbital sinus were measured using ELISA. As shown in Figs. 1c and 2d, both psoriatic mice models produced higher levels of IL-6 and IL-8 and ISL treatment led to significant decrease in their levels in a dose-dependent manner. In addition, as shown in Supplemental Figure 1b and Supplement Figure 2e, the levels of IL-2 and IL-12p70 in K14 mice and IL-23 and IL-4 in IMQ-induce mice were tested by ELISA, respectively. We found that the expression of cytokines tested was decreased after ISL treatment. Furthermore, we also measured serum IL-17 levels of mice treated with ISL; however, the levels were too low to be detected (data not shown).
ISL decreased psoriasis-like skin inflammation in mice
To elucidate the possible mechanisms whereby ISL attenuated psoriasis-like skin inflammation in mice, we characterized psoriasis-like skin inflammation, resembling clinical features of the human psoriatic lesions including increased epidermal proliferation, abnormal differentiation, epidermal accumulation of neutrophils in microabcesses, neoangiogenesis, infiltrated with CD4+ T cells, CD11c+ dendritic cells, and plasmacytoid dendritic cells in the K14/VEGF transgenic mice and IMQ-induced psoriasis mice. In this study, several critical inflammatory markers including CD4, CD8, CD11b/c, F4/80, and VEGF in the ear and back skin of psoriatic mice were determined by immunohistochemical analysis in these two psoriasis animal models. Results showed that ISL could decrease expressions of CD4, CD8, CD11b/c, F4/80, and VEGF in the ear and back skin of psoriatic mice in a dose-dependent manner compared to those in control mice (Figs. 1e–f and 2e–f, also see Supplemental Figure 2c–d). These results suggested that ISL attenuated the psoriatic disease process by decreasing psoriasis-like ear or skin inflammation in mice.
ISL inhibited the proliferation of inflammatory keratinocytes in vitro
Accumulating studies have demonstrated that keratinocytes play a key role in the regulation of skin inflammation, and they respond to environmental and inflammatory factors as well as multiple cytokines stimuli, such as IL-1, IL-6, IL-17, IL-22, TNFα, and OSM [28–32]. The immortal human keratinocyte cell line (HaCaT) and normal human epidermal keratinocytes (NHEK) are commonly used as a paradigm for in vitro skin keratinocytes study [33, 34]. To further investigate the therapeutic potential of ISL in vitro, inflammatory keratinocytes were established using HaCaT and NHEK cells to mimic the inflammatory response between keratinocytes and immune cells in the pathogenesis of psoriasis according to the method described previously [28]. CCK-8 assay was used to examine the effect of ISL on the proliferation of keratinocytes with or without IL-1α, IL-17A, IL-22, TNFα, and OSM (named M5 combination) treatment. After treatment with ISL for 24, 48, and 72 h, ISL showed more significant dose- and time-dependent inhibitory effects on the growth of keratinocytes stimulated with M5 as compared with the untreated ones (Fig. 3a). Additionally, we also observed significant morphological changes of inflammatory keratinocytes treated with different concentrations of ISL for 48 h compared to the control group (Supplemental Figure 4b). These results indicated that ISL not only attenuated the inflammation in mouse psoriasis model through inhibiting the expression of IL-6 and IL-8 but also inhibited the proliferation of inflammatory keratinocytes in vitro.
ISL inhibited the proliferation of inflammatory keratinocytes in vitro. a Effects of ISL on the proliferation of normal keratinocytes and inflammatory keratinocytes, respectively. After treatment with ISL at different concentrations for 24, 48, and 72 h, ISL showed more significant dose- and time-dependent inhibitory effects on the growth of keratinocytes stimulated with M5 as compared with the one stimulated without M5. b After M5 treatment, both IL-6 and IL-8 level of keratinocytes were significantly upregulated versus normal one as well as NF-κB and p-NF-κB
ISL downregulated the levels of IL-6 and IL-8 in inflammatory keratinocytes in vitro
As shown above, ISL could attenuate the inflammation in psoriasis mouse models, and the significant decrease of IL-6 and IL-8 contributed to ISL anti-inflammation effects. To further confirm this finding, we evaluated the secretion levels of IL-2, IL-6, IL-8, and IL-12p70 by ELISA assay in keratinocytes stimulated with and without M5. As shown in Fig. 4b (left panel), apparent fold changes of IL-6 and IL-8 were observed in keratinocytes after M5 treatment versus normal one, while, IL-2 and IL-12p70 were below the detection limits of the assay (data not shown). To evaluate the effects of ISL on the inhibition of IL-6 and IL-8 expression in normal keratinocytes and inflammatory keratinocytes, respectively, ELISA was employed to determine the changes of IL-6 and IL-8 levels in the culture supernatant. Consistent with the observation in psoriatic mice, ISL also significantly downregulated the expression of IL-6 and IL-8 in inflammatory keratinocytes (Fig. 5a, Supplemental Figure 4c–d), while there were no significant changes in the expression of IL-6 and IL-8 in normal keratinocytes after treatment with ISL (Fig. 5a, Supplemental Figure 4c–d). These results further supported the idea that ISL assuaged inflammation by inhibiting the expression of IL-6 and IL-8.
ISL inhibited the expression of NF-κB in psoriasis animal models. a Protein levels of NF-κB, p-NF-κB, and IκBα were determined by Western blotting in K14/VEGF transgenic mice; results showed that ISL could distinctly decreased protein levels of NF-κB and p-NF-κB in the ear of the K14/VEGF transgenic mice (top panel) but not IκBα. While in wild-type Balb/c mice (bottom panel), none of the differences reached statistical significance. b Protein levels of NF-κB, p-NF-κB, and IκBα were determined by Western blotting in skin of IMQ-induced psoriasis mouse model. Consistent with the data above, results showed that ISL could effectively decreased protein levels of NF-κB and p-NF-κB in back skin of the IMQ-induced mice but not IκBα. Pairs of total protein were normalized by β-actin. Correspondingly, quantification of Western blot analysis images was performed using Image J software (NIH) and was presented in graphical form (*P < 0.05)
ISL downregulated the expression of IL-6 and IL-8 at both protein and mRNA levels. a Effects of ISL on levels of IL-6 and IL-8 in supernatant of keratinocytes stimulated with and without M5, respectively. Results demonstrated that ISL significantly downregulated the IL-6 and IL-8 levels of inflammatory keratinocytes simultaneously, but not the normal keratinocytes. Results are represented as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). b In normal keratinocytes and inflammatory keratinocytes, RNA level of NF-κB was detected by quantitative PCR analysis, respectively. Data illustrated that ISL reduced the RNA level of NF-κB in inflammatory keratinocytes; however, in normal keratinocytes, none of the differences reached statistical significance. c Protein levels of NF-κB, p-NF-κB, and IκBα were determined by Western blotting in vitro, compared with normal keratinocytes, ISL could obviously reduce levels of NF-κB and p-NF-κB in inflammatory keratinocytes. Pairs of total protein were normalized by β-actin. Correspondingly, quantification of Western blot analysis images was performed using Image J software (NIH) and was presented in graphical form (*P < 0.05)
ISL decreased the expression of NF-κB in psoriasis in vivo and in vitro
NF-κB is a crucial mediator involved in the pathogenesis of psoriasis, and psoriasis is marked by elevated levels of active, phosphorylated NF-κB. Accumulating studies have also linked psoriasis to mediators in the NF-κB pathway. NF-κB has been hypothesized to connect the altered keratinocyte and immune cell behaviors that characterize the psoriatic milieu. Consistent with previous reports, both NF-κB and phosphorylated NF-κB in inflammatory keratinocytes were significantly upregulated after stimulation with M5 (Fig. 3b, right panel). To test the influence of ISL on NF-κB pathway, we monitored the changes of NF-κB signaling by Western blotting. Results showed that both NF-κB and phosphorylated NF-κB were significantly reduced after ISL treatment in psoriatic models in vivo and in vitro in a dose-dependent manner, typically in the group treated with 2 mg/kg ISL in vivo (Fig. 4a–b, Supplemental Figure 3) and in the group treated with 16 μg/ml ISL in vitro (Fig. 5c). Additionally, no significant changes of IκBα were detected in vivo (Fig. 4a, b) as well as in vitro (Fig. 5c). Immunohistochemical analysis also showed that ISL could significantly downregulate expression of NF-κB in the ear or back skin of psoriatic mice in a dose-dependent manner (Figs. 1g and 2g and Supplemental Figure 2d). Interestingly, there were no significant changes of NF-κB, p-NF-κB, or IκBα either in wild-type Balb/c mice or in normal keratinocytes treated with different concentrations of ISL (Figs. 4a and 5c). Furthermore, qPCR revealed that ISL not only inhibited NF-κB protein expression but also repressed NF-κB mRNA levels in inflammatory keratinocytes (Fig. 5b, right panel). However, we did not detect any significant changes in normal keratinocytes after ISL treatment (Fig. 5b, left panel). Together, ISL inhibited NF-κB at both the protein and mRNA levels only in the psoriasis model, but not in wild-type Balb/c mice or normal human keratinocytes, suggesting that ISL could be a promising candidate for chronic inflammatory disorders such as psoriasis.
ISL suppresses the expression of IL-6 and IL-8 by downregulating NF-κB activation
To further explore the inhibitory effects of ISL on the expression of IL-6, IL-8, and NF-κB, two individual experiments were performed. Briefly, we measured the expression of IL-6 and IL-8 by ELISA. To this end, inflammatory keratinocytes were first established using human keratinocyte cell line HaCaT by treatment with M5. To induce the expression of IL-6 and IL-8, inflammatory keratinocytes were stimulated with recombinant human NF-κB (rHuNF-κB). In the meantime, we treated cells with bay11-7082 which could selectively and irreversibly inhibit NF-κB activation in the presence or absence of ISL. The abolishment of NF-κB signaling by bay11-7082 significantly decreased the expression of IL-6 and IL-8, and ISL treatment also showed similar IL-6 and IL-8 reduction albeit to a lesser degree (Fig. 6a). Notably, rHuNF-κB treatment could not rescue the expression of IL-6 and IL-8 in inflammatory keratinocytes treated with ISL or bay11-7082 (Fig. 6a). The rescue failure by rHuNF-κB was due to the inhibition of NF-κB activation by ISL and bay11-7082, as rHuNF-κB successfully activated NF-κB signaling indicated as NF-κB phosphorylation at Ser536 in the absence of ISL and bay11-7082 (Fig. 6b). These results further supported our finding that ISL inhibited the expression of IL-6 and IL-8 by blocking NF-κB activation.
The suppression of NF-κB activation by ISL is responsible for downregulation of IL-6 and IL-8. a ISL downregulated the expression of IL-6 and IL-8 by suppressing NF-κB activity in HaCaT stimulated with M5. After stimulation with human recombinant NF-κB (rHuNF-κB, 10 ng/ml) for 24 h, the expression of IL-6 and IL-8 of inflammatory keratinocytes were induced, simultaneously. In contrast, blocking NF-κB signal by bay11-7082 (10 μM) for 24 h led significant decreases of IL-6 and IL-8 levels. Importantly, rHuNF-κB treatment could not rescue the expression of IL-6 and IL-8 in psoriasis-like cells after treated with ISL or bay11-7082. Data are shown as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). b Effects of ISL on NF-κB, p-NF-κB, and IκBα in the presence of rHuNF-κB or bay11-7082. Western blotting showed that upon rHuNF-κB stimulation on inflammatory keratinocytes, NF-κB signaling substantially activated, and this was also partially abolished by ISL or bay11-7082 treatment. Pairs of total protein were normalized by β-actin. Correspondingly, quantification of Western blot analysis images was performed using Image J software (NIH) and was presented in graphical form (*P < 0.05)
Discussions
Licorice products are consumed daily in many countries [6, 35]. In traditional Chinese medicine, licorice is one of the most frequently employed botanicals [5]. Several components of licorice, such as glycyrrhetinic acid and glycyrrhizin, have been used clinically as an antiallergic and antihepatitis agent in UK and Japan [1]. Although its relatively low contents, ISL, a flavonoids in licorice, has been reported to possess anti-inflammatory, antioxidant, and antitumor properties [9–11, 36]. Of note, ISL exhibited antitumorigenic activity and estrogen receptor (ER) α-dependent growth promoting effects on breast cancer cells [37]. Animal study indicated that the LD50 of ISL in mouse is approximately 6 g/kg (i.g.), additionally, at the concentrations employed in this study, no changes in gross measures, such as weight loss, feeding, behavior, or other significantly side effects were observed, suggesting that ISL might be of low toxicity [38].
Recently, increasing evidence has shown the potential of ISL in preventing and treating disease. ISL lowers VEGF levels of breast cancer via PI3K/AKT signaling, targets GPR78 to chemo-sensitize breast cancer stem cells, and downregulates EGFR signaling to induce apoptosis both in wild-type and L858R/T790M mutant human lung cancer [39–41]. However, the precise mechanisms have not been fully investigated. Previously, we demonstrated that ISL could suppress IL-6 expression in multiple myeloma [11], implying that ISL might have potential therapeutic effects on inflammatory disease such as psoriasis. Studies have indicated that the serious impairment of the quality of life caused by high incidences of psoriasis equals or even exceeds that of other major illnesses such as rheumatoid arthritis or cancer [12, 14]. While, traditional treatments have limited effects, with inconvenience and toxicity. Hence, developing more effective and reliable approaches to psoriatic therapy is urgently needed. In this study, the results showed that ISL could effectively prevent the inflammatory progress in psoriasis models both in vitro and in vivo.
Aberrant cytokine expression has been proposed as an underlying cause of psoriasis. In addition to IL-2, IL-12, IL-23, the proinflammatory cytokines IL-6 and IL-8 also play key roles in the pathogenesis and the course of psoriasis [37, 42]. NF-κB, an inducible transcription factor, plays a pivotal role in the induction and function of many cytokines in psoriasis. It produces and regulates many pro-inflammatory cytokines, including IL-6 and IL-8, which expresses in high levels in psoriasis [20, 43–45]. Hence, either specifically targeting NF-κB signaling or decreasing levels of pro-inflammatory cytokines and regulating balance among them might be helpful for improving the pathologic process of psoriasis. Here, the experimental results have shown that ISL could effectively prevent the inflammatory progress in psoriasis in vivo and in vitro. In the mouse model of psoriasis, results displayed that ISL could significantly improve psoriatic lesion and slow down the pathologic process of psoriasis by decreasing IL-6 and IL-8 levels in the serum in addition to the reduction of CD4, CD8, CD11b/c, F4/80, and VEGF expression in the ear and back skin of psoriatic mice in a dose-dependent manner, respectively. Furthermore, we found that ISL not only significantly suppressed NF-κB expression but also inhibited its activation by correspondingly downregulating phosphorylated levels of NF-κB, which subsequently resulted in the reduction of pro-inflammatory cytokines IL-6 and IL-8, while IκBα had no obvious changes. Similar results were also obtained in vitro, and ISL not only reduced NF-κB at the protein level but also reduced mRNA levels in vitro. However, obvious inhibitory effects of ISL on NF-κB, IL-6, and IL-8 were not observed in normal keratinocytes and wild-type Balb/c mice, implying that ISL had low toxicity both in vitro and in vivo.
In conclusion, our studies indicated that ISL had significantly anti-inflammatory effects by downregulating both IL-6 and IL-8 via suppression of NF-κB activity, suggesting that ISL might be a promising NF-κB inhibitor and a potential candidate for treatment of psoriasis and other autoimmune inflammatory diseases.
References
Shibata S (2000) A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zsshi 120:849–862
Olukoga A, Donaldson D (1998) Historical perspectives on health - the history of liquorice: the plant, its extract, cultivation, commercialisation and etymology. J R Soc Health 118:300–304
Reid DP (1995) A handbook of Chinese healing Herbs. SHAMBHALA PUB Incorporated.
Huseman PA (1944) Licorice: putting a weed to work. Heffer & Sons, Ltd, London
Wang ZY, Nixon DW (2001) Licorice and cancer. Nutr Cancer 39:1–11
Gibson M (1978) Glycyrrhiza in old and new perspectives. Lloydia 41:348–354
Fenwick G, Lutomski J, Nieman C (1990) Liquorice, Glycyrrhiza glabra L.—composition, uses and analysis. Food Chem 38:119–143
Fu B, Li H, Wang X, Lee FS, Cui S (2005) Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J Agr Food Chem 53:7408–7414
Yamamoto S, Aizu E, Jiang H, Nakadate T, Kiyoto I, Wang JC, Kato R (1991) The potent anti-tumor-promoting agent isoliquiritigenin. Carcinogenesis 12:317–323
Yadav VR, Prasad S, Sung B, Aggarwal BB (2011) The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int Immunopharmacol 11:295–309
Chen X, Wu Y, Jiang Y, Zhou Y, Wang Y, Yao Y, Yi C, Gou L, Yang J (2012) Isoliquiritigenin inhibits the growth of multiple myeloma via blocking IL-6 signaling. J Mol Med 90:1311–1319
Schon MP, Boehncke WH (2005) Psoriasis. New Engl J Med 352:1899–1912
Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, H-g Z, Wang T, Zheng J (2011) Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35:596–610
Christophers E (2001) Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol 26:314–320
Nestle FO, Kaplan DH, Barker J (2009) Psoriasis. New Engl J Med 361:496–509
Gottlieb AB (2005) Psoriasis: emerging therapeutic strategies. Nat Rrev Drug Discov 4:19–34
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. New Engl J Med 336:1066–1071
Goldminz A, Au S, Kim N, Gottlieb A, Lizzul P (2013) NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci 69:89–94
Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB, Gottlieb AB (1989) Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A 86:6367–6371
Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M (2010) Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med 207:2255–2269
Wang H, Peters T, Sindrilaru A, Scharffetter-Kochanek K (2009) Key role of macrophages in the pathogenesis of CD18 hypomorphic murine model of psoriasis. J Invest Dermatol 129:1100–1114
Schon MP (1999) Animal models of psoriasis—what can we learn from them? J Invest Dermatol 112:405–410
Detmar M, Brown LF, Schön MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK (1998) Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 111:1–6
Baker B, Brent L, Valdimarsson H, Powles A, Al‐Imara L, Walker M, Fry L (1992) Is epidermal cell proliferation in psoriatic skin grafts on nude mice driven by T‐cell derived cytokines? Brit J Dermatol 126:105–110
Li J, Li X, Zhang Y, Zhou X, Yang H, Chen X, Wang Y, Wei Y, Chen L, Hu H et al (2010) Gene therapy for psoriasis in the K14‐VEGF transgenic mouse model by topical transdermal delivery of interleukin‐4 using ultradeformable cationic liposome. J Gene Med 12:481–490
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845
Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F (2010) Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1 alpha, and TNF-alpha recapitulates some features of psoriasis. J Immunol 184:5263–5270
Banno T, Gazel A, Blumenberg M (2004) Effects of tumor necrosis factor-α (TNFα) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem 279:32633–32642
Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E (2007) Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol 178:4615–4622
Mee JB, Johnson CM, Morar N, Burslem F, Groves RW (2007) The psoriatic transcriptome closely resembles that induced by interleukin-1 in cultured keratinocytes: dominance of innate immune responses in psoriasis. Am J Pathol 171:32–42
Nograles K, Zaba L, Guttman‐Yassky E, Fuentes‐Duculan J, Suárez‐Fariñas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson K, White T (2008) Th17 cytokines interleukin (IL)‐17 and IL‐22 modulate distinct inflammatory and keratinocyte‐response pathways. Brit J Dermatol 159:1092–1102
Schoop VM, Mirancea N, Fusenig NE (1999) Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol 112:343–353
Boniface K, Lecron JC, Bernard FX, Dagregorio G, Guillet G, Nau F, Morel F (2005) Keratinocytes as targets for interleukin-10-related cytokines: a putative role in the pathogenesis of psoriasis. Eur Cytokine Netw 16:309–319
Spinks E, Fenwick G (1990) The determination of glycyrrhizin in selected UK liquorice products. Food Addit Contam 7:769–778
Andres RM, Montesinos MC, Navalon P, Paya M, Terencio MC (2013) NF-kappa B and STAT3 inhibition as a therapeutic strategy in psoriasis: in vitro and in vivo effects of BTH. J Invest Dermatol 133:2362–2371
Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, Menichini F, Amdò S (2002) Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem 82:315–322
Chen G, Zhu L, Liu Y, Zhou Q, Chen H, Yang J (2009) Isoliquiritigenin, a flavonoid from licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. Phytother Res 23:498–506
Hsia SM, Shieh TM, Shih YH (2012) Effects of isoliquiritigenin (ISL) on VEGF secretion in human breast cancer cell line MDA-MB-231. Cancer Res 72:1991–1991
Wang KL, Hsia SM, Chan CJ, Chang FY, Huang CY, Bau DT, Wang PS (2013) Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin Ther Tar 17:337–349
Jung SK, Lee MH, Kim JE, Singh P, Lee SY, Jeong CH, Lim TG, Chen H, Chi YI, Kundu JK (2014) Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J Biol Chem 289:35839–35848
Di Cesare A, Di Meglio P, Nestle FO (2009) The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129:1339–1350
Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, Osemlak P, Paszkowski T, Roliński JM (2008) Cytokines and anticytokines in psoriasis. Clin Chim Acta 394:7–21
Perera GK, Di Meglio P, Nestle FO (2012) Psoriasis. Annu Rev Pathol-Mech Dis 7:385–422
Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF (2013) NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci 69:89–94
Acknowledgments
We thank Dr. Xiu Teng for the human keratinocyte cell line HaCaT and helpful suggestions in the vitro studies, Congcong Shen for excellent discussions, Xuewen Jin for real-time quantitative PCR analysis and expert suggestions, Weiyong Xu and Juan He for excellent technical assistance in HE staining, Fanghua Li for immunohistochemistry analysis, and Min Wu and John Yun-Chung Chen for the revision of the manuscript.
Conflict of interest
No potential conflicts of interest were disclosed.
Grant support
This study was supported by National Science and Technology Major Projects (No. 2012ZX09103-301-045).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yangping Wu and Xiangzheng Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
PDF 644 kb
Rights and permissions
About this article
Cite this article
Wu, Y., Chen, X., Ge, X. et al. Isoliquiritigenin prevents the progression of psoriasis-like symptoms by inhibiting NF-κB and proinflammatory cytokines. J Mol Med 94, 195–206 (2016). https://doi.org/10.1007/s00109-015-1338-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1338-3