Abstract
Interleukin-33 (IL-33), a cytokine belonging to the IL-1 family, is crucially involved in inflammatory pathologies including liver injury and linked to various modes of cell death. However, a link between IL-33 and necroptosis or programmed necrosis in liver pathology remains elusive. We aimed to investigate the regulation of IL-33 during necroptosis-associated liver injury. The possible regulation of IL-33 during liver injury by receptor-interacting protein kinase 1 (RIPK1) and poly(ADP-ribose) polymerase 1 (PARP-1) was investigated in mice in vivo and in hepatic stellate cells in vitro. The liver immunohistopathology, flow cytometry, serum transaminase measurement, ELISA, and qPCR-based cytokine measurement were carried out. By using a chemical approach, we showed that pretreatment of mice with Necrostatin-1 (Nec-1) (inhibitor of RIPK1) and/or PJ34 (inhibitor of PARP-1) significantly protected mice against concanavalin A (ConA) liver injury (aspartate amino-transferase (AST)/alanine amino-transferase (ALT)) associated with down-regulated hepatocyte-specific IL-33 expression. In contrast, the expression level of most systemic cytokines (except for IL-6) or activation of liver immune cells was not altered by chemical inhibitors rather an increased infiltration of neutrophils in the liver. During polyinosine-polycytidylic acid (Poly(I:C))-induced acute hepatitis, liver injury and hepatocyte-specific IL-33 expression was also inhibited by PJ34 without any protective effect of PJ34 in CCl4-induced liver injury. Moreover, PJ34 down-regulated the protein expression of IL-33 in activated hepatic stellate cells by cocktail of cytokines or staurosporine in vitro. In conclusion, we evidenced that the Nec-1/PJ34 is a potent inhibitor of liver injury and Nec-1/PJ34 down-regulated hepatocyte-specific IL-33 expression in the liver in vivo or in hepatic stellate cells in vitro, suggesting IL-33 as a possible readout of necroptosis-involved liver pathologies.
Key message
-
Necroptosis inhibitors can protect mice against liver injury induced by ConA or Poly(I:C).
-
IL-33 expression in liver injury in vivo is inhibited by PJ34.
-
IL-33 expression in hepatic stellate cells in vitro is inhibited by PJ34.
-
Hepatocyte-specific IL-33 expression is down-regulated by Nec-1/PJ34 during hepatitis.
-
IL-33 is a new marker of necroptosis-associated liver injuries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver injuries involve different mechanisms of cell death like apoptosis, necrosis, or necroptosis (also called as programmed necrosis) depending upon the death stimuli, cellular environment, and immune responses [1]. These cellular death mechanisms determine the fate of the liver. The caspase-independent programmed necrosis is a novel form of cell death that is dependent on receptor-interacting protein (RIP) kinases, RIPK1 and RIPK3 activities, upon engagement of several stimuli like the death receptors of tumor necrosis factor (TNF)-α superfamily [2–6]. In the liver, the hepatocytes undergo non-apoptotic cell death during partial hepatectomy [7] and in steatohepatic livers [8] with induction of RIPK3 expression in hepatocytes. The concanavalin A (ConA)-induced hepatic injury mimics immune cell-mediated fulminant hepatitis or autoimmune hepatitis in human [9, 10]. In mice, ConA induces activation of liver natural killer T (NKT) cells [1] and T cells that lead to hepatocyte death via tumor necrosis factor-related apoptosis inducing ligand (TRAIL), a TNF family member [11–13]. Most of the studies reported that ConA-induced hepatic cell death was rather necrotic [14] than apoptotic (independent of caspases) [15]. The ConA-induced hepatic cell death was shown to be associated with RIPK1 and RIPK3, and conditional deficiency of hepatocyte caspase-8 sensitized the mice to increased ConA liver injury associated with increased formation of necrosome [16]. A protective effect of Necrostatin-1 (Nec-1), inhibitor of RIPK1, has found in ConA-induced acute hepatic injury in mice [17]. Furthermore, the poly(ADP-ribose) polymerase 1 (PARP-1) is now known to be involved in a RIPK1-RIPK3-dependent cascade of TRAIL-induced necroptosis, and we have shown that ConA liver injury is a relevant TRAIL-induced necroptotic model [18]. The polyinosine-polycytidylic acid (Poly(I:C)) is a synthetic analog of viral double-stranded RNA (dsRNA) and mediates liver injury through Toll-like receptor 3 (TLR3) [19]. The liver injury induced by Poly(I:C) involves TNF receptor-associated factor 6 (TRAF6), receptor-interacting protein 1 (RIP1), and NF-κB activation [20]. The carbon tetrachloride (CCl4) induced acute liver toxicity in mice and was used as a model of liver fibrosis with cytochrome-lipid-peroxidation-dependent free radical injury [21, 22].
In the liver, the interleukin-33 (IL-33), a member of the IL-1 family, also proposed as an alarmin cytokine [23], is expressed by hepatocytes in ConA hepatitis [24], in Poly(I:C)-induced liver injury [25] and in liver ischemia/reperfusion injury [26]. Furthermore, NKT cells are responsible for the specific hepatocyte-IL-33 expression [24] and TRAIL is required to induce IL-33 in hepatocytes in this hepatic model [27]. At a functional level, IL-33 interacts via its specific receptor ST2 and common chain IL-1RAcP [28, 29] to mediate its cytokine activity. In murine ConA hepatitis, Volarevic et al. [30] have shown that administration of recombinant IL-33 induced protective effects by triggering anti-apoptotic effects, by decreasing pro-inflammatory cytokines, and by recruiting regulatory T cells (Tregs) in the liver.
IL-33 is proposed to be released in the functional/active form during necrotic cell death as an alarmin while it is inactivated during apoptotic cell death by caspases [31–35], and a link between IL-33 and necroptotic cell death remains elusive. In the present study, we investigated the expression of IL-33 and its chemical inhibition in ConA and Poly(I:C)-induced acute liver injury. We used chemical inhibition approach by selective inhibition of RIPK1 activity by Nec-1 and PARP-1 inhibition by PJ34 during ConA-induced, Poly(I:C)-induced, and CCl4-induced acute hepatitis. The consequence in activation and recruitment of liver immune cells and cytokine level during ConA hepatitis was investigated following Nec-1 and PJ34 treatment in mice. We showed that RIPK1/PARP-1 inhibition by Nec-1 + PJ34 protected mice against ConA liver injury. In conclusion, we evidenced that the pharmacological inhibition of liver injury by Nec-1/PJ34 down-regulated hepatocyte-specific IL-33 expression in the liver in vivo or in hepatic stellate cells in vitro.
Materials and methods
Animals and treatment protocol
The C57Bl/6 8–10-week-old wild-type (WT) mice (Janvier, Le Genest-sur-isle, France) were injected by intravenous (i.v.) route with ConA (Sigma-Aldrich) to induce acute hepatitis at a dose of 12 mg/kg body weight. Mice were sacrificed at 10 (for liver enzymes, messenger RNA (mRNA) and protein expression of IL-33), 12, and 24 h (for flow cytometry experiments) following ConA administration. The Poly(I:C) (Invivogen) was intravenously injected with a dose of 30 μg/mouse, and mice were sacrificed at 8 and 16 h of postinjection. The control mice received a similar volume of vehicle in each treatment group. For Necrostatin-1 (Nec-1) and PJ34 experiment, C57Bl/6 WT mice were pretreated with intraperitoneal (i.p.) administration of 10 mg/kg of PJ34 (Alexis Biochemicals, Enzo Life Sciences, Villeurbanne, FR) (1 h before) or i.v. administration of 125 μg/mouse of Nec-1 (Sigma-Aldrich, N9037) (15 min before), or with both inhibitors prior to ConA injection (12 mg/kg) or Poly(I:C) injection. C57Bl/6 mice were treated with PJ34 (i.p.) at 10 mg/kg body weight in PBS or PBS alone (10 μl/g body weight) 1 h before oral gavage of CCl4 in oil to induce acute hepatitis at a dose of 2.4 g/kg body weight. After 24 h of first injection, mice were treated with PJ34 and were sacrificed at 48 or 72 h following CCl4 injection.
All mice were bred in specific pathogen-free conditions at the local animal house facilities. The study was conducted in compliance with French laws and the institution’s guidelines for animal welfare. All efforts were made to minimize suffering and the number of animals involved. The protocol was approved by the “Comité Rennais d’Ethique en matière d’Expérimentation Animale” that is the county committee agreed by the Ministry of Research and Higher Education (protocol agreement number: R-2012-CPP-Ol, researcher agreement for M samson #35-96 and C. Piquet-Pellorce #35-82).
Histopathological, biochemical, and immunohistochemical analysis
The histopathological (hematoxylin and eosin (H&E) staining) and serum biochemical analyses, immunolocalization of IL-33, and the counting of IL-33-positive hepatocytes were performed as described earlier [24]. Briefly, serum aspartate amino-transferase (AST)/alanine amino-transferase (ALT) was measured according to the IFCC primary reference procedures using Olympus AU2700 Autoanalyzers (Olympus Optical, Tokyo, Japan). For histopathology, H&E staining of liver tissues was carried out to access the liver injury. Paraformaldehyde-fixed and paraffin-embedded mouse liver sections followed by antigen retrieval were incubated with primary antibody (goat IgG anti-murine IL-33, R&D Systems) in a Ventana CT/09/021 automated machine (Ventana Medical Systems, USA). Revelation of the primary antibody was carried out using HRP-conjugated rabbit polyclonal anti-goat (Dako, USA) secondary antibody followed by diaminobenzidine and hematoxylin coloration. The counting of IL-33-positive hepatocytes was carried in at least 20 different microscopic fields corresponding to 2.67-mm2 surface area using image analysis software (Simple PCI, Compix, Hamamatsu, Japan). The truly IL-33-positive hepatocytes were counted on the basis of diameter of cells and intensity of signals/degree of immunopositivity/staining (differential interference contrast) by Simple PCI software.
In vitro hepatic stellate cell stimulation and Western blot
Hepatic stellate cells (HSCs) of human were prepared by collagenase/pronase digestion of liver using a perfusion system and subsequent fractionation of the heterogeneous cell suspension on continuous density gradient with Percoll. The viability of HSC was >90 % as estimated by Trypan blue test. HSCs were grown in RPMI 1640 medium supplemented with 10 % fetal calf serum [36]. The HSCs were stimulated by a cocktail of cytokines (CCs) containing 1 ng/ml IL-1β, 10 ng/ml IL-6, 20 ng/ml TNF-α (R&D Systems, Minneapolis, MN, USA), and 10 ng/ml interferon (IFN)-γ (BioSource Europe, Nivelles, Belgium) or with staurosporine (STS, 10 and 25 nM) for 24 h. The effect of varying concentrations of PJ34 (10, 50, and 100 μM) was tested upon activated HSC. The protein was extracted from control and stimulated HSCs for Western blot analysis, and detection of IL-33 and β-actin proteins was carried out as previously described [22]. The ratio of IL-33/β-actin band intensity was measured by LAS-3000 imager analysis (Fujifilm Europe, Düsseldorf, Deutschland).
RNA isolation and RT-qPCR
Total RNA was extracted from mice livers using TRIzol (Invitrogen) reagent. First-strand complementary DNA (cDNA) was synthesized using the SuperScript™ II Reverse Transcriptase (Invitrogen). The cDNA or RT amplification was further verified by PCR amplification using the house-keeping gene, GAPDH. The protocol and conditions for RT-PCR and qPCR were same as reported earlier by our laboratory using specific primers for 18S, IL-33, IL-6, IL-1β, IFN-γ, TNF-α, and CXCL1 [24].
Cytokine measurement
The mouse Th1/Th2 10plex (IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17, TNF-α, IFN-γ, and GM-CSF) ready-to-use FlowCytomix sample kit (eBioscience) was used to measure cytokines in sera according to prescribed protocol of the supplier (eBioscience, France).
Isolation of liver immune cells and flow cytometry analysis
The liver immune cells were isolated as described earlier [37, 38] with a viability of >95 %. Cells were resuspended in staining buffer (PBS with 10 % FCS) and preincubated with anti-CD16/32 (BD Pharmingen) to block non-specific binding. The cells were then labeled with appropriate fluorochrome-conjugated antibodies/reagents (BD Pharmingen, MD Bioproducts, and eBioscience): anti-CD3-V500 (clone 500-A2), anti-CD4-PE-Cy7 (clone RM4-5), anti-CD8-APC-Cy7 (clone 53-6.7), anti-NK1.1-PerCP-Cy-5.5 (clone PK136), CD45-V500 (clone 30-F11), anti-CD11b-PE Cy™7 (clone M1/70), CD11c-APC (clone HL3), anti-Gr1-V450-Ly.6G/C (clone RB6-8C5), CD25-FITC (7D4), Foxp3-PE (clone MF23), TCRβ-V450 (clone H57-597), and anti-CD69-PE (clone H1.2F3). The stained cells were analyzed on FACSAria™ II flow cytometer using BD FACSDiva software (BD Bioscience), and data were analyzed by CXP software (Beckman Coulter). Dead cells and doublet cells were excluded on the basis of forward and side scatter.
Statistical analysis
The results are representative of three independent experiments with n = 5–7 animals in each experimental group, and data was expressed as means ± SEM. Mann-Whitney U test was used for comparison of control group parameters with treatment group, and multiple group analysis was carried out by one-way ANOVA. The correlation between continuous variables was analyzed by using GraphPad Prism 5 software. For all statistical analyses, *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Pretreatment of Nec-1 and PJ34 protected mice against ConA hepatitis and inhibited IL-33 expression in liver
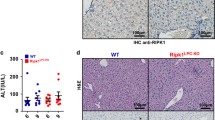
To evaluate the effect of Nec-1 (inhibitor of RIPK1 kinase activity) and PJ34 (inhibitor of PARP-1) to inhibit hepatocyte cell death in vivo and IL-33 as a possible readout, we investigated the effect of Nec-1 + PJ34 pretreatment in ConA-induced liver injury. The Nec-1 or PJ34 alone or in combination did not induce any hepatic injury (as evaluated by serum transaminase levels) (Fig. 1a, S1). Nec-1 + PJ34 pretreatment effectively protected mice against ConA liver injury with a significant decrease in serum AST and ALT levels at 10 h (Fig. 1a, S1) compared to the ConA-alone group. The combined inhibition of liver injury by Nec-1 + PJ34 was more significant (Fig. 1a, S1). The hepatocytes specifically expressed IL-33 in ConA hepatitis as we demonstrated previously [24, 27]; however, the pretreatment of Nec-1 + PJ34 down-regulated liver mRNA and hepatocyte-specific expression of IL-33 in ConA-treated mice at 10 h (Fig. 1b, c). These results suggested that necroptotic inhibitors can block the mRNA and protein IL-33 expression. The hepatocyte-specific IL-33 expression was not evident in Nec-1/PJ34 control mice livers (Fig. 1c). The number of IL-33-expressing hepatocytes was significantly down-regulated by Nec-1, PJ34, and combination of Nec-1 + PJ34 in comparison to ConA-treated mice (Fig. 1d). These results demonstrate that the ConA hepatic injury is a relevant necroptotic model and hepatocyte-specific IL-33 appears as a marker during ConA hepatic injury.
Liver injury and IL-33 expression in WT mice treated with Nec-1, PJ34, and/or ConA. a Level of serum ALT (IU/L) in mice treated with Nec-1, PJ34, and/or ConA. b Relative mRNA expression of IL-33 in the livers of mice treated with Nec-1, PJ34, and/or ConA 10 h postinjection. The PBS-treated mice served as a reference for mRNA expression. c Immunolocalization of IL-33 was performed using primary antibody goat IgG anti-mouse-IL-33 and secondary HRP-conjugated rabbit anti-goat antibody with hematoxylin counterstaining in sections of mice livers following Nec-1, PJ34, and/or ConA treatment. d Comparison of number of IL-33-expressing hepatocytes in mice after Nec-1, PJ34, and/or ConA treatment. Scale bar was 200 or 50 μm
Nec-1 + PJ34 administration selectively inhibited pro-inflammatory cytokines during ConA liver injury
While the ConA hepatic injury is known to be associated with the increased influx of pro-inflammatory cytokines produced by immune cells [9, 12], we wondered if the inhibition of injury by Nec-1 + PJ34 affected the cytokine expression. We found that the combined pretreatment of Nec-1 + PJ34 selectively inhibited only the mRNA expression of IL-6 (Fig. 2a), while the sustained pro-inflammatory cytokine/chemokine levels of IL-1β, TNF-α, IFN-γ, chemokine (C-X-C motif ligand 1), and CXCL1/KC were not inhibited significantly by Nec-1 + PJ34 treatment (Fig. 2a). In parallel, the circulating cytokine level of IL-6 was significantly decreased by Nec-1 + PJ34 pretreatment in ConA-induced hepatitis in mice but not IFN-γ (Fig. 2b). These findings suggest that IL-33 and IL-6 shared same profile while other measured cytokine expression was not altered by Nec-1 + PJ34 during immune cell-mediated hepatitis.
Cytokine expression of IL-1β, IL-6, TNF-α, IFN-γ, and CXCL1 in WT mice treated with Nec-1, PJ34, and/or ConA. a Relative fold change in mRNA expression of IL-1β, IL-6, TNF-α, IFN-γ, and CXCL1 in the liver of mice treated with Nec-1, PJ34, and/or ConA 10 h postinjection. The PBS-treated mice served as a reference for mRNA expression. b Measurement of serum (pg/ml) IFN-γ and IL-6 by ELISA in WT mice treated with vehicle control or ConA 10 h postinjection
The activation and number of immune cells in the liver were not inhibited by Nec-1 + PJ34 pretreatment during ConA liver injury
To delineate the impact of Nec-1 + PJ34 on liver immune cell activation and their number following ConA administration (at 12 and 24 h), we characterized the immune cell profile in the liver by flow cytometry (Fig. 3a). We first demonstrated that Nec-1 + PJ34 significantly inhibited ConA liver injury (AST/ALT levels) after 12 or 24 h of ConA injection (Fig. S2). The immune cells in liver such as T cells (CD3+), NK cells (CD3−NK1.1+), NKT cells (CD3+NK1.1+), and B lymphocytes (CD19+) remained highly activated after 12 or 24 h of ConA administration as evident from an early marker of activation (CD69+) which was not significantly inhibited by Nec-1 + PJ34 pretreatment (Fig. 3b). Hence, the Nec-1 + PJ34 did not directly affect immune cell activation.
The number of immune cells in the liver of WT mice following Nec-1 + PJ34 and ConA treatment. a Flow cytometry analysis of number of NK cells, NKT cells, T cells, B cells, macrophages and neutrophils in the liver of mice following Nec-1 + PJ34 and ConA treatment. b Representation of the activation marker (CD69+) of T, NK, NKT, and B cells in the liver of mice treated with Nec-1+ PJ34 and ConA (12 and 24 h) or vehicle control. c The number of NK cells, NKT cells, T cells, B cells, neutrophils, and macrophages in the liver of mice following Nec-1 + PJ34 and ConA treatment
The number of intrahepatic NK cells, B lymphocytes, and macrophages (CD11bhiGr-1int), but not of NKT cells or T cells, was increased in ConA-alone and Nec-1 + PJ34-pretreated mice without any significant difference between these groups (Fig. 3c). The neutrophil (CD11b+CD11c−Gr-1+ cells or CD11bhiGr-1hi cells) count in the liver increased significantly with a difference between Nec-1 + PJ34-pretreated and ConA-treated mice at 12 h but not at 24 h of postadministration (Fig. 3c). Thus, the inhibition of hepatocyte cell death by Nec-1 + PJ34 did not impact the activation or the number of infiltrate cells during ConA hepatitis, except neutrophils that were significantly increased.
PJ34 prevented onset of Poly(I:C)-induced liver injury and IL-33 expression in the liver but not of CCl4-induced liver injury
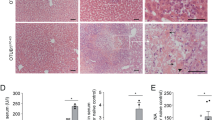
To look whether IL-33 inhibition by PJ34 was not restricted to ConA hepatitis model only, we investigated the protective effect of PJ34 in Poly(I:C)-induced liver injury, a dsRNA viral mimetic hepatic model in mice. The PJ34 more significantly inhibited the ConA liver injury than Nec-1; therefore, in Poly(I:C)-mediated liver injury, we only tested effect of PJ34. A comparative decrease in ALT/AST level was observed following 8 h of Poly(I:C) + PJ34 administration, an acute onset of liver injury; however, this protective effect was not evident following 16 h of PJ34 injection (Fig. 4a, S3). These findings were in accordance with liver histology, which showed very mild or no liver injury in Poly(I:C) + PJ34 compared with Poly(I:C)-alone treatment at 8 h (Fig. 4b). At 16 h of Poly(I:C) administration, the liver histology did not reveal any liver injury (Fig. S3). The hepatocyte-specific IL-33 expression was induced at 8 h of Poly(I:C) administration but not in PBS control mice or at 16 h of Poly(I:C) administration (Fig. S3). Interestingly, the IL-33 expression in hepatocytes was greatly decreased in Poly(I:C) + PJ34-treated mice (Fig. 4b). The hepatocyte-specific IL-33 count was significantly decreased by PJ34 treatment in Poly(I:C)-treated mice compared to Poly(I:C)-positive control mice (Fig. 4c) suggesting an inhibitory effect of PJ34 on IL-33 regulation in the liver. In contrast, PJ34 did not significantly inhibited CCl4-induced hepatotoxicity/liver injury (ALT, AST level) at 24, 48, and 72 h (Fig. S4a). Non-significant difference was observed in PBS vs PJ34-CCl4-treated mice in terms of ratio of liver weight over total body weight or in percentage of body weight (Fig. S4b, c).
Liver injury and IL-33 expression in WT mice following Poly(I:C) and PJ34 treatment. a Levels of serum ALT (IU/L) in WT mice following PBS, Poly(I:C) (30 μg/mouse i.v.), and Poly(I:C) + PJ34 treatment at 8 and 16 h of postinjection. b Liver histology (H&E) and immunostaining of IL-33 in the livers of WT mice treated with PBS, Poly(I:C), and/or Poly(I:C) + PJ34 at 8 h. c Comparison of number of IL-33-expressing hepatocytes in WT mice following PBS, Poly(I:C), and/or Poly(I:C) + PJ34 treatment at 8 h of postinjection
IL-33 expression was down-regulated by PJ34 in stimulated human HSCs in vitro
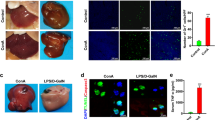
Our previous data evidenced that the stimulated human HSCs were a potent IL-33 induction model in vitro [22]; therefore, we tested the effect of PJ34 on a cocktail of cytokines (CCs)- or staurosporine (STS)-stimulated HSCs at different doses. The CC significantly augmented the protein expression of IL-33 (Fig. 5a), as expected, which was inhibited by PJ34 in a dose-dependent manner. A complete inhibition of IL-33 protein expression was observed at 100-μM dose of PJ34 in vitro. In parallel, the STS induced an increase in protein expression of IL-33 in stimulated HSC (Fig. 5b) and the IL-33 expression was down-regulated by PJ34 treatment. These data showed that the chemical inhibitor PJ34 significantly blocked the expression of IL-33 in vitro.
Western blot analysis of IL-33 in stimulated human hepatic stellate cells in vitro. a The HSCs were stimulated with vehicle control; CCs alone; and/or CCs with 10, 50, and 100-μM concentrations of PJ34. IL-33 and β-actin protein expression was detected in Western blot, and ratio of IL-33/β-actin band intensity was measured by LAS-3000 imager analysis (Fujifilm Europe, Düsseldorf, Deutschland). b The HSCs were stimulated with vehicle control; staurosporine (STS) 10 and 25 nM; and STS with different concentrations of PJ34 like 10, 50, and 100 μM. IL-33 and β-actin protein expression was detected in Western blot, and ratio of IL-33/β-actin was quantified by LAS-3000 imager analysis software
Discussion
The RIPK1/3-mediated programmed necrosis or necroptosis has been involved in various diseases and inflammatory conditions like viral infection [3, 39], systemic inflammatory response syndrome (SIRS) and sepsis [40], acute pancreatitis [4], ischemia-reperfusion injury [41], and intestinal inflammation [42, 43]. Interestingly, the potent inhibition of RIPK1 by chemical inhibitor Nec-1 provides an excellent tool to decipher the implication of necroptosis pathway in different pathologies and cell death models in vitro. We previously evidenced that Nec-1 and PJ34 inhibitors can block RIPK1 and PARP-1 expression during ConA-associated necroptotic liver injury in mice [18]. In another study, it was confirmed that Nec-1 inhibits ConA-induced hepatitis [17] and a protective effect of Nec-1 in acetaminophen (APAP)-induced liver injury in mice was found via inhibition of ROS production [44–46]. The mechanisms of ConA-induced liver injury were firstly known to be caspase-dependent apoptosis [47, 48] and caspase-independent necrosis [14–16], and we showed ConA-mediated liver injury as a relevant necroptotic model [18]. We further evidenced the regulation of IL-33 by TRAIL (an initiator molecule of necroptosis) in ConA-induced acute liver injury [27], and the present study demonstrated the inhibitory effect of Nec-1/PJ34 on the regulation of IL-33 during liver injury in vivo (ConA and Poly(I:C) hepatic models) or in human HSCs in vitro.
This study showed that inhibition of RIPK1 kinase activity and PARP-1 activity by Nec-1 and PJ34 protected mice against ConA hepatitis. The Nec-1 and PJ34 pretreament synergistically inhibited early markers of liver injury (serum AST and ALT) in ConA hepatitis. We recently showed the induction of hepatocyte-specific IL-33 expression during Poly(I:C)-induced acute hepatitis [25], and here, we evidenced that PJ34 inhibited serum AST/ALT and hepatocyte-specific IL-33 expression during Poly(I:C)-induced liver injury. Thus, the activation of TLR3 by viral dsRNA mimetic could lead to necroptotic cell death [49] and IL-33 was induced in the liver in this hepatic model which was blocked by PJ34. The ConA and Poly(I:C) hepatic models have mimicry with human hepatitis (fulminant or immune cell dependent) indicating the plausible therapeutic effect of PJ34 and Nec-1 in acute hepatitis. PJ34 did not significantly inhibit CCl4-induced liver injury (ALT, AST level) that may explain different modes of liver cell death (cytochrome activation dependent) in CCl4 hepatic model than ConA or Poly(I:C), i.e., immune cell dependent. Further, we have already shown that hepatocyte-specific IL-33 expression was not evident during CCl4-induced liver injury [24]. The human fibroblasts/HSC strongly induced the expression of IL-33 following stimulation with CCs in vitro as we reported previously [22], and here, we demonstrated that PJ34 can down-regulate the expression of IL-33 in stimulated HSC by CC or staurosporine.
A storm of pro-inflammatory cytokines and activation/infiltration of immune cells in the liver are associated with ConA-induced fulminant hepatitis [9, 12, 50, 51]. Accordingly, we found that even in the absence of liver injury (Nec-1 + PJ34-protected mice), the expression of TNF-α, IFN-γ, IL-1β, and CXCL1 as well as the activation of intrahepatic immune cells (CD69+) following ConA hepatitis remained sustained which may explain a sterile inflammatory response in the liver. In other words, the hepatitis phenomenon can be bifurcated as inflammation of the liver and hepatolysis. In the liver, there is an early wave (1–4 h) of pro-inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) following ConA administration and then a second wave (late phase) of cytokines like IL-1β and IL-6 resulting from release of damage-associated molecular patterns (DAMPs) [52, 53]. Among the late-phase cytokines, the IL-6 was known to be involved in repairment of the liver following liver injury. In the liver, the cytokines IL-1β and TNF-α are secreted by activated Kupffer cells and IFN-γ by NKT and T lymphocytes; however, IL-6 is massively produced by Kupffer cells, hepatic fibroblasts, and endothelial cells and is involved in hepatocyte proliferation and tissue repair in the liver [54]. As the PJ34 inhibits the liver injury, the decreased level of IL-6 may be due to inhibition of liver injury in our study. The Nec-1/PJ34 slightly but non-significantly reduced IFN-γ level (mRNA and protein) without inhibition of TNF-α during ConA hepatitis. As these cytokines (TNF-α, IFN-γ) are majorly produced by macrophages and NK/T cells, Nec-1-PJ34 did not inhibit activation of these cells; therefore, it seems that the necroptotic inhibitors specifically inhibit TRAIL-dependent hepatocyte cell death without affecting immune cells.
In consequence to liver injury, the secretion of intracellular contents or endogenous danger signals (DAMPs) including DNA or microRNAs from dying cells (e.g., hepatocytes) can cause inflammation and auto-immunity, which can be used as surrogate markers of liver injury during viral, drug, alcohol, and TLR-dependent hepatitis [55–57]. We showed that in ConA liver injury, the endogenous alarmins IL-33 and HMGB1 were highly expressed in the liver and particularly in the hepatocytes [23]; thus, in consequence to hepatocyte death, these alarmins can signal to immune system. Indeed, the blockage of RIPK1 kinase activity did not affect the induction of pro-inflammatory cytokines [58, 59]; this seemed true in our model of liver injury except for IL-6.
The numbers of intrahepatic T cells, NK cells, NKT cells, B lymphocytes, and macrophages were not affected by Nec-1/PJ34 treatment, suggesting that the cell death inhibition by Nec-1 + PJ34 did not affect the immune cell counts during ConA hepatitis. The number of liver NKT cells dramatically decreased in ConA- or Nec-1/PJ34-treated mice that represented ConA-mediated NKT cell activation and subsequent death (activation-induced cell death (AICD)) as described earlier [9, 60]. However, the increase in the number of neutrophils at 12 h of Nec-1 + PJ34 treatment could be explained by the fact that Nec-1 + PJ34 that inhibited liver injury may relatively prolong the normal half-life (6–8 h) of neutrophils [61], in association with increased expression of CXCL1 and MIP-2 (neutrophil-specific recruitment chemokines) in the liver. Hence, the up-regulated cytokine and chemokine expression and the activation or infiltration of immune cells (NK, NKT, T, and B cells and macrophages) appear in upstream of hepatocyte necroptotic cell death in consequence to ConA stimulation.
In summary, the chemical inhibitors Nec-1 or PJ34 inhibit liver injury/inflammation and can down-regulate the expression of IL-33 in hepatocytes or HSC, suggesting Nec-1/PJ34 as a protector of liver injury and IL-33 as a readout in necroptosis-involved liver injuries.
Abbreviations
- AST:
-
Aspartate amino-transferase
- ALT:
-
Alanine amino-transferase
- ConA:
-
Concanavalin A
- IL-33:
-
Interleukin 33
- IL-1RAcP:
-
Interleukin-1 receptor accessory protein
- i.v.:
-
Intravenous
- i.p.:
-
Intraperitoneal
- Nec-1:
-
Necrostatin-1
- PARP-1:
-
Poly(ADP-ribose) polymerase 1
- P.I.:
-
Postinjection
- RIPK:
-
Receptor-interacting protein kinase
- Poly(I:C):
-
Polyinosine-polycytidylic acid
References
Malhi H, Guicciardi ME, Gores GJ (2010) Hepatocyte death: a clear and present danger. Physiol Rev 90:1165–1194
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science (New York, NY) 325:332–336
Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M (2011) TNF-alpha induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. The Journal of membrane biology 239:115–122
Zorde-Khvalevsky E, Abramovitch R, Barash H, Spivak-Pohis I, Rivkin L, Rachmilewitz J et al (2009) Toll-like receptor 3 signaling attenuates liver regeneration. Hepatology 50:198–206
Csak T, Dolganiuc A, Kodys K, Nath B, Petrasek J, Bala S et al (2011) Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology 53:1917–1931
Tiegs G, Hentschel J, Wendel A (1992) A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90:196–203
Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K (2000) Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A 97:5498–5503
Zheng SJ, Wang P, Tsabary G, Chen YH (2004) Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest 113:58–64
Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K et al (2008) Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A 105:10895–10900
Beraza N, Malato Y, Sander LE, Al-Masaoudi M, Freimuth J, Riethmacher D et al (2009) Hepatocyte-specific NEMO deletion promotes NK/NKT cell- and TRAIL-dependent liver damage. J Exp Med 206:1727–1737
Ni HM, Chen X, Ding WX, Schuchmann M, Yin XM (2008) Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice. Am J Pathol 173:962–972
Kunstle G, Hentze H, Germann PG, Tiegs G, Meergans T, Wendel A (1999) Concanavalin A hepatotoxicity in mice: tumor necrosis factor-mediated organ failure independent of caspase-3-like protease activation. Hepatology 30:1241–1251
Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ et al (2011) Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 141:2176–2187
Zhou Y, Dai W, Lin C, Wang F, He L, Shen M et al (2013) Protective effects of necrostatin-1 against concanavalin A-induced acute hepatic injury in mice. Mediat Inflamm 2013:706156
Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F et al (2012) TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ 19:2003–2014
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Kawai T, Akira S (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143:1–20
Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33:105–136
Marvie P, Lisbonne M, L’Helgoualc’h A, Rauch M, Turlin B, Preisser L et al (2010) Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med 14:1726–1739
Arshad MI, Piquet-Pellorce C, Samson M (2012) IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int 32:1200–1210
Arshad MI, Rauch M, L’Helgoualc’h A, Julia V, Leite-de-Moraes MC, Lucas-Clerc C et al (2011) NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur J Immunol 41:2341–2348
Arshad MI, Patrat-Delon S, Piquet-Pellorce C, L’Helgoualc’h A, Rauch M, Genet V et al (2013) Pathogenic mouse hepatitis virus or poly(I:C) induce IL-33 in hepatocytes in murine models of hepatitis. PLoS One 8:e74278
Sakai N, Van Sweringen HL, Quillin RC, Schuster R, Blanchard J, Burns JM et al (2012) Interleukin-33 is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology 56:1468–1478
Arshad MI, Piquet-Pellorce C, L’Helgoualc’h A, Rauch M, Patrat-Delon S, Ezan F et al (2012) Tumor necrosis factor related apoptosis inducing ligand (TRAIL), but not FasL and tumor necrosis factor alpha (TNFa), regulates interleukin (IL)-33 expression in murine hepatocytes during acute hepatitis. Hepatology 56:2353–2362
Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L et al (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 104:282–287
Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU (2007) IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci U S A 104:18660–18665
Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S et al (2012) Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol 56:26–33
Lamkanfi M, Dixit VM (2009) IL-33 raises alarm. Immunity 31:5–7
Cayrol C, Girard JP (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106:9021–9026
Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C et al (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31:84–98
Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G (2009) Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 284:19420–19426
Ali S, Nguyen DQ, Falk W, Martin MU (2010) Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun 391:1512–1516
Bourd-Boittin K, Basset L, Bonnier D, L’Helgoualc’h A, Samson M, Theret N (2009) CX3CL1/fractalkine shedding by human hepatic stellate cells: contribution to chronic inflammation in the liver. J Cell Mol Med 13:1526–1535
Lisbonne M, L’Helgoualc’h A, Nauwelaers G, Turlin B, Lucas C, Herbelin A et al (2011) Invariant natural killer T-cell-deficient mice display increased CCl(4)-induced hepatitis associated with CXCL1 over-expression and neutrophil infiltration. Eur J Immunol 41:1720–1732
Diana J, Beaudoin L, Gautron AS, Lehuen A (2011) NKT and tolerance. Methods Mol Biol 677:193–206
Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P et al (2011) Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A 108:15312–15317
Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T et al (2011) RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35:908–918
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M et al (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477:330–334
Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H et al (2011) Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477:335–339
An J, Mehrhof F, Harms C, Lattig-Tunnemann G, Lee SL, Endres M et al (2013) ARC is a novel therapeutic approach against acetaminophen-induced hepatocellular necrosis. J Hepatol 58:297–305
Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S et al (2014) Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS open bio 4:777–787
Zhang YF, He W, Zhang C, Liu XJ, Lu Y, Wang H et al (2014) Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett 225:445–453
Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K et al (2006) The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 124:601–613
Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S et al (2009) Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30:56–66
Han J, Zhong CQ, Zhang DW (2011) Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol 12:1143–1149
Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G (1995) Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21:190–198
Kusters S, Gantner F, Kunstle G, Tiegs G (1996) Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology 111:462–471
Galluzzi L, Kroemer G (2011) Necroptosis turns TNF lethal. Immunity 35:849–851
Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL et al (2012) Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol 18:119–125
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V et al (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science (New York, NY) 274:1379–1383
Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z et al (2009) Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A 106:4402–4407
Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H et al (2010) Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 56:1830–1838
Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J et al (2012) Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. doi:10.1002/hep.25873
Lee TH, Shank J, Cusson N, Kelliher MA (2004) The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem 279:33185–33191
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G (1995) Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol 22:226–229
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER (2010) Neutrophil kinetics in health and disease. Trends Immunol 31:318–324
Acknowledgments
This work was supported by INSERM, the Ministère de l’Education Nationale de la Recherche et de la Technologie, the University of Rennes 1, the Région Bretagne, the “Ligue contre le cancer, comités du grand Ouest”. Muhammad Imran Arshad was supported by a PhD fellowship from the Government of Pakistan and serving as an assistant professor under tenure track system (Higher Education Commission, University of Agriculture, Faisalabad). Sandrine Jouan-Lanhouet was supported by the Association pour la Recherche sur le Cancer (doctoral fellowship). For immunohistochemistry, cytometry analysis, and animal house facilities, we would like to thank the dedicated platforms (i.e., H2P2 (Pascale Bellaud and Roselyne Viel), cytometry platform (Dr. Gersende Lacombe), and animal house platforms (Laurence Touami) of SFR BIOSIT, University of Rennes 1, France.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 539 kb)
Rights and permissions
About this article
Cite this article
Arshad, M.I., Piquet-Pellorce, C., Filliol, A. et al. The chemical inhibitors of cellular death, PJ34 and Necrostatin-1, down-regulate IL-33 expression in liver. J Mol Med 93, 867–878 (2015). https://doi.org/10.1007/s00109-015-1270-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1270-6