Abstract
We have investigated genetic/pathogenetic factors associated with a new clinical entity in patients presenting with pheochromocytoma/paraganglioma (PHEO/PGL) and polycythemia. Two patients without hypoxia-inducible factor 2α (HIF2A) mutations, who presented with similar clinical manifestations, were analyzed for other gene mutations, including prolyl hydroxylase (PHD) mutations. We have found for the first time a germ-line mutation in PHD1 in one patient and a novel germ-line PHD2 mutation in a second patient. Both mutants exhibited reduced protein stability with substantial quantitative protein loss and thus compromised catalytic activities. Due to the unique association of patients’ polycythemia with borderline or mildly elevated erythropoietin (EPO) levels, we also performed an in vitro sensitivity assay of erythroid progenitors to EPO and for EPO receptor (EPOR) expression. The results show inappropriate hypersensitivity of erythroid progenitors to EPO in these patients, indicating increased EPOR expression/activity. In addition, the present study indicates that HIF dysregulation due to PHD mutations plays an important role in the pathogenesis of these tumors and associated polycythemia. The PHD1 mutation appears to be a new member contributing to the genetic landscape of this novel clinical entity. Our results support the existence of a specific PHD1- and PHD2-associated PHEO/PGL-polycythemia disorder.
Key message
• A novel germ-line PHD1 mutation causing pheochromocytoma/paraganglioma and polycythemia.
• Increased EPOR activity and inappropriate hypersensitivity of erythroid progenitors to EPO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia-inducible factors (HIFs) function as central players in the cell response to hypoxia [1]. HIF-α protein degradation is initiated through its hydroxylation by prolyl hydroxylase domain proteins (PHDs), of which there are three main isoforms: PHD1, PHD2, and PHD3 (encoded by EGLN2, EGLN1, and EGLN3, respectively) [2, 3]. In normoxia, PHDs use O2 and α-ketoglutarate to hydroxylate HIF prolyl residues that are subsequently recognized by the von Hippel-Lindau (VHL) protein, a component of the E3 ubiquitin ligase complex, which modifies HIFs for their degradation in proteasomes [4]. Abnormal regulation of the HIF pathway (e.g., during hypoxia) is tightly linked to the induction of many downstream genes and promotes cell transformation and tumorigenesis [1, 4].
Genes regulated specifically by HIF-1α or HIF-2α have been reported; one well-studied example is erythropoietin (EPO), which was recently discovered to be mainly regulated by HIF-2α [5]. Elevated EPO levels cause secondary polycythemia, a disease where the absolute red cell mass is increased [6]. Secondary polycythemia is caused by genetic defects in the oxygen-sensing pathway, impaired oxygen delivery, local tissue hypoxia, or it can be tumor- and drug-induced [7]. Mutations in PHD2, VHL, and HIF2A have been implicated in the pathogenesis of polycythemia in humans [8–10]. This is in contrast to primary polycythemia where intrinsic abnormalities of hematopoeietic progenitors or their EPO receptors (EPORs) result in the constitutive overproduction of red cell mass without EPO elevations [11, 12]. Among genetically induced polycythemias, PHD-related polycythemias typically present with normal or slightly increased EPO levels [13].
Paragangliomas (PGLs) are neural crest tumors derived either from the adrenal medulla (called pheochromocytomas (PHEOs)) or extra-adrenal chromaffin cells [14]. These tumors produce catecholamines, often with serious consequences, including hypertension, stroke, or arrhythmia. Ladroue et al. first described a germ-line PHD2 mutation in a patient presenting with recurrent abdominal PGLs and polycythemia [15]. VHL and somatic HIF2A mutations were later reported in patients with an early onset of polycythemia induced by high EPO followed by the development of PHEOs/PGLs, often recurrent and multiple [16, 17]. The presence of a germ-line HIF2A mutation in a patient with PGL and polycythemia was described by Lorenzo et al. [18].
However, some patients with similar clinical phenotypes have not been found to be associated with the mutations described above, even though up-regulation of HIF-1α or HIF-2α is present in their PHEOs/PGLs. Given their clinical presentation of early-onset polycythemia with slightly elevated EPO levels followed by the development of PHEO/PGL, we decided to screen for mutations in other HIF regulators, including PHDs, in these patients.
In this study, we have found for the first time a PHD1 (EGLN2) germ-line mutation in one patient and a PHD2 (EGLN1) germ-line mutation in another patient, who would be the second patient reported to date with a PHD2 mutation. Both patients shared similar clinical phenotypes. Patients with PHD1 and PHD2 mutations associated with polycythemia appear to have borderline or mildly elevated EPO levels, in contrast to HIF2A mutation patients who have markedly elevated EPO levels. PHD1 and PHD2 mutations caused loss of protein function, triggering activation of HIF-1α and HIF-2α and their downstream-regulated genes (EDN1, EPO, GLUT1, GNA14, LDHA, POU5F1, SOX2, and VEGFA) in the tumors. Increased EPOR activity and inappropriate hypersensitivity of erythroid progenitors to erythropoietin resulted in polycythemia with borderline or mildly elevated EPO in these patients. Our study provides important evidence indicating the existence of a specific association of PHD1 and PHD2 mutations with PHEO/PGL and polycythemia and may help explain the molecular pathogenetic mechanism of this disease.
Case series
Patient 1
A 48-year-old female referred herself to NIH for evaluation of recurrent PHEOs/PGLs and polycythemia with borderline or mildly elevated EPO levels (Table 1). The patient was diagnosed with polycythemia at age 6, followed by phlebotomies at age 10 (Table 1). At age 14, she presented with a left adrenal PHEO and later with recurrent PHEOs along with a thoracic periaortic lymph node positive for metastatic PHEO/PGL. After each operation, her polycythemia improved but did not normalize; usually after 2–4 months, it worsened and the patient again required phlebotomies. At age 48, she was found to have 2 lesions in the right adrenal gland, 1 lesion in the aortocaval region, and 1 in the right pelvic area suggestive of multiple and metastatic PHEOs/PGLs. Furthermore, a urinary bladder tumor was detected, surgically removed, and confirmed as PGL.
Patient 2
A 60-year-old female was referred to NIH for evaluation of multiple PGLs and a right adrenal PHEO (Table 1). The diagnosis of polycythemia was made at age 16, and the patient was treated with phlebotomies from then on. Her EPO levels were found to be only borderline or mildly elevated. At age 39, she was found to have a left adrenal PHEO and 2 PGLs near the left renal artery. After resection of the tumors, her polycythemia significantly improved, but worsened after 6 months. At NIH, the patient was found to have multiple tumors; surgical resection was performed in 2013 and histopathology confirmed the presence of a right adrenal PHEO and 3 periaortic PGLs.
No relative of either patient was found to have any history of polycythemia or PHEO/PGL (Table 1). The parents of patient 2 are deceased and were therefore not evaluated.
Materials and methods
This study was approved by the IRB of the Eunice Kennedy Shriver NICHD/NIH, and all patients gave written informed consent.
DNA sequencing
Genomic DNA was extracted from tumors and white blood cells. Exon sequencing was performed to screen for mutations in VHL, HIF1A, HIF2A, JAK2, PHD1, PHD2, and PHD3 using polymerase chain reaction (PCR). DNA sequences of each exon were determined by Sanger DNA sequencing.
Loss of heterozygosity analysis
Loss of heterozygosity (LOH) was determined through microsatellite analysis of the PHD1 and PHD2 gene loci; the primer pairs for the microsatellite markers are summarized in Supplementary Table S1.
Real-time PCR
Total RNA was extracted from tumor specimens. Messenger RNA (mRNA) expression of hypoxia-related genes was measured using quantitative real-time PCR (qPCR). The primers used in present study include EDN1 (QT00088235), EPO (QT00001484), GLUT1 (QT00068957), GNA14 (QT00099379), LDHA (QT00001687), POU5F1 (QT00210840), SOX2 (QT00237601), VEGFA (QT01010184), and ACTB (QT00095431).
PHD1 and PHD2 plasmid and mutagenesis
PHD1 and PHD2 mutations were introduced into the pCMV6-PHD1/2 vector (Origene) using the Quikchange Lightning Site-Directed Mutagenesis Kit (Agilent). DNA sequences of the plasmids were verified by DNA sequencing.
Luciferase assay
A luciferase assay was performed as previously described [19]. A hypoxia-responsive element (HRE)-driven luciferase gene was introduced into Hep3B cells through lentivirus infection and puromycin selection. Zero to 25 ng of PHD1 or PHD2 expression vectors were co-transfected with a HIF2A expression vector (50 ng) into the Hep3B HRE-Luc cell line using Lipofectamine 2000 (Life Technologies, Invitrogen). A pcDNA3 vector was used as a control plasmid. The HRE-associated transcriptional activity was determined after 36 h using the ONE-Glo luciferase assay system (Promega).
Cycloheximide pulse-chase assay
HeLa cells were transfected with recombinant vectors encoding PHD1 or PHD2 mutants using Lipofectamine 2000 transfection reagent (Invitrogen). After 48 h, the cells were treated with cycloheximide (50 μM). Cells were collected after 0, 3, 6, 12, or 24 h. The quantity of protein residue was determined by Western blot.
Immunohistochemistry
Five-micrometer tissue sections were probed with anti-HIF-1α (Sigma-Aldrich), anti-HIF-2α (Novus Biologicals), anti-EPOr (Santa Cruz), anti-phosphorylated EPOR, anti-PHD1, and anti-PHD2 (Abcam) antibodies and counterstained with hematoxylin. The intensity of the staining was scored as follows: 0 = negative, 1+ = weak, 2+ = intermediate, 3+ = strong; original magnification, ×200. Immunohistochemistry (IHC) data were reviewed in a double-blinded manner.
Microarray processing and analysis
PHEO/PGL tissue and normal adrenal medulla were collected for the study. One sample from the PHD2 patient and six from two HIF2A patients were used for microarray analysis, as previously described [20, 21].
In vitro assay of erythroid progenitor’s sensitivity to EPO
An in vitro sensitivity assay of erythroid progenitors to EPO was performed, as previously described [22]. Mononuclear cells isolated from the peripheral blood were plated in methylcellulose media (MethoCult, StemCell Technologies) supplemented with various concentrations of EPO (0.015 to 3.0 U/ml; StemCells Technologies). Cell cultures were maintained in a humidified atmosphere of 5 % CO2 at 37 °C for 14 days. Erythroid burst-forming unit colonies (BFU-Es) were scored by standard morphological criteria.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test. Results were expressed as mean ± SEM with p values <0.05 considered significant.
Results
Mutation and allelic deletion analyses of PHD1 and PHD2
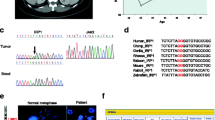
In both blood and tumor specimens, we identified a novel heterozygous PHD1 mutation (c.188T>A, p.Ser61Arg (S61R)) in one patient and PHD2 mutation (c.682G>T, p.Ala228Ser (A228S)) in another patient [Fig. 1a, Supplementary Figure S1]. LOH analysis was performed with two sets of microsatellite markers flanking the PHD1 and PHD2 genes, and both tumors showed allelic loss for the markers at the PHD1 (Q LOH = 0.33–0.37) and PHD2 (Q LOH = 0.04–0.10) gene loci, respectively (Fig. 1b). The allelic deletions were also noticed in a sequencing study, with mutant nucleotide signals more dominant compared to wild-type nucleotides (Supplementary Figure S2).
PHD1 and PHD2 mutations, LOH, peptide sequencing and stability, and ubiquitination in PHEO/PGL and other samples. a Sanger sequencing showing heterozygous PHD1 and PHD2 mutations in blood samples. b DNA fragment analysis showing LOH in PHD1 or PHD2 loci, respectively, in tumors. Loss of allele is indicated by asterisk. c Amino acid sequence alignment of mutation sites in different species of PHD1 and PHD2. d CHX assay measuring PHD1 and PHD2 stability and showing the shortened protein stability in mutant proteins. e Immunoprecipitation assay showing enhanced Siah2 recognition and protein ubiquitination of PHD1 and PHD2 mutants. f Luciferase assay showing that reduction of HIF-2α induced target gene expression was significantly less diminished after transduction with the mutant than the WT PHDs (*p < 0.01, PHD wild type vs. PHD mutant, 12.5 ug transfection; † p < 0.01, PHD wild type vs. PHD mutant, 25 ug transfection)
Protein stability analysis of PHD1 and PHD2 mutants
Alignment of the amino acid sequences of PHD1 residues 41 to 65 and PHD2 residues 216 to 240 in different species showed that the mutations were located in the evolutionarily conserved area (Fig. 1c). The amino acid substitutions were likely to affect the folding and stability of the PHD1 and PHD2 proteins, as previously reported [15]. To test this hypothesis, we performed a cycloheximide pulse-chase assay. Mutations in PHD1 and PHD2 resulted in remarkably reduced protein half-lives, indicating quantitative losses of the PHD1 and PHD2 proteins due to their reduced stability (Fig. 1d). Reduced stability of mutant PHD1 and PHD2 was evidenced by the rapid degradation over time after the addition of cycloheximide (CHX) to block protein synthesis as seen on Western blot. The wild-type PHD1 and PHD2 proteins showed half-lives of 5.33 and 10.27 h, respectively. In contrast, PHD1-S61R and PHD2-A228S were less stable, with half-lives of 1.52 and 1.7 h, respectively. Enhanced protein degradation of the mutant PHD1 and PHD2 was supported by increased association of the mutant PHDs with E3 ubiquitin ligase 3 (Siah2) and protein ubiquitination compared to the wild-type PHDs in an immunoprecipitation assay (Fig. 1e).
Measurement of HIF-2α transcriptional activity
We next studied HIF-2α induction of transcriptional activity in the presence of wild-type and mutant PHDs using a luciferase assay (Fig. 1f). HIF-2α transcriptional activity was effectively reduced by an overexpression of wild-type PHD1 or PHD2 with an increasing amount of PHDs. Overexpression of PHD1-S61R or PHD2-A228S mutants led to a much less pronounced effect on HIF-2α-induced transcriptional activity, indicating reduced HIF degradation due to decreased protein half-lives of the mutant proteins (Supplementary Figure S3).
Expression of PHD1 and PHD2 proteins in tumors with PHD1 and PHD2 mutations in comparison to human adrenal medulla and a sporadic non-PHD1- or non-PHD2-mutated PHEO
The tumor from the PHD1 patient shows negative staining for PHD1, while the expression of PHD2 is heterogeneous, with areas of negative staining (0) and areas with weak (1+) staining. PHD1 expression in the tumor from the PHD2 patient was scored 1–2+; PHD2 staining in this sample was weak (1+). In the sporadic PHEO, PHD1 staining exhibited intermediate/strong (2–3+) intensity and PHD2 staining was scored 1–2+. Human adrenal medulla shows strong (3+) staining for PHD1 and intermediate (2+) staining for PHD2 (Fig. 2). A PHEO sample was incubated without primary antibody as a negative control (Supplementary Figure S4).
Up-regulation of HIF-1α and HIF-2α and downstream hypoxia-related gene expression
HIF-1α and HIF-2α levels were increased in tumor tissue, as detected by IHC analysis (Fig. 3a). The figure shows positive cytoplasmic immunostaining for HIF-1α and nuclear staining for HIF-2α. The tumor mass from the PHD1 mutation patient showed weak (1+) staining for HIF-1α and intermediate (2+) heterogeneous staining for HIF-2α. HIF-1α and HIF-2α expression in the sample from the PHD2 mutation patient was scored 1–2+. In comparison, in the tissue of a patient with a HIF2A mutation, expression of HIF-1α was weak (1+) and HIF-2α intensity was scored 2+. In normal adrenal medulla, HIF-1α was absent and only rarely did nuclei stain for HIF-2α. Increased activity of hypoxia pathways was confirmed by induction of HIF-1α and HIF-2α target gene expression relative to normal adrenal medulla, namely EPO, EDN1, GLUT1, GNA14, LDHA, POU5F1, SOX2, and VEGFA, in tumors from both patients by qPCR (Fig. 3b).
Effects of PHD1 and PHD2 mutations on HIFs, HIF-regulated gene expression, microarray gene profiling, EPO receptor activity, and in vitro analysis of native erythroid progenitors in tumor and blood samples. a IHC of HIF-1α and HIF-2α in PHEOs with PHD1 and PHD2 mutations in comparison to a PGL with a HIF2A mutation. Original magnification, ×200. Scale bar, 10 μm. b qPCR assay showing the expression of eight hypoxia-related genes—EDN1, EPO, GLUT1, GNA14, LDHA, POU5F1, SOX2, and VEGFA—in tumor specimens from PHD1 (P1) and PHD2 (P2) patients. A normal adrenal medulla (NM) was used as a control. c Heatmap of 176 differentially expressed genes in normal adrenal medulla (N1-7) vs. HIF2A (H1-6) and PHD2 (P1) PHEOs/PGLs identified by significance analysis of microarray with an FDR of 0.01. P1 showed an expression pattern more similar to HIF2A PHEOs/PGLs than normal adrenal medulla. However, expression differences also existed between HIF2A and PHD2 tumors, as can be seen based on the different color distributions (red, up-regulation; blue, down-regulation). Rank product analysis identified 20 genes to be significantly differentially expressed between HIF2A and PHD2 (p < 0.05; genes are indicated in Supplemental Table 3). d IHC showing EPOR expression in tumor specimens. Scale bar, 10 μm. e BFU-E assay showing EPO sensitivity of hematopoietic precursor cells from patients. The y-axis represents the absolute number of clones per 1,000,000 blood cells from patient blood.
Comparison of gene expression profiles in tumors associated with PHD2 and HIF2A mutations
We further compared the gene expression profiles of PHD2 and HIF2A tumors with normal adrenal medulla by microarray (Fig. 3c). Genes that showed similar expression levels in the PHD2 tumor and normal adrenal medulla were excluded (ratio of 0.5 to 1.5), resulting in 1940 genes that passed the filter. Then, differentially expressed genes between a combination of HIF2A and PHD2 PHEOs/PGLs vs. normal adrenal medulla were estimated by significance analysis of microarray (SAM) with a two-class comparison option. False discovery rates were estimated with 1000-fold permutations, and significant expression changes were selected at a 1 % false discovery rate, revealing 176 genes (y-axis). Figure 3c shows a heat map of these 176 genes, which indicates differences in the expression pattern of HIF2A (H1-6)- and PHD2 (P1)-related PGLs compared to normal medulla (N1-7). Twenty significantly changed genes between HIF2A and PHD2 PHEOs/PGLs were identified by rank product analysis (p < 0.05). Sample information is presented in Supplementary Table 2; gene identifiers and expression values are given in Supplementary Table 3. All data are available at the GEO database (GSE 39716). Of note, we only had the chance to analyze one tumor with the PHD2 mutation, since adequate RNA from PHD1-mutated tumors was not available, because these tumors were removed outside the NIH and frozen tissue was not collected.
EPO receptor IHC and in vitro analysis of native erythroid progenitors
We determined the expression of EPOR in PHEO specimens by IHC (Fig. 3d). We identified up-regulation of both the receptor and its phosphorylation in tumor specimens from PHD1 and PHD2 patients compared to a normal adrenal medulla. A BFU-E assay was carried out to assess erythroid progenitor sensitivity to EPO. Progenitor cells from both PHD1 and PHD2 patients’ peripheral blood exhibited hypersensitivity to EPO compared to progenitor cells from control persons (Fig. 3e).
Discussion
The present study shows for the first time a PHD1 germ-line mutation associated with the pathogenesis of PHEO/PGL-polycythemia and further extends the findings of PHD2 mutations in this disease. Both mutations caused amino acid substitutions, abnormal recognition of intrinsic E3 ligase Siah2, and resulted in substantial loss of protein stability of both PHD1 and PHD2. The dysregulation of the HIF-α signaling pathway caused by a mutation in either PHD1 or PHD2 affected HIF-α protein degradation and HIF-1α and HIF-2α target gene expression. We also found increased EPO and EPOR activity in PHD1 and PHD2 tumors compared to normal adrenal medullary tissue. Furthermore, we discovered hypersensitivity of erythroid progenitors to EPO in both patients. These findings are consistent with the clinical presentation that in addition to activation of the HIF-α signaling pathway, both patients also developed polycythemia, at least partially, due to the high activity and hypersensitivity of the EPOR signaling pathway to EPO.
PHDs and VHL are known to play essential roles in regulating HIFs. The pioneering studies of Ratcliffe and Kaelin showed that HIF ubiquitination and proteasomal removal are preceded by its proper hydroxylation by PHDs [2, 3]. Mutations in PHDs, VHL, and HIF2A can result in HIF-α stabilization, which may play a central role in the pathogenesis of some hereditary PHEOs/PGLs [23].
Almost all tumors are hypoxic, and tumor hypoxia, specifically via up-regulation of HIFs, contributes to tumor growth, progression, migration, metastasis, and resistance to various cancer treatments. Although the role of PHDs in cancer development remains inconclusive, in neural crest tumors such as PHEOs/PGLs, PHDs may act as tumor suppressor genes [24]. PHDs use 2-oxoglutarate as an electron donor, which is oxidized into succinate. High succinate levels inhibit PHD activity [25]. The accumulation of succinate is a typical metabolic feature of succinate dehydrogenase-deficient PHEOs/PGLs, and it is hypothesized that their tumorigenesis is also linked to the inhibition of PHDs, resulting in the up-regulation of HIF-α target genes. PHDs may also affect tumorigenesis in HIF-independent signaling, as reviewed by Jokilehto and Jaakkola [24]. This can involve the regulation of the NF-κB, NOTCH, and perhaps mTOR signaling pathways, resulting in abnormal cell differentiation and proliferation, angiogenesis, apoptosis, and immune responses [24, 26]. Finally, both mutations cause abnormal recognition of intrinsic E3 ligase Siah2 and result in the quantitative loss of both PHD1 and PHD2, further contributing to dysregulation of the HIF signaling pathway [27]. In summary, the precise role of PHDs as tumor suppressor genes or oncogenes depends on their relative abundance in a specific tissue, interplay among the three isoforms, as well as other genetic and epigenetic mechanisms.
Furthermore, a very recent study provided new evidence that PHD mutations are indeed involved in the pathogenesis of polycythemia via HIF-2α up-regulation and hypersensitivity of erythroid progenitors to EPO [28]. Consistent with these findings, one case of a germ-line PHD2 mutation has been described in the pathogenesis of PGL associated with polycythemia, presenting with normal EPO levels [15]. Nevertheless, the precise pathogenic mechanism of polycythemia, particularly extramedullary and extratumoral sources of erythropoiesis, is still unclear in our patients. As evident from the medical history of our patients, removal of their PHEOs/PGLs only partially improved their polycythemia or normalized their condition for a short period of time, despite no evidence of residual or additional tumors by biochemical and imaging assessment. This suggests that other sources of extratumoral and extramedullary erythropoiesis could exist. A recent study by Arsenault et al.[28] showed that heterozygous Phd2 loss in the neural crest-derived renal interstitial EPO-producing cells alone accounts for a significant portion of the erythrocytosis in both Phd2 P294R/+ and Phd2 +/− mice. Furthermore, other neural crest cells, beside chromaffin cells, may be additional sources of EPO. This is well documented in previous studies that suggest astrocytes, retinal cells, and, perhaps, enterochromaffin cells in the gastrointestinal tract, among others [29]. Thus, PHD germ-line mutations in these cells could lead to abnormal EPO production, further supporting the recent data of Arsenault et al. in a Phd2 knock-in mouse model [28].
Our findings regarding up-regulation of EPOR and its hypersensitivity to EPO support the notion that these two mechanisms may contribute to tumorigenesis in both patients. Some studies demonstrated that EPO could stimulate proliferation, growth, and metastatic spread as well as inhibit apoptosis in cancer cells via JAK2-STAT, ERK1/2, and the PI3K/AKT pathways [30, 31]. These effects can be further potentiated during hypoxia. Interestingly, and related to the present study, Um et al. found that EPO via EPOR exerts an antiapoptotic effect on rat PHEO (PC-12) cells [32]. Nevertheless, detailed molecular mechanisms related to EPO/EPOR signaling and tumor growth remain to be established in this population of patients, as suggested by Yoon et al. [33].
Mutations in different HIF regulators and HIF itself appear to have similar disease manifestations but also present with some different clinical phenotypes, especially the recently described HIF2A- and now PHD1- and PHD2-associated PHEO/PGL and polycythemia [16]. The differences could result from the subtly distinct impacts of these gene mutations in the activation of HIF-1α and HIF-2α target genes and their effects on tumorigenesis [34]. For this reason, we decided to look at the differential expression of several HIF target genes in PHD2-associated tumors. We found that the PHD2 mutation affected both HIF-1α and HIF-2α target genes in PHEO/PGL. In contrast to HIF2A mutations where HIF-2α target genes are preferentially affected [16], there seems to be an interplay in the induction of both HIF-1α and HIF-2α target genes in PHD1 and PHD2 loss-of-function mutations. To gain a more global perspective, microarray-based gene expression profiling was performed. A total of 176 genes in PHD2- and HIF2A-related PHEOs/PGLs were identified as differentially expressed compared to normal adrenal medulla. In addition to commonly expressed genes, however, there was clear evidence for differential expression between PHD2 and HIF2A tumors. None of these genes was identified as an exclusive HIF-1α or HIF-2α target gene; thus, transcriptional co-factors or other mechanisms in the complex pathway of HIF transcriptional regulation may contribute to the differential expression pattern. This is in agreement with previous studies showing that PHDs affect both HIF-1α and HIF-2α signaling pathways [24]. When well-established human PHEO/PGL-derived cell lines become available, several experiments performed in the present study should be validated and performed under both normoxic and hypoxic conditions. Moreover, further experiments with additional PHD1 and PHD2 samples are needed to derive more conclusive information.
Patients with PHD1, PHD2, or HIF2A mutations share some clinical similarities, including multiple or recurrent PHEOs/PGLs, noradrenergic phenotypes, and an early onset of polycythemia, but they also differ in several aspects of their clinical presentations. First, patients with PHD1 and PHD2 mutations present with borderline or mildly elevated serum EPO levels compared to those with HIF2A mutations, where EPO levels are usually several-fold increased. Polycythemia does not seem to occur at birth. Second, HIF2A mutation patients present with another neuroendocrine tumor—somatostatinoma—that has not yet been detected in patients with PHD1 and PHD2 mutations. Third, in patients with HIF2A mutations, metastatic PHEO/PGL has not yet been described. Finally, both these diseases diverge from primary polycythemias where the EPOR/JAK-STAT pathway is constitutively activated, resulting in polycythemia with inappropriately low EPO levels.
In conclusion, the PHD1 mutation is the latest inherited abnormality that contributes to the genetic landscape of PHEOs/PGLs. The present study further provides evidence that the PHD-HIF-EPOr pathway plays a role in the pathogenesis of these tumors and their association with polycythemia. Its close link to the HIF-1α and HIF-2α signaling pathways further supports the hypothesis that these and other hereditary PHEOs/PGLs could be therapeutically approached by modulating HIF signaling in these tumors [23]. The discovery reported here suggests that patients with polycythemia associated with borderline or mildly elevated EPO levels should be screened for the presence of PHD1 and PHD2 mutations. Finally, patients carrying a germ-line mutation in genes of the HIF signaling pathway should have stringent follow-up to detect and treat PHEO/PGL early.
References
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ et al (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW et al (2002) Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A 99:13459–13464
Kaelin WG Jr (2008) The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8:865–873
Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA (2005) HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105:3133–3140
Prchal JT (2005) Polycythemia vera and other primary polycythemias. Curr Opin Hematol 12:112–116
McMullin MF (2010) HIF pathway mutations and erythrocytosis. Expert Rev Hematol 3:93–101
Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D et al (2002) Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32:614–621
Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TR et al (2008) Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood 111:5400–5402
Prchal JT, Gordeuk VR (2008) The HIF2A gene in familial erythrocytosis. N Engl J Med 358:1966, author reply 1966–1967
de la Chapelle A, Traskelin AL, Juvonen E (1993) Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A 90:4495–4499
Sokol L, Luhovy M, Guan Y, Prchal JF, Semenza GL, Prchal JT (1995) Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood 86:15–22
Albiero E, Ruggeri M, Fortuna S, Finotto S, Bernardi M, Madeo D, Rodeghiero F (2012) Isolated erythrocytosis: study of 67 patients and identification of three novel germ-line mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Haematologica 97:123–127
Lenders JW, Eisenhofer G, Mannelli M, Pacak K (2005) Phaeochromocytoma. Lancet 366:665–675
Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouyssegur J, Richard S, Gardie B (2008) PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med 359:2685–2692
Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K (2012) Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 367:922–930
Capodimonti S, Teofili L, Martini M, Cenci T, Iachininoto MG, Nuzzolo ER, Bianchi M, Murdolo M, Leone G, Larocca LM (2012) Von hippel-lindau disease and erythrocytosis. J Clin Oncol 30:e137–e139
Lorenzo FR, Yang C, Ng Tang Fui M, Vankayalapati H, Zhuang Z, Huynh T, Grossmann M, Pacak K, Prchal JT (2013) A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 91:507–512
Yang C, Huntoon K, Ksendzovsky A, Zhuang Z, Lonser RR (2013) Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Rep 3:52–59
Shankavaram U, Fliedner SM, Elkahloun AG, Barb JJ, Munson PJ, Huynh TT, Matro JC, Turkova H, Linehan WM, Timmers HJ et al (2013) Genotype and tumor locus determine expression profile of pseudohypoxic pheochromocytomas and paragangliomas. Neoplasia 15:435–447
Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M, Fehm T, Solomayer E, Riess O, Wallwiener D et al (2006) Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res 66:5278–5286
Lanikova L, Lorenzo F, Yang C, Vankayalapati H, Drachtman R, Divoky V, Prchal JT (2013) Novel homozygous VHL mutation in exon 2 is associated with congenital polycythemia but not with cancer. Blood 121:3918–3924
Jochmanova I, Yang C, Zhuang Z, Pacak K (2013) Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst 105:1270–1283
Jokilehto T, Jaakkola PM (2010) The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med 14:758–770
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7:77–85
Takeda K, Fong GH (2007) Prolyl hydroxylase domain 2 protein suppresses hypoxia-induced endothelial cell proliferation. Hypertension 49:178–184
Tian YM, Mole DR, Ratcliffe PJ, Gleadle JM (2006) Characterization of different isoforms of the HIF prolyl hydroxylase PHD1 generated by alternative initiation. Biochem J 397:179–186
Arsenault PR, Pei F, Lee R, Kerestes H, Percy MJ, Keith B, Simon MC, Lappin TR, Khurana TS, Lee FS (2013) A knock-in mouse model of human PHD2 gene-associated erythrocytosis establishes a haploinsufficiency mechanism. J Biol Chem 288:33571–33584
Juul SE, Yachnis AT, Christensen RD (1998) Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev 52:235–249
Miyake M, Goodison S, Lawton A, Zhang G, Gomes-Giacoia E, Rosser CJ (2013) Erythropoietin is a JAK2 and ERK1/2 effector that can promote renal tumor cell proliferation under hypoxic conditions. J Hematol Oncol 6:65
Shi Z, Hodges VM, Dunlop EA, Percy MJ, Maxwell AP, El-Tanani M, Lappin TR (2010) Erythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell line. Mol Cancer Res 8:615–626
Um M, Gross AW, Lodish HF (2007) A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal 19:634–645
Yoon D, Agarwal N, Prchal JT (2008) Does erythropoietin promote tumor growth? Clin Cancer Res 14:1920, author reply 1920–1921
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23:9361–9374
Acknowledgments
We would like to acknowledge the clinical and technical assistance of Victoria Martucci, Karen Adams, Pauline Dmitriev, and Joan Nambuba in the production of this manuscript. We also thank both patients and their families for their participation and assistance. This research was supported, in part, by the Intramural Research Program of the NIH, Eunice Kennedy Shriver NICHD, NINDS, NCI, and NHGRI.
Conflict of interest
The authors declare no conflict of interests related to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chunzhang Yang and Zhengping Zhuang contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 6711 kb)
Rights and permissions
About this article
Cite this article
Yang, C., Zhuang, Z., Fliedner, S.M.J. et al. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J Mol Med 93, 93–104 (2015). https://doi.org/10.1007/s00109-014-1205-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1205-7