Abstract
The existence of a link between estrogen deprivation and osteoarthritis (OA) in postmenopausal women suggests that 17β-estradiol (17β-E2) may be a modulator of cartilage homeostasis. Here, we demonstrate that 17β-E2 stimulates, via its receptor human estrogen receptor α 66 (hERα66), type II collagen expression in differentiated and dedifferentiated (reflecting the OA phenotype) articular chondrocytes. Transactivation of type II collagen gene (COL2A1) by ligand-independent transactivation domain (AF-1) of hERα66 was mediated by “GC” binding sites of the −266/−63-bp promoter, through physical interactions between ERα, Sp1/Sp3, Sox9, and p300, as demonstrated in chromatin immunoprecipitation (ChIP) and Re-Chromatin Immuno-Precipitation (Re-ChIP) assays in primary and dedifferentiated cells. 17β-E2 and hERα66 increased the DNA-binding activities of Sp1/Sp3 and Sox-9 to both COL2A1 promoter and enhancer regions. Besides, Sp1, Sp3, and Sox-9 small interfering RNAs (siRNAs) prevented hERα66-induced transactivation of COL2A1, suggesting that these factors and their respective cis-regions are required for hERα66-mediated COL2A1 up-regulation. Our results highlight the genomic pathway by which 17β-E2 and hERα66 modulate Sp1/Sp3 heteromer binding activity and simultaneously participate in the recruitment of the essential factors Sox-9 and p300 involved respectively in the chondrocyte-differentiated status and COL2A1 transcriptional activation. These novel findings could therefore be attractive for tissue engineering of cartilage in OA, by the fact that 17β-E2 could promote chondrocyte redifferentiation.
Key messages
• 17β-E2 up-regulates type II collagen gene expression in articular chondrocytes.
• An ERα66/Sp1/Sp3/Sox-9/p300 protein complex mediates this stimulatory effect.
• This heteromeric complex interacts and binds to Col2a1 promoter and enhancer in vivo.
• Our findings highlight a new regulatory mechanism for 17β-E2 action in chondrocytes.
• 17β-E2 might be an attractive candidate for cartilage engineering applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mature articular cartilage harbors a predominant cell type, the chondrocyte, which is responsible for the extracellular matrix (ECM) turnover regulation by synthesizing and secreting cartilage-specific matrix markers such as type II collagen and large aggregating proteoglycans, aggrecans. The role played by type II collagen, a phenotypic marker of healthy articular cartilage, in the homeostasis of ECM is critical, as illustrated by the loss of the physicomechanical properties of the tissue leading to a variety of joint diseases such as osteoarthritis (OA) and rheumatoid arthritis. During OA development and/or when cells are cultured as monolayers or passaged, chondrocytes undergo dedifferentiation process: they lose their normal cartilage phenotype and have been found to progressively reduce their type II collagen synthesis in favor of nonspecific articular cartilage collagens (isotypes I, III, V, and X) [1, 2]. As a consequence, it is of special interest to get insight into the molecular mechanisms that govern type II collagen gene expression, in order to better understand the process of phenotype alteration in chondrocytes during OA.
Type II collagen is first synthesized as a procollagen triple-helix molecule including identical α1(II) chains encoded by the COL2A1 gene [3]. Sequence analysis of COL2A1 has revealed crucial DNA regulatory elements within both the short promoter and the first intron regions [4–6] (Fig. 1). Thus, several binding sites of the intronic enhancer have been shown to interact with transcription factors, such as Sox-9, Sox-6, and L-Sox-5, acting cooperatively and consequently activating expression of COL2A1 in 10T1/2 and MC615 cells [7]. Moreover, zinc finger transcription factors Sp1, Sp3, and C-Krox are able to bind not only to this region but also to several DNA sites localized in a 266-bp promoter of COL2A1. Interestingly, Sp1 was found to be a potent activator of COL2A1 transcription by binding to GC-rich sequences in the promoter and in the intronic enhancer, whereas Sp3 prevents this action by competing Sp1 for its GC-responsive elements [5, 8].
Schematic representation of the human COL2A1 gene. Localization of binding sites of some of the transcription factors which have been characterized to interact with the promoter or the enhancer region (in the first intron) of COL2A1. CIIS type II collagen silencer; TBP TATA binding protein; C/EBP CCAAT enhancer binding protein; NFYA, nuclear transcription factor Y α
From epidemiological studies, the prevalence of OA is known to increase dramatically in women around the age of 50, coinciding with the onset of menopause and suggesting the potential for estrogens in preventing OA. The menopause is associated with an increase in the urinary levels of CTX-II, a telopeptide fragment of type II collagen, and this increase correlates with collagen II degradation and joint damage and can be antagonized by estrogens [9, 10]. Although there are some conflicting results among these studies, most of the clinical observations show that hormone replacement therapy (HRT) is associated with reduced severity and prevalence of OA in postmenopausal women [11, 12]. Another study demonstrated that the localized production of 17β-E2 by synovial cells inhibits interleukin (IL)-6 secretion by osteoarthritic chondrocytes in postmenopausal women [13] highlighting the anti-inflammatory properties of 17β-E2. This chondroprotective effect of HRT was confirmed in animal models. For instance, a long-term (3 years) estrogen replacement therapy in ovariectomized monkeys significantly decreases the severity of OA lesions by maintaining intact articular cartilage structure and reducing number of osteophytes, chondrocyte cloning, and proteoglycan loss [14]. As estrogens are known to down-regulate the matrix metalloproteinases (MMPs) that are critical for cartilage collagenolysis, the main effect of estrogens relies upon the inhibition of the catabolic processes in articular chondrocytes. However, 17β-E2 can also stimulate the anabolic pathway by interacting with synthesis and secretion of transforming growth factor-β1 (TGF-β1) and insulin-like growth factor-I (IGF-I). Indeed, compared with chondrocytes from nontreated ovariectomized monkeys, 17β-E2-treated animals have twofold higher synovial fluid levels of IGF-I [15]. This growth factor is critical for the regulation of chondrocyte proliferation, aggrecan synthesis, and COL2A1 expression [16, 17] as well as TGF-β whose expression is influenced by 17β-E2 in dose-dependent and biphasic manner [18]. In this context, molecular mechanisms by which estrogens mediate a chondroprotective action through type II collagen regulation remain to be elucidated.
Identification of two isoforms of estrogen receptor, alpha (ERα) and beta (ERβ), in articular chondrocytes from several species including rabbits [19] and human [20] provides strong evidence that cartilage can respond to estrogens. Deletion of both ERs leads to increased osteophytosis in 6-month-old knockout mice [21]. The majority of ERs are nuclear proteins belonging to the steroid receptor family of transcription factors, structurally organized in six distinct domains (A to F). According to the “classical genomic model,” ligand-activated ERα binds to specific DNA sequences called “estrogen-responsive elements” (ERE); so that two transactivation functions, AF-1 (ligand-independent) and AF-2 (ligand-dependent), located respectively in B and E receptor domains, act cooperatively to modulate transcriptional activity of 17β-E2-target genes. Nevertheless, 35 % of the categorized human 17β-E2-responsive genes are transcribed via indirect ER-DNA association through protein–protein interactions with several trans factors such as Sp1, nuclear factor-kappaB (NF-κB), or activator protein-1 (AP-1) [22, 23]. For instance, Sp1 and ERα can form a complex in vivo to mediate E2-induced activation of kisspeptin 1 promoter in hypothalamic GT-1 cells [24].
The 46-kDa human estrogen receptor α (hERα) isoform (hERα46), which has been first identified in MCF-7 breast carcinoma cell line, lacks the N-terminal 173 amino acids of A and B domains and is consequently devoided of AF-1 transactivation sequence. In a cell context where AF-1-mediated transactivation predominates over AF-2, hERα46 is an effective competitive inhibitor of hERα66. Indeed, when both isoforms are co-expressed (as it seems to be the most frequent situation in vivo), hERα46 efficiently represses the transcription of estradiol-target genes induced by hERα66 by suppressing AF-1 activity of full-length estrogen receptor [25]. Expression of hERα46 was also reported in human primary osteoblasts at similar levels compared to hERα66 [26]. To our knowledge, no information exists on the exact function of hERα46 in articular chondrocytes, except our previous study demonstrating that, unlike hERα66, hERα46 isoform is not able to stimulate uridine diphosphate (UDP)-glucose dehydrogenase gene activity in primary rabbit articular chondrocytes [27].
In the present study, we aimed to investigate the role of 17β-E2 in the regulation of COL2A1 expression in rabbit articular chondrocytes (RAC). We demonstrated for the first time that COL2A1 up-regulation by 17β-E2 involves a −266/−63-bp promoter region and that this effect was mediated by AF-1 region of hERα66 in both differentiated and dedifferentiated chondrocytes. Moreover, we showed that hERα66 requires Sp1, Sp3, Sox-9, and p300 cooperation to increase COL2A1 transcription in RAC. Therefore, association of 17β-E2/hERα66 complex to COL2A1 regulatory sequences may contribute to reverse the loss of type II collagen expression during chondrocyte dedifferentiation, such an alteration currently observed in OA.
Materials and methods
General materials
Chemical reagents were of the highest grade from Sigma-Aldrich Co. (Saint Quentin-Fallavier, France), as well as IL-1β, TGF-β1, tamoxifen, and mithramycin A. Trichloroacetic acid, Tris and glycine were purchased from Acros Organics (Noisy-le-Grand, France). Fetal calf serum (FCS), Dulbecco’s modified Eagle’s medium (DMEM), di-deoxynucleoside triphosphate (dNTPs), RNAse inhibitor, trypsin, and phosphate-buffered saline (PBS) were purchased from Invitrogen (Cergy-Pontoise, France). Protein assay kit was from Bio-Rad (Marnes-la-Coquette, France). 2X SYBR Green Master Mix was purchased from Applied Biosystems (Courtabœuf, France). All the primers and oligonucleotides used in this study (real-time PCR, electrophoretic mobility shift assays (EMSAs), chromatin immunoprecipitation assays (ChIP)) were synthesized by Eurogentec Corporation (Seraing, Belgium) (Table 1). [3H]proline and [γ-32P]dATP were purchased from PerkinElmer Life Sciences (Courtabœuf, France) and double charcoal-treated FCS was from Abcys (Paris, France).
Antibodies
Rabbit polyclonal anti-type II collagen was from Novotec (Lyon, France). Rabbit monoclonal anti-β-actin (C-2), anti-ERα (MC-20), anti-Sp1 (PEP-2), anti-Sp3 (D-20), anti-Sox-9 (P-20), anti-p300 (N-15), anti-RNA polymerase II (H-224), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit were purchased from Tebu-Bio (Le Perray en Yvelines, France).
Chondrocyte culture
RAC were isolated from articular cartilage slices of shoulders and knees of 3-week-old male rabbits, by sequential digestion with hyaluronidase (1,088 U/ml, Serva, Heidelberg, Germany), trypsin (124 U/ml, Coger, Paris, France), and type I collagenase (410 U/ml, Invitrogen). The cells were seeded generally at 3.5 × 105 cells per 9.6-cm2 dishes or 1.5 × 106 cells per 55-cm2 dishes in 2 or 8 ml of DMEM containing 10 % heat-inactivated FCS, supplemented with glutamine (2 mM), fungizone (0.25 μg/ml), and antibiotics: penicillin (100 UI/ml) and erythromycin (100 μg/ml). They were grown for 8–12 days at 37 °C in a 5 % CO2-humidified atmosphere, with medium change every 2–3 days. For dedifferentiation, in order to mimic some of OA phenotype characteristics (i.e., decrease of type II and increase of type I collagen productions), RAC cultures were passaged three times with 0.25 % trypsin (Invitrogen) after reaching confluency. When chondrocytes were treated by 17β-E2 (Sigma-Aldrich Co), they were incubated in phenol red-free DMEM medium supplemented with 2 % double charcoal-treated FCS. In some experiments, articular chondrocytes were incubated for 24 h with tamoxifen (1 μM), a partial antagonist of estrogen receptors or mithramycin A (100 nM), a binding inhibitor to GC-rich sequences, in the same medium as for 17β-E2 treatment. MCF-7 cell line was cultured in the same conditions as primary RAC.
Collagen labeling and assay
To assay newly synthesized collagen, series of chondrocytes in six-well plates were transiently transfected, at 80 % confluency, with 10 μg of the expression vector pCR3.1-hERα66 or pCR3.1-hERα46, encoding the long or the short estrogen receptor α isoforms, respectively (pCR3.1 plasmid was used as control). Second series were not transfected and used to study the effect of increasing concentrations of 17β-E2. Fifteen hours later, all the cell culture media were changed to 10 % FCS/DMEM supplemented with 100 μg/ml sodium ascorbate. Twenty-four hours later, cells were incubated for 24 h in 2 % double charcoal-treated FCS/DMEM medium supplemented with 50 μg/ml sodium ascorbate, 100 μg/ml β-aminopropionitrile, and 2 μCi/ml [3H]proline, containing or not 17β-E2 (0–5 nM), TGF-β1 (3 ng/ml), or IL-1β (1 ng/ml). Only the chondrocytes which were not submitted to transient transfection experiments were treated by the hormone or the cytokines. Twenty-four hours after treatments or 48 h post-transfection, the culture medium and the cell extracts were collected, and the amount of labeled collagen was assayed as previously described [28].
Western blotting
Twenty-four hours after incubation with 17β-E2 or 48 h post-transfection, RAC cultures were washed three times with PBS and whole cell extracts were prepared as previously described [27]. The cell extract-associated proteins (20 μg) were resolved on a 8 % polyacrylamide gel, using 1 % sodium dodecyl sulfate (SDS)/Tris glycine buffer. The proteins were then electrotransferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Molsheim, France). Free protein-binding sites of the PVDF membranes were blocked for 1 h in Tris buffer saline (TBST, 20 mM Tris, 137 mM NaCl, 0.1 % Tween 20) containing 10 % nonfat dry milk, and the membranes were incubated with anti-type II procollagen, anti-ERα, anti-Sp1, anti-Sp3, or anti-β-actin antibodies. After overnight incubation at 4 °C, the membranes were rinsed and incubated for 1 h with a secondary antibody (HRP-conjugated goat anti-rabbit antibody at 1:6,000 dilution). Protein signals were revealed using a Western Blotting Chemiluminescence Luminol Reagent (Tebu-Bio) and quantified with the Image Quant software (Amersham Biosciences, Orsay, France). The type II collagen signal was normalized to that of β-actin.
Real-time RT-PCR
Total RNAs were extracted using TRIzol® reagent, according to the manufacturer’s instructions (Invitrogen). Two micrograms of RNAs, previously treated with DNase I (Invitrogen), was reverse transcribed into cDNA using 50 pmol of oligo dT, 5 mM of each dNTPs, reverse transcriptase buffer 5×, 40 units/μl of RNase inhibitor, and 200 units/μl of Moloney Murine Leukemia Virus enzyme (Invitrogen) in a final volume of 25 μl. Real-time PCR amplifications were carried out with 5 μl of cDNA (diluted 1:100) on an “ABI Prism 7000 sequence detection system” (Applied Biosystems), using sequence-specific primers (Table 1) and 2X SYBR Green Master Mix protocol as outlined by the manufacturer. Each sample was run in triplicate, and analysis of relative gene expression was done by using the 2−ΔΔCT method.
Transient transfection experiments
Eighty percent confluent RAC in six-well plates were transiently transfected by the calcium phosphate precipitation method. Luciferase reporter plasmids (10 μg) containing various deletions of COL2A1 promoter/first intron were co-transfected with 10 μg of pCR3.1 vector containing or not the cDNA encoding the hERα. The reporter constructs pGL2-3.374 kb, pGL2-3.316 kb, pGL2-0.387 kb, and pGL2-0.110 kb cover, respectively, the −932/+2,842, −932/+2,384, −266/+121, and −63/+47 bp of the promoter and first intron of COL2A1, as previously described [6]. Expression vectors used for hERα encode either long form of estrogen receptor α or short mutated inoperative form of the receptor (A/B domain deleted), denoted hERα66 or hERα46, respectively. After 12–15 h, the medium was changed to 10 % FCS/DMEM. Twenty-four hours later, the samples were harvested, and the protein content and luciferase activities were assayed on whole cell extract (Promega, Charbonnières les Bains, France) in a luminometer (Berthold Lumat LB 9501, Bad Wildbad, Germany). The protein amount was determined by the Bradford colorimetric method (Bio-Rad). Luciferase activities were normalized to protein content and represented as mean ± SD of three independent samples. For all the transcriptional activity assays, the Escherichia coli lacZ gene, encoding β-galactosidase (β-gal vector), was systematically transfected in combination with COL2A1 reporter constructs and hERα expression plasmids to achieve for transfection efficiencies. In an additional experiment (Fig. 5c), 10 μg of pGL2-0.387 kb COL2A1 reporter construct was co-transfected with 2 μg of small interfering RNA (siRNA) directed against ERα (Tebu-Bio). The corresponding negative siRNA (scrambled sequence of the siRNA target) was used as control. After 15 h of transfection, the medium was changed to 10 % FCS/DMEM. Twenty-four hours later, the samples were harvested and analyzed as described above.
siRNA transfection
In knock-down experiments, hERα66 and hERα46 expression vectors were also co-transfected with siRNAs (Tebu-Bio) directed against the human Sp1, Sp3, and Sox-9 mRNAs. At 80 % confluency, RAC were transiently transfected by the calcium phosphate precipitation method using 5 μg of hERα66 or hERα46 expression vectors in combination with 2 μg of Sp1, Sp3, or Sox-9 siRNAs. After an overnight transfection period, the cells were incubated for 24 h in absence or in presence of 17β-E2. The corresponding insertless plasmid pCR3.1 and negative siRNA were used as controls. Relative expression of Col2a1, Sp1, Sp3, Sox-9, and hERα66 genes was determined by real-time RT-PCR.
EMSAs
Nuclear proteins from RAC treated or not with increasing concentrations of 17β-E2 or transfected with estrogen receptors expression vectors, as described above, were extracted by a minipreparation procedure [29]. EMSAs were performed with oligonucleotides presented in Table 1, which were end-labeled with [γ-32P]dATP using T4 polynucleotide kinase (Promega). RAC nuclear extracts (10 μg) were incubated for 20 min at room temperature with 2 fmol of radioactive probe in a specific binding buffer (for +2,817/+2,846 probe, −96/−66 probe, −225/−188 probe and ERE/mutant ERE probes, 20 % glycerol, 40 mM Tris pH 7.5, 2 mM dithiothreitol (DTT), 200 mM NaCl, 0.01 % BSA, 0.2 % Nonidet P-40; for +2,353/+2,415 probe or +2,383/+2,415 probe, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.9, 50 mM KCl, 10 % glycerol, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM DTT, 1 mM PMSF, 0.05 % Nonidet P-40) in the presence of 2 μg of poly(dI-dC) (for +2,817/+2,846 probe, −96/−66 probe, −225/−188 probe or ERE probes) or 2 μg of poly(dG-dC) (for +2,353/+2,415 or +2,383/+2,415 probe), used as nonspecific DNA competitors. For EMSAs performed with ERE and mutant ERE probes, samples were submitted for 20 min to a supplemental incubation in the presence or absence of a ERα antibody (4 μg) or molecular excess (×100) of wild-type nonlabeled ERE probe (cold probe). Samples were fractionated on a 7 % polyacrylamide electrophoresis gel for 2 h at 150 V in 0.5× TBE buffer (Tris borate 450 mM, 10 mM EDTA) and visualized by autoradiography.
Immunoprecipitation assays
Protein nuclear extracts (200 μg) from control or 1 nM 17β-E2 treated chondrocytes were pre-cleared with 2.5 mg of protein A-Sepharose (Sigma-Aldrich Co) for 2 h at 4 °C. Pre-cleared extracts, relieved of protein A-Sepharose, were immunoprecipitated for 15 h at 4 °C using an anti-Sp1 antibody. Then, protein–antibody complexes were captured by adding 3 mg of protein A-Sepharose, and unbound proteins were removed by washing the solid phase four times with hypertonic lysis buffer (119 mM Tris pH 6.8, 5 % glycerol, 0.4 % SDS, 1 % β-mercaptoethanol, 0.02 % bromophenol blue). Samples were then resuspended in 50 μl of 2× sample loading buffer (20 mM HEPES, 25 % glycerol, 1 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA) and boiled for 5 min. Remaining nuclear proteins were centrifuged at 14,000×g for 2 min (4 °C), and supernatants were submitted to Western blotting.
ChIP
RAC, incubated or not with 17β-E2 (1 nM), were cross-linked, scraped, and lysed according to the manufacturer’s instructions. Resulting chromatin was used in ChIP assays using a commercial chromatin immunoprecipitation kit (Active Motif, Rixensart, Belgium). In Re-ChIP experiments, a supplemental overnight immunoprecipitation with ERα antibody was performed to isolate specific DNA–protein complexes. ChIP primers (Table 1) were designed to amplify in PCR a 171-bp specific fragment of the COL2A1 core promoter or a 229-bp fragment of the COL2A1 intronic enhancer region.
Statistical analysis
Results were representative of at least three experiments with similar results, performed in triplicates unless otherwise precisions. Statistically significance was assessed by using the Student’s t test. Statistically significant differences were set at *** p < 0.001, ** p < 0.01, and * p < 0.05.
Results
17β-E2 and its receptor hERα66 stimulate type II collagen production in primary articular chondrocytes
We first determined whether 17β-E2 and its receptors hERα66 or hERα46 modulate collagen synthesis in primary RAC. 17β-E2 was found to enhance total collagen neosynthesis (Fig. 2a), mainly composed of type II collagen (80 %) in primary RAC. The stimulating effect of the hormone, mainly observed in the cell extract-associated fraction compared to supernatant culture medium, was biphasic with a maximal peak for concentrations of 0.5 and 2 nM. As expected, incubation of the cells with TGF-β1 resulted in a marked increase (about 175 %) of [3H]proline labeling in the cell extract fraction, whereas IL-1β reduced the incorporation to 50 % of the control value. Next, we found that hERα66 stimulated total cell extract and culture medium associated collagen production in RAC, whereas mutated receptor hERα46 did not induce any significant effect on both fractions (Fig. 2b). However, a weak enhancing effect of hERα46 was observed on the total production of neosynthesized collagens. Then, we specifically investigated the effect of 17β-E2 on type II collagen synthesis by Western blotting. By comparison to untreated cells, we found that 17β-E2 stimulated type II procollagen protein synthesis by approximately 100 % (Fig. 3a) in primary RAC. In the same way, forced expression of hERα66 induced a twofold increase in type II collagen protein amounts, whereas hERα46 slightly enhanced these amounts by approximately 30 % (Fig. 3c). In dedifferentiated chondrocytes, a low dose of 17β-E2 (0.01 nM) remained activator for type II procollagen production, but to a lower level compared to primary RAC, since the induction produced by the hormone was only of 22 % (Fig. 3b). In contrast, for higher concentrations (0.1–5 nM), 17β-E2 significantly decreased type II collagen amounts in a dose-dependent manner.
Effect of 17β-E2, hERα66, and hERα46 on collagen neosynthesis in primary chondrocytes. a Collagen radiolabeling assay on both medium and cell extract fractions from primary RAC cultured for 24 h in the presence or absence of TGF-β, IL-1β, or 17β-E2. b Collagen radiolabeling assay on primary RAC transiently transfected with hERα66 or hERα46 cDNA. The values, normalized to the amount of total protein, were expressed as percentage of the control (absence of 17β-E2 (a) or empty pCR3.1 plasmid (b)) and represent the mean ± SD of three (a) and four (b) independent experiments performed in triplicate. Statistical significance was assessed by using the Student’s t test, and differences were set at *** p < 0.001, ** p < 0.01, and * p < 0.05
Effect of 17β-E2, hERα66, and hERα46 on type II collagen protein expression in primary and dedifferentiated RAC. a Primary chondrocytes were treated with increasing concentrations of 17β-E2 for 24 h, and type II collagen expression was assayed using Western blotting analysis of whole cell extracts. β-actin was used as a loading control. The same experiment as in (a) was performed on dedifferentiated articular chondrocytes (b) or on primary RAC transiently transfected with hERα66 or hERα46 expression vectors (c). Histograms represent the relative expression of type II procollagen normalized to β-actin protein signal and estimated after densitometric analysis of the blots using the ImageJ software. Results are representative of two (b) and three (a and c) independent experiments
17β-E2 and its receptor hERα66 up-regulate Col2a1 mRNAs steady-state levels in primary articular chondrocytes
Similarly to collagen neosynthesis results, the steady-state levels of Col2a1 mRNAs were increased under 17β-E2 treatment in a biphasic manner (i.e., only doses ranging between 1 and 5 nM increased the amounts of type II procollagen transcripts) (Fig. 4a), suggesting a transcriptional control. As shown in Fig. 4b, the stimulatory effect of 17β-E2 was dependent on estrogen receptors. Indeed, the addition of tamoxifen, a partial antagonist of estrogen receptors, induced about 55–60 % decrease of type II collagen mRNAs steady-state levels and prevented the stimulatory action of 17β-E2 on the transcript levels. According to these results, we found that intermediate amounts (5–10 μg) of hERα66 strongly increased Col2a1 mRNAs steady-state levels, whereas higher quantity (15 μg) of expression vector induced an approximate 30 % decrease of mRNA levels (Fig. 4c, d). Moreover, these last data have a similar pattern as those observed in panel A where RAC were incubated with 17β-E2, indicating once again that the hormone mediates its effects on Col2a1 gene through hERα66.
Effect of 17β-E2 and hERα66 on the steady-state levels of type II collagen mRNAs in primary chondrocytes. a Real-time RT-PCR analysis of Col2a1 mRNA expression using total RNAs isolated from primary RAC, treated or not with increasing concentrations of 17β-E2 for 24 h. Results were normalized to 18S rRNAs. b Primary RAC were treated or not for 24 h with tamoxifen (Tam.), a partial antagonist of estrogen receptors in our model, and assayed for real-time RT-PCR analysis. c Articular chondrocytes were transiently transfected with increasing amounts of hERα66 expression vector and Col2a1 expression was measured by real-time RT-PCR. Results were normalized to glyceraldehyde 3-phospho dehydrogenase (GAPDH) mRNAs. d Verification of hERα66 overexpression using specific primers for the long isoform of human estrogen receptor. Results are representative of three independent experiments and values are the means ± SD of triplicate samples. Statistically significance was assessed by using the Student’s t test and differences were set at *** p < 0.001, ** p < 0.01, and * p < 0.05
A −266/−63-bp region of COL2A1 promoter mediates the up-regulatory effect of hERα66 in both primary and dedifferentiated RAC
To delineate the genomic sequences involved in hERα-mediated effect, in both the promoter and/or the first intron regions of COL2A1, we co-transfected different COL2A1 reporter constructs together with hERα66 or hERα46 expression vectors. Whatever the construct size, the transcriptional activity of the reporter gene was always found to be enhanced by hERα66, compared to the respective controls, except for the shortest construct −63/+47 bp (Fig. 5a, b). Therefore, it is likely that a minimal −266/−63-bp region of COL2A1 promoter mediates hERα66-induced COL2A1 transactivation in both primary and dedifferentiated RAC. Moreover, hERα46 isoform did not significantly modify the basal expression of the reporter COL2A1 (except for the shortest construct −63/+47 bp), indicating that hERα66 stimulation of COL2A1 is specific of this isoform and depends directly on AF-1 ligand-independent transactivation domain of hERα.
Effect of 17β-E2, hERα66, hERα46, and ERα siRNA on the transcription activity of human COL2A1 in cultured chondrocytes. Luciferase assays on primary (a) or three-passage dedifferentiated (b) chondrocytes transiently transfected with different COL2A1 reporter constructs together with either hERα66 (wild type) or hERα46 (mutated isoform). Transcriptional activities of each construct were corrected for protein amounts. c Luciferase assays on primary chondrocytes transiently transfected with 10 μg of pGL2-0.387 kb reporter construct (covering the −266/+121-bp sequence of COL2A1) together with 2 μg of ERα siRNA (siERα). Results represent the mean ± SD of triplicate samples. d Primary RAC were incubated for 24 h with 17β-E2 (1 nM), mithramycin A (Mith., 100 nM) or both and type II collagen mRNA expression was assayed by RT-PCR as described under “Materials and methods.” Values are the means of two independent experiments performed in triplicate. CIIS, type II collagen silencers. Statistically significance was assessed by using the Student’s t test, and differences were set at *** p < 0.001, ** p < 0.01, and * p < 0.05
Then, in order to check whether ERα66 was involved in basal transregulation of type II collagen gene, we co-transfected the pGL2-0.387 kb COL2A1 reporter construct together with an ERα-specific siRNA. ERα66 silencing was accompanied by a 75 % down-regulation of COL2A1 transcription levels compared to control siRNA (Fig. 5c), demonstrating the central role played by ERα66 to mediate 17β-E2-induction of COL2A1 transcription in articular chondrocytes.
Additionally, since no ERE is present in the −266/−63-bp sequence of COL2A1 and since we have identified several GC boxes binding Sp1 and Sp3 in this region [5, 6], experiments were performed with mithramycin A, a binding inhibitor to GC-rich sequences. The objective of such an assay was to determine if hERα66 effect on COL2A1 could involve a protein/protein type of interaction with Sp1/Sp3, these latter trans factors binding to their proper cis-elements. The results showed that, not only incubation of primary RAC with mithramycin A strongly decreased basal expression of Col2a1 mRNAs but also prevented, at least in part, the 17β-E2-induced stimulation of type II collagen mRNAs expression (Fig. 5d). Thus, these data indicate that hERα66 has a transactivating effect on COL2A1, probably through an interaction with Sp factors binding to their cognate cis-elements.
17β-E2 increases the DNA-binding activity of Sp1 and Sp3 to the COL2A1 proximal promoter
As previously shown in Fig. 5a, b, 17β-E2 and its receptor hERα66 stimulate transcriptional activity of COL2A1 by the −266/−63-bp region. Six specific C-Krox/Sp1/Sp3-binding sites have been characterized in this promoter region (Fig. 1) [5, 6]. To determine the capacity of 17β-E2 to modulate Sp1/Sp3-binding sites activity of COL2A1, EMSAs were carried out with the −96/−66 and −225/−188 COL2A1-radiolabeled oligonucleotides which were already proven to contain both one GC-rich sequence (Fig. 6c). As shown in Fig. 6a, b, the binding activity of Sp1/Sp3 proteins involved in the formation of the different complexes was increased by 17β-E2 in a biphasic manner. Indeed, the intermediate concentrations 0.1 and 1 nM induced a significant increase in the Sp1/Sp3 binding activities to −96/−66 and −225/−188 COL2A1-radiolabeled probes, whereas lower or higher doses of the hormone induced a weaker effect. These data highlight the potential for 17β-E2 to increase transcriptional activity of COL2A1 by probably promoting the binding of Sp1 and, to a lesser extent, of Sp3 to COL2A1 promoter.
17β-E2 increases the DNA-binding activity of Sp1 and Sp3 to the COL2A1 promoter. a Electrophoretic mobility shift assays (EMSAs) were performed using the 5′ end-radiolabeled −96/−66 COL2A1 probe, incubated with nuclear extracts (NE) from primary RAC treated with increasing concentrations of 17β-E2 for 24 h. Negative controls lacking nuclear extracts are shown (probe). Sp1/Sp3 and Sp3 protein–DNA complexes are indicated by an arrow. b EMSAs were performed as described in a, using the 5′ end-radiolabeled −225/−188 COL2A1 probe. c Sequences of the −96/−66 and −225/−188 COL2A1-radiolabeled probes used. Sp1/Sp3 binding site is highlighted in bold characters
17β-E2 and its receptor hERα66 increase the DNA-binding activity of Sp1 and Sp3 to the COL2A1 first intron
First, to obtain additional information on the ability of 17β-E2 and hERα66 to modify Sp1/Sp3-binding sites activity of COL2A1, EMSAs were also performed with the +2,817/+2,846 COL2A1-radiolabeled oligonucleotide (Fig. 7c). This enhancer probe was already demonstrated to represent a high affinity binding site for Sp1/Sp3. Two retarded protein–DNA complexes were previously observed; a major one designated “Sp1/Sp3” and another minor involving Sp3 [5, 6]. As shown in Fig. 7a, the DNA-binding activity of Sp1 and Sp3 involved in the formation of the first complex was found to be increased by 17β-E2 in a dose-dependent manner in primary RAC. To a lesser extent, the specific binding activity of Sp3 was also increased by the hormone as reflected by the modulation of the second “Sp3 complex.” Moreover, a strictly similar pattern also applied for three-passage dedifferentiated chondrocytes (Fig. 7b).
Effect of 17β-E2, hERα66, and hERα46 on the DNA-binding activity of Sp1 and Sp3 to the COL2A1 enhancer. a Electrophoretic mobility shift assays (EMSAs) were performed using the 5′ end-radiolabeled +2,817/+2,846 COL2A1 probe, incubated with nuclear extracts (NE) from primary RAC transfected with either hERα66 or hERα46 expression vectors or treated with increasing amounts of 17β-E2 for 24 h. b Three-passage dedifferentiated RAC were incubated with increasing concentrations of 17β-E2 for 24 h, and resulting nuclear extracts were analyzed by EMSA as described in a. Negative controls lacking nuclear extracts are shown (probe). Sp1/Sp3 and Sp3 protein–DNA complexes are indicated by an arrow. Nuclear extracts from MCF-7 cell line were also used as control. c Sequence of the +2,817/+2,846 COL2A1-radiolabeled probe used. Sp1/Sp3 binding site is highlighted in bold characters
Secondly, the presence of ERα and its binding specificity in articular chondrocytes were validated by EMSA experiments using a consensus ERE or mutated ERE probes and RAC nuclear extracts incubated or not with antibody directed against ERα (Fig. 8). As shown in Fig. 8a, forced expression of hERα66 was effective, and its binding is specific as demonstrated by the addition of a molar excess of cold wild-type ERE probe that decreases the binding of hERα66. The specificity was also evidenced by using a mutated ERE probe in direct binding experiments leading to the prevention of hERα66 binding. Furthermore, the addition of a hERα66 antibody provoked a huge decrease in the hERα66/probe complex, indicating that this nuclear receptor is involved in the formation of such a complex. In the same sense, hERα66 binding is increased in dedifferentiated chondrocytes under 17β-E2 exposure and it was also specific (Fig. 8b).
a ERα66 binds specifically to an ERE consensus probe in primary articular chondrocytes. EMSA analysis was carried out with the nuclear extracts from primary RAC transfected with either 10 μg of the expression vector hERα66 or hERα46. Double-stranded radiolabeled wild-type ERE consensus or mutant ERE probes were incubated with nuclear extracts (10 μg) in the presence or absence of ERα antibody (4 μg) or molar excesses (×100) of wild-type nonlabeled ERE probe (cold probe). Negative controls lacking nuclear extracts are shown (probe). ERα-DNA complexes are indicated by an arrow. Nuclear extracts (NE) from MCF-7 cell line (positive cells for estrogen receptors hERα) were also used as control. b 17β-E2 increases ERα DNA-binding to an ERE consensus probe in dedifferentiated chondrocytes. Three-passage dedifferentiated RAC were incubated with 17β-E2 for 24 h at concentrations ranging from 0 to 10 nM, and resulting nuclear extracts were analyzed by EMSA as described in a
Altogether, these EMSA assays indicate that 17β-E2 and hERα66 enhance transcription of COL2A1 by increasing the binding activity of Sp1 and to a lesser extent of Sp3, not only to COL2A1 promoter but also to COL2A1-specific enhancer.
17β-E2 increases the binding activity of the Sox proteins to the COL2A1-specific enhancer
Since IGF-I stimulatory effects on COL2A1 transcription have been already shown by our laboratory to be mediated by an increase in DNA-binding activity of Sox-9/Sox-6/L-Sox-5 to their functional cis-elements in the enhancer of the gene [17], EMSA analyses were performed using the +2,353/+2,415 bp (four “high mobility group” (HMG) binding sites) or +2,383/+2,415 bp (Sox-9 cis-elements and one HMG binding site only) probes (Fig. 9c). As shown in Fig. 9a, b, the binding activity of Sox-9/Sox-6/L-Sox-5 involved in the formation of the different complexes was found to be increased by 17β-E2 in primary articular chondrocytes as well as in dedifferentiated chondrocytes. By contrast to transient transfection data obtained with reporter construct pGL2-3.316 kb, these last results suggest that Sox-9/Sox-6/L-Sox-5, as well as the COL2A1 enhancer region, might also participate in the anabolic effect of 17β-E2 on COL2A1 in primary and dedifferentiated chondrocytes since the hormone significantly increases their DNA-binding activity to this region.
17β-E2 enhances the binding activity of the Sox proteins to the +2,353/+2,415 and +2,383/+2,415 sequences of the COL2A1 enhancer. a, b EMSA analysis was carried out with the 5′ end-radiolabeled +2,353/+2,415 (four Sox DNA-binding sites) or +2,383/+2,415 (two Sox DNA-binding sites) COL2A1 double-stranded probes incubated with nuclear extracts from primary (a) or dedifferentiated (b) articular chondrocytes treated with increasing amounts of 17β-E2 for 24 h. Protein-DNA complexes are indicated by an arrow. c Sequences of the +2,353/+2,415 and +2,383/+2,415 COL2A1-radiolabeled probes used. HMG binding sites are highlighted in bold characters
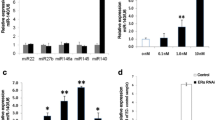
Sp1, Sp3, or Sox-9 siRNAs prevent hERα66-induced stimulation of Col2a1 gene expression
To finally demonstrate that Sp1, Sp3, and Sox-9 proteins are required for hERα66-induced up-regulation of type II collagen expression, we knocked down their expression by siRNA experiments. First, hERα66 produced a ∼3-fold increase in type II collagen mRNAs steady-state levels under control and 17β-E2-treated conditions (Fig. 10a, b). Next, we verified the siRNA efficiency to silence Sp1, Sp3, and Sox-9 expression by RT-PCR analysis (Supplemental Fig. 1A). As expected, cell transfection with Sp1 siRNA produced a decrease of about 65 % of Sp1 mRNA levels in the control and 17β-E2-treated samples. In parallel, endogenous Sp3 and Sox-9 silencing was accompanied by an important down-regulation of Sp3 and Sox-9 mRNAs levels (about 75 % in presence of 17β-E2 and 90 % in its absence). Then, the effect of Sp1, Sp3, and Sox-9 silencing on hERα66-induced activation of Col2a1 expression was evaluated (Fig. 10a, b). Our data demonstrate that Sp1, Sp3, and Sox-9 siRNAs inhibit hER66-induced stimulation of type II collagen mRNA steady-state levels, in the presence or not of 17β-E2, providing further evidence that these factors play a central role to mediate hERα66 stimulation of COL2A1 transcription in articular chondrocytes. Additionally, the efficiency of hERα66 overexpression in RAC was effective (Supplemental Fig. 1B), in agreement with previous experiments (Fig. 4c).
Sp1, Sp3, and Sox-9 siRNAs prevent the hERα66-induced stimulation of type II collagen mRNA expression. Primary RAC were transfected with 2 μg of siRNAs directed against Sp1, Sp3, or Sox-9 (respectively, siSp1, siSp3, and siSox-9) in combination with 5 μg of hERα66 expression vector. After an overnight transfection period, the cells were incubated for 24 h in the presence (b) or absence (a) of 17β-E2. At the end of the experiment, RT-PCR analysis of Col2a1 mRNA expression was performed, and expression of type II collagen transcripts was normalized to GAPDH mRNA. Results are representative of two independent experiments and each represents the mean ± SD of triplicate samples. Statistically significant differences relative to the controls pCR3.1 plasmid (denoted by asterisk) or to hER66 expression vector (denoted by number sign) were obtained by the Student’s t test (*** p < 0.001, ** p < 0.01, ### p < 0.001, and ## p < 0.01)
Sp1 interacts with ERα66 and Sp3 in primary articular chondrocytes
Since ERα66 appears as a key regulatory factor in the modulation of DNA-binding activity of Sp trans factors to COL2A1, we therefore investigated the potential protein–protein interactions between these factors by immunoprecipitation (IP) experiments using an anti-Sp1 antibody. First, ERα66 was found to physically interact with Sp1, independently of the presence of 17β-E2 (Fig. 11a). Furthermore, Sp1 was also co-immunoprecipitated with Sp3 (Fig. 11b (i), b(ii)) in the same conditions. Western blots were carried out with an anti-Sp1 antibody and a negative IgG, as positive and negative controls of the assay, respectively (Fig. 11c, d). Additionally, nuclear extracts bound to pre-cleared protein A-Sepharose beads, protein A-Sepharose beads incubated with an anti-Sp1 antibody in absence of nuclear extracts, and finally protein A-Sepharose beads incubated with nuclear extracts in absence of anti-Sp1 antibody were also loaded on SDS-PAGE gel as a supplementary control of antibody specificity (Fig. 11b (ii)). All together, these data strongly support the hypothesis that ERα66 can regulate type II collagen expression by physically interacting with a heteromeric Sp1/Sp3 complex and therefore modulating Sp1/Sp3 DNA-binding activity to COL2A1.
ERα66 and Sp3 transcription factors interact with Sp1 in the nucleus of primary articular chondrocytes. Nuclear extracts from primary chondrocytes cultured in presence or absence of 17β-E2 for 24 h were submitted to immunoprecipitation (IP) procedure with anti-Sp1 antibody (IP Sp1). Immunoprecipitated complexes were incubated 2 h with protein A-Sepharose beads and then submitted to Western blotting (W.B.) in order to detect ERα (a) and Sp3 (b(i)) proteins. Twenty micrograms of nuclear extracts, which were not submitted to IP (W.B. w/o IP), was also loaded onto SDS-PAGE gels. b(ii) As a supplementary control of the specificity, a Western blotting experiment using an anti-Sp3 antibody was performed on nuclear extracts bound to pre-cleared protein A-Sepharose beads, on protein A-Sepharose beads incubated with an anti-Sp1 antibody in absence of nuclear extracts and finally, and on protein A-Sepharose beads incubated with nuclear extracts in absence of anti-Sp1 antibody. Positive and negative controls of the experiment were realized by immunoprecipitating Sp1 and submitting immunoprecipitated complexes to Western blotting using an anti-Sp1 antibody (c, positive control) and IgG (d, negative control)
ERα66, Sp1, Sp3, and Sox-9 proteins interact and bind to Col2a1 promoter in vivo
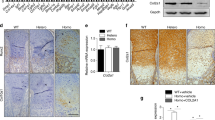
Computer sequence analysis of COL2A1 has revealed that the promoter region did not contain ERE binding sites (data not shown). Therefore, in order to confirm our in vitro data and to determine if ERα66 was effectively implicated in the regulation of COL2A1 by 17β-E2, we further clarified ERα66 involvement by ChIP assays. Experimental results demonstrated for the first time that, despite the absence of ERE binding sites, ERα66 can be recruited in vivo onto the Col2a1 short promoter in primary and dedifferentiated articular chondrocytes (Fig. 12a, b). Similar amounts of ERα66 seem to be recruited onto the promoter both in control and 17β-E2-treated conditions. As expected, Sp1 and Sp3 factors also bind to Col2a1 promoter, confirming our previous work [8]. Moreover, although type II collagen gene promoter does not contain HMG-like binding sites, we demonstrated that Sox-9 was able to bind indirectly to Col2a1 promoter in primary RAC, via protein–protein interactions with Sp1/Sp3 (this study and reference [17]) and/or changes in spatial conformation/folding between the intronic enhancer and the promoter of the gene (Fig. 13). The PCR primers used in ChIP assays map the −119/+52-bp region of COL2A1 promoter, including three specific Sp cis-elements (−119/−112, −81/−74, and −41/−33 bp).
ERα66, Sp1, Sp3, Sox-9, and p300 interact in vivo on the Col2a1 promoter and enhancer. Chromatin immunoprecipitation (ChIP) assays were performed on primary (a, c) or three-passage dedifferentiated (b) rabbit articular chondrocytes (RAC) incubated in the presence or absence of 1 nM 17β-E2 for 24 h, according to the manufacturer’s instructions. Resulting DNA was submitted to PCR analysis (40 cycles) using specific primers flanking the −119/+52-bp Col2a1 region (a, b) or the +2,383/+2,611-bp Col2a1 region (c). PCR products were separated on a 2.5 % agarose gel and visualized by UV illumination following ethidium bromide staining. As positive control, the same primers were used on the genomic DNA (Input) and DNA samples immunoprecipitated with anti-RNA polymerase II antibody (RNA pol. II). Nonspecific immunoglobulin antibody (IgG) was used as negative control. d, e In order to perform Re-ChIP experiments, the sheared chromatin isolated from primary or dedifferentiated chondrocytes was first immunoprecipitated with 6 μg of antibody directed against ERα, followed by a second IP using antibodies raised against Sp1, Sp3, and Sox-9 (d) or p300 (e). The released DNA, treated with proteinase K, was amplified by PCR (35 cycles) using specific primers flanking −119/+52 bp Col2a1 region or +2,383/+2,611 bp Col2a1 region and visualized as described above. IP, immunoprecipitation; C, control
Schematic representation of 17β-E2 and hERα66 genomic pathway involved in the up-regulation of type II collagen gene in articular chondrocytes. COL2A1 does not contain ERE binding sites, so that hERα66 interacts with COL2A1 promoter in a nonclassical genomic pathway involving protein–protein interactions with transcription factors Sp1, Sp3, and Sox-9. Some co-activators such as p300 are also involved in the transactivation process of COL2A1
To further demonstrate if an interaction between ERα66 and these three trans factors could be effective on Col2a1 promoter, Re-ChIP assays were performed using an anti-ERα antibody. As shown in Fig. 12d, Sp1, Sp3, and Sox-9 were always recruited onto the Col2a1 promoter after specific protein interaction with ERα66 receptor. When the dedifferentiated chondrocytes are incubated in the presence of 17β-E2, although Sp1 was still bound to the Col2a1 promoter in the same amounts, it seems that lower amounts of Sp3 and Sox-9 interact with this sequence compared to the control conditions. These novel findings strongly suggest that an ERα66/Sp1/Sp3/Sox-9 heteromeric complex formation could be necessary to mediate the stimulatory effect of 17β-E2 on type II collagen gene expression (Fig. 13).
ERα66, Sp1, Sp3, and Sox-9 proteins interact and bind to Col2a1 first intron in vivo
As previously suggested in EMSA experiments carried out with COL2A1 first intron probes (Figs. 7 and 9), a multimeric complex composed of ERα66/Sp1/Sp3/Sox-9/L-Sox-5 and Sox-6 transcription factors could interact on the COL2A1 enhancer region to mediate 17β-E2-induced COL2A1 transactivation. Therefore, in order to validate our in vitro hypothesis and to determine whether these transcription factors were effectively able to bind in vivo to Col2a1-specific enhancer, we performed ChIP assays in primary chondrocytes using primers mapping the +2,383/+2,611-bp region of COL2A1 first intron. This region includes a Sox-9 cis-element (+2,384/+2,404 bp), one HMG binding site (+2,388/+2,394 bp), and one Sp1/Sp3 binding sequence (+2,440/+2,485 bp) but does not harbor ERE cis-sequence. After PCR amplification, a 229-bp fragment was observed in samples that were immunoprecipitated with anti-Sp1, anti-Sp3, and anti-Sox-9 antibodies, demonstrating that these factors are recruited on endogenous +2,383/+2,611 bp region of Col2a1 (Fig. 12c). Furthermore, we demonstrated, for the first time, that despite the absence of ERE cis-sequences, ERα66 is also recruited in vivo onto the Col2a1 enhancer in articular chondrocytes.
As COL2A1 does not contain any ERE region, ERα66 necessarily interacts with the +2,383/+2,611-bp Col2a1 enhancer in an indirect way. To identify what protein partners could be involved in the ERα66 recruitment, we performed Re-ChIP experiments using an anti-ERα antibody. Our data showed that ERα66 was bound indirectly to Col2a1 enhancer through protein–protein interactions with Sp1, Sp3, and Sox-9 transcription factors in primary as well as dedifferentiated articular chondrocytes (Fig. 12d). As expected, and since COL2A1 enhancer region does not represent a binding site for RNA polymerase II, even though some molecules of RNA polymerase II are transcripting the gene, ERα66 does not interact with this enzyme. These results suggest that ERα66 acts in concert with Sp1, Sp3, and Sox-9 transcription factors to bind the +2,383/+2,611-bp region of Col2a1 enhancer in vivo.
p300 is also involved in the regulation of Col2a1 transcription by ERα66
Presumably, the ERα66 binding to Sp and Sox proteins induces the recruitment of co-regulatory proteins required for transactivation. Nevertheless, no identified hERα66 co-regulatory proteins have been characterized in articular chondrocytes. To further investigate whether hERα66 co-activators can occupy the same Col2a1 promoter/enhancer together as part of a complex, we performed a sequential chromatin immunoprecipitation (Re-ChIP) assay using primers mapping the −119/+52-bp COL2A1 promoter and +2,383/+2,611-bp enhancer regions. Chromatin from both primary and dedifferentiated RAC was first immunoprecipitated with an anti-ERα antibody and then with a second antibody directed against p300. Our results clearly indicate that ERα66 and p300 are present as a complex on the Col2a1 promoter and enhancer (Fig. 12e). In addition, it seems that 17β-E2 increases the protein interactions between ERα66 and p300 co-activator on both Col2a1 promoter and enhancer, supporting the stimulatory role of this hormone on COL2A1 transcription.
Taken together, these results support a pivotal role for Sp1, Sp3, and Sox-9 proteins in indirect ERα66 recruitment on Col2a1 and strongly suggest that up-regulation of type II collagen by 17β-E2/ERα66 complex is mediated by these transcription factors which interact with euchromatin-associated co-factors such as p300 on both enhancer and promoter regions of the gene.
Discussion
In the degenerative pathologies of cartilage such as OA, alteration of type II collagen expression is a critical step leading to the loss of differentiated articular chondrocyte phenotype. From epidemiological and biochemical studies, there is already a substantial evidence for a role of estrogens in cartilage matrix homeostasis. Nevertheless, these studies still do not identify molecular mechanisms by which articular chondrocytes or synoviocytes might be directly impacted by low levels of 17β-E2.
The main novel findings of the present study were that ERα66-induced activation of COL2A1 transcription is mediated by Sp1/Sp3 together with Sox-9 and euchromatin-associated factors such as p300 and that these factors are recruited in vivo both to the first intron-specific enhancer and to the promoter region, the two major regulatory regions of this gene. We particularly investigated and compared the effects mediated by 17β-E2 and its receptor ERα66 on type II collagen expression in primary and dedifferentiated articular chondrocytes. In view of the contradictory reports concerning the action of the hormone on the ECM components, we first focused on the biological effect of 17β-E2 on type II collagen production in chondrocytes. Our results demonstrate that 17β-E2 stimulates type II collagen neosynthesis and Col2a1 mRNAs steady-state levels in primary RAC, suggesting that 17β-E2 acts at the transcriptional level through a genomic pathway mediated by ERα66. Furthermore, because of a possible autocrine TGF-β stimulation in mediating 17β-E2 activity on type II collagen, we have evaluated the dose–response effect of 17β-E2 on the gene expression of TGF-β1 and TGF-β type II receptor (TβRII), known as some of the major components of the TGF-β system which play key roles in the physiopathology of articular chondrocytes. 17β-E2 at concentrations ranging from 0.01 to 5 nM is not able to modulate the steady-state amounts of the transcripts encoding TGF-β1 and TβRII (data not shown), whereas this estrogen enhances the Col2a1 mRNA amounts between 1 and 5 nM. These results suggest that even though 17β-E2 was demonstrated in other cell models to induce TGF-β expression that could in turn modulate target genes expression, the observed induction of type II collagen in our study does not seem to be mediated through autocrine stimulation of TGF-β in articular chondrocytes.
In a second time, because of a putative 17β-E2 genomic action mediated by ERα66, we focused on the transcriptional action of this receptor on COL2A1 regulation. The present data revealed that hERα66-induced stimulation of COL2A1 activity is mediated by the −266/−63-bp promoter region. From a transcriptional point of view, nucleotide sequence analysis of this promoter region revealed six C-Krox/Sp1/Sp3-binding sites [5, 6]; in particular, two of them were specific for Sp proteins (−119/−112 and −81/−74 bp). Our particular interest for hERα66 function in articular chondrocytes comes from the fact that this receptor has been previously described as a partner of Sp1, a key transactivator of COL2A1, in several different cell lines [30, 31]. Indeed, the interaction of hERα66 with other transcription factors such as AP-1, NF-κB, and Sp1 which, in turn, bind their cognate DNA elements, modulates gene expression and transcription factor activity by stabilizing DNA–protein complexes and/or recruiting co-activators [31]. Our data established that hERα66 interacts physically with Sp1 in chondrocytes. This is in agreement with the observations of Porter et al. [32] who showed that the major site of interaction of hERα66 with Sp1 protein is associated with the C-terminal DNA-binding domain of Sp1, which is a region within Sp1 that can also bind a multitude of other transcription factors, including steroid hormone receptors such as progesterone or androgen receptors [33, 34]. In addition, hERα66/Sp1 complex can activate transcription through a consensus GC-rich Sp1 binding site in transient transfection studies in MCF-7 human breast cancer cells, and this response is also observed with hERα66 variants lacking the DNA-binding domain [35].
In our cellular model, we demonstrated that ERα66 increases the DNA-binding activity of Sp1 and, to a lesser extent, of Sp3 to the COL2A1 promoter and enhancer, in both differentiated and dedifferentiated chondrocytes. Interestingly, in primary RAC, we also found that 17β-E2 increases significantly Sp1 protein levels in a biphasic manner without affecting Sp3 protein expression, except for a 10 nM concentration (Supplemental Fig. 2). Similar results were observed in dedifferentiated RAC for Sp1 protein levels, whereas protein expression of long (110 kDa) and short (70 kDa) Sp3 isoforms is significantly reduced, in a dose-dependent manner, following 17β-E2 treatment. These results indicate therefore that ERα66 increases the Sp1/Sp3 ratio, which is in accordance with the positive effect of 17β-E2 on type II collagen expression levels at low- or medium-range concentrations. For high-concentration treatment, it is possible that the strong inhibition of Sp1 expression by 17β-E2, while Sp3 expression is more slightly reduced, may lead to a reduction in the Sp1/Sp3 ratio and therefore to a suppression of the stimulatory effect on type II collagen expression. This “dose-related effect” may be due in part to the ability of 17β-E2 to modulate differentially Sp1 and Sp3 protein levels in articular chondrocytes. Indeed, Sp1/Sp3 modulation has already been shown as a critical parameter for the regulation of COL2A1 transcription: for instance, a decrease of Sp1/Sp3 ratio is required to mediate IL-1β, interleukin-6, and TGF-β1 inhibition of COL2A1 transcription in RAC [5, 8, 28, 36]. So far, interactions of ERα66 with other Sp proteins different from Sp1 have not been well characterized; however, ERα66 interacts with multiple domains of Sp3. Interestingly, the interacting domains of both proteins are different from those previously observed for interactions between Sp1 and hERα protein [37]. Thus, the ability of ERα66 to differentially modulate DNA-binding activity of these Sp proteins could be explained, in part, by the capacity of ERα66 to bind different domains of Sp proteins. Furthermore, the present work, together with previous results from our laboratory [8], established that Sp1 and Sp3 proteins interact each other in chondrocytes. Taken together, these data strongly suggest that a heteromeric Sp1/Sp3/ERα66 complex could mediate the transactivating effect of 17β-E2 on COL2A1 promoter. These results are in agreement with those obtained by Schultz et al. [38] who reported that hERα66 confers estrogen responsiveness to the progesterone gene by enhancing Sp1 interaction with a “GC box” in the −80/−34-bp region of the human progesterone gene.
By ChIP assays, we showed for the first time that ERα66 indirectly binds to −119/+52 bp sequence of the Col2a1 promoter in primary or dedifferentiated chondrocytes. Higgins et al. [39] showed that hERα66 is constitutively associated with 17β-E2-responsive GC-rich promoter elements that also bind Sp1, Sp3, and Sp4 factors. We demonstrated here the DNA-binding of ERα66 in combination with Sp1 and Sp3 factors to the −119/+52-bp promoter region of COL2A1, which includes three specific Sp cis-elements (−119/−112, −81/−74, and −41/−33 bp). Thus, these ChIP data combined with transient transfection experiments of COL2A1 reporter vectors indicate that the two upstream Sp binding sites mediate the hERα66 transactivating effect in both primary and dedifferentiated chondrocytes. Besides, despite the absence of HMG-like binding sites in type II collagen gene promoter, Sox-9 also binds indirectly to the −119/+52-bp Col2a1 region in primary RAC (this study and reference [17]). This transcription factor was demonstrated to be an activator of COL2A1 transcription [40]. Sox9 is co-expressed with type II collagen during chondrogenesis in the mouse and in cultured chondrocytes and has been shown to be absolutely essential for early events in chondrocyte differentiation [41] and for expression of the cartilage-specific matrix genes in human articular chondrocytes [42]. In addition, it was recently demonstrated through the development and use of mice misexpressing Sox-9 specifically in hypertrophic chondrocytes that Sox-9 is an important negative regulator of cartilage vascularization and endochondral ossification [43].
Concerning the enhancer region, we reported for the first time an indirect interaction between ERα66 and the +2,383/+2,611 Col2a1 sequence in both primary and dedifferentiated chondrocytes. Our EMSA analysis clearly indicated that 17β-E2 increased Sox-9, Sox-6, and L-Sox-5 DNA-binding to their cis-elements in the first intron of COL2A1, strongly suggesting that this region was involved in the up-regulation of COL2A1 by the hormone. Moreover, Re-ChIP experiments demonstrated that ERα66/Sp1, ERα66/Sp3, and ERα66/Sox-9 complexes were all recruited onto the Col2a1 promoter and enhancer in the presence or the absence of 17β-E2. In dedifferentiated RAC, Sp3 recruitment to the Col2a1 gene seemed to be decreased after interaction with ERα66 following exposure to the hormone. This modulation could be a way by which ERα66 may finely regulate the DNA-binding activity of the well-known Sp1/Sp3 heteromer and favor Sp1 recruitment to the detriment of Sp3 leading to the transactivation of COL2A1. Finally, from our siRNA experiments, we deduced that inhibition of Sp1, Sp3, or Sox-9 expression was sufficient to prevent hERα66-induced stimulation of Col2a1 mRNA levels. Interestingly, some of our previous experiments revealed that Sp1 is an activator of Sox9 DNA-binding activity and Sox9 gene transcription in chondrocytes, but reciprocal combination is also effective, i.e., Sox9 increases the transcription of Sp1 gene [17]. Therefore, considering that transcription factors are known to act in concert, it is conceivable that 17β-E2 in vivo effect could be mediated by a bridging complex, composed of ERα66, Sp1, Sp3, Sox-9, and certainly of several other transcription factors, which could establish a physical link between the two main transregulatory regions of the gene, i.e., the proximal promoter and the first intron, and consequently, may facilitate chromatin decondensation and its accessibility to the transcriptional machinery in order to initiate transcription process (Fig. 13). Indeed, a relationship between COL2A1 promoter and first intron is required for type II collagen expression in cartilage during chondrogenesis [44, 45]. It has been reported that the expression of a human COL2A1 transgene in a mouse model is governed by a 309-bp enhancer intronic fragment (+2,388 to +2,696 bp) and requires the presence of a 90-bp promoter region for tissue-specific expression in the developing cartilaginous skeleton [45]. Hence, stimulation of the synthesis and/or binding activity by 17β-E2 of one of the transcription factors involved in this main molecular complex may facilitate the formation of a “loop structure” and would increase type II collagen gene expression in RAC.
Despite their different transcriptional functions, Sp1 and Sp3 generally compete for the same DNA-binding sites. They form homotypic and heterotypic complexes with numerous transcriptional co-regulators which determine the role of Sp proteins as either transcriptional activators or repressors. Presumably, ERα66 binding to Sp proteins induces recruitment of co-regulatory proteins required for the transactivation. Thus, the mechanism proposed to explain 17β-E2 transactivating effect in chondrocytes is related to the interaction of its receptor hERα66 with co-activators, such as steroid receptor co-activators (SRC), CREB binding protein (CBP), or p300. To date, no identified hERα66 co-regulatory proteins have been characterized in articular chondrocytes. Nevertheless, our Re-ChIP experiments performed on Col2a1 promoter and enhancer raised the hypothesis that p300 is the major co-activator of ERα66 in both primary and dedifferentiated RAC. ERα66-p300 interactions on Col2a1 are significantly increased following 17β-E2 stimulation, which may contribute to COL2A1 transactivation. Indeed, p300 acts as a bridging factor to form multicomponent complexes and connects DNA-binding transcription factors to the transcription machinery. Furthermore, p300 is known to interact directly with the AF-2 domain and can potentiate the synergistic activity that results from AF-1 and AF-2 interaction by facilitating binding of TATA binding protein (TBP) on estrogen target promoters [46–48]. Otherwise, p300/CBP also presents an intrinsic histone acetyltranferase activity and facilitates the action of associated transcription factors by modulating histone acetylation. For instance, p300 amplifies stimulatory effects of Sox-9 during chondrogenesis. Additionally, p300 physically binds to C-terminal activation domain of Sox-9 and acts in synergy with this one to activate COL2A1 transcription by connecting its specific enhancer sequences with the transcription initiation complex [17, 49, 50]. Thus, we may postulate without ambiguity that ERα66 acts as a co-activator/linker in a transcription factor crosstalk between Sp/Sox proteins and other co-activators such as p300 or SRC-3 in articular chondrocytes.
As demonstrated by using the AF-1 dominant negative action of hERα46, the transcriptional activation of human COL2A1 by hERα66 requires the AF-1 transactivation domain of the receptor. Moreover, preliminary studies from our laboratory using hERα66 mutants harboring progressive deletions of A/B domain indicate that the entire AF-1 domain (i.e., Box1, Box2, and Box3) is required for hERα66-induced type II collagen mRNA stimulation (Supplemental Figs. 3A and 3B). Corroborating these results, several studies clearly demonstrated the importance of AF-1 activity in the estrogenic induction of genes, as well as the requirement of AF-1 region for full hERα66 activity [37, 39]. For example, the progressive deletion of the AF-1 domain of hERα66 leads to a corresponding decrease in the activity of the MMP-13 promoter [51]. The AF-1 domain is well characterized as the ligand-independent transactivation domain of the hERα66 and synergizes with the AF-2 domain to elicit the overall estrogen response on promoters. Nevertheless, recent data from Billon-Gales et al. [52] reported that AF-1 domain of hERα66 is completely dispensable for mediating the major atheroprotective effects of estrogens using a mouse model missing the hERα66 A/B domains. This discrepancy between our results and this last study could be in part explained by the fact that the respective contribution of AF-1 and AF-2 to the transcriptional activity of the full-length hERα66 varies in a promoter and cell type-specific manner [53, 54]. As our results failed to show a stimulatory transactivity when hERα46 expression vector is used, we can postulate that the AF-1 domain of hERα66 probably plays the major positive regulatory role in modulating the activity of COL2A1 promoter/enhancer.
In order to better understand the exact role of hERα46 isoform, additional experiments were performed, where primary RAC were co-transfected with hERα66 expression vector in combination with increasing amounts of hERα46 expression vector. These data show for the first time that hERα46 has the ability to repress hERα66-induced Col2a1 and Sp1 gene expression in a dose-dependent manner in primary articular chondrocytes (Supplemental Fig. 4). In vivo, both isoforms are frequently co-expressed. In this situation, hERα46 efficiently represses the transcription of estradiol-target genes induced by hERα66 by suppressing AF-1 activity of full-length estrogen receptor [25]. In terms of transcription, we can postulate that hERα46 competes with hERα66 for its binding to Sp1 and Soxs proteins on both the promoter and the first intron of Col2a1 gene and prevents hERα66 from binding to these factors and exerting its stimulatory effect on Col2a1 transcription. However, since we observed a faint but significant stimulatory effect of hERα46 on type II collagen synthesis in collagenase and Western blot assays, a weaker positive regulatory role can likely be assigned to AF-2 domain too. Additionally, our data reveal that the deletion of any of the functional domains of hERα66 suppresses the positive regulatory effect of this receptor on Col2a1 transcription, indicating that all of these functional domains are indispensable in hERα66 action (Supplemental Fig. 3). In addition, as the transfection of hERα-deleted isoforms also decreases Sp1 mRNA levels, this confirms that the receptor involves Sp1 in its regulation of Col2a1 gene. Altogether, our results demonstrate that articular chondrocytes are mainly sensitive to the AF-1 function of hERα66, however, with low permissiveness to AF-2.
Another novel and relevant finding in our study is that 17β-E2 as well as hERα66 are able to increase and therefore reactivate type II procollagen expression in dedifferentiated RAC through a transcriptional control of type II collagen by increasing the DNA-binding activities of Sp1 and Sox9 to their cis-responsive elements in COL2A1. In fact, we previously demonstrated that these two transcription activators are able to allow recovering, at least in part, of the differentiated phenotype in dedifferentiated cells [5, 40]. For example, Sp1 is an activator of COL2A1 transcription in differentiated and dedifferentiated chondrocytes, mediating at least the partial recovery of chondrocyte phenotype, once it has been lost [6]. Besides, our previous study showed that 17β-E2 and hERα66 stimulate UDP-glucose dehydrogenase gene activity in primary rabbit articular chondrocytes [27]. Consequently, 17β-E2 would be not only able to reactivate type II collagen expression in dedifferentiated chondrocytes but could also elicit a cellular redifferentiation through an increased glycosaminoglycans synthesis in these cells. As stem cells used for cartilage repair seem to have a similar fate as chondrocytes that drive endochondral ossification, making the repair tissue inadequate for normal joint function, our data are therefore attractive in view of the estrogens use in articular cartilage engineering.
Preliminary studies from our laboratory indicate that changes within the respective levels of expression of hERα isoforms are likely to occur during chondrocyte dedifferentiation process (data not shown), as it already occurs in MCF-7 cell line where hERα66/hERα46 ratios change with the cell growth status [25]. Recent works indicate that differences in the expression of hERα isoforms can influence gene regulation and cell growth and/or apoptosis [51, 55]. Altered expression of hERα66/hERα46 may account for differential effects of 17β-E2 on COL2A1 expression and could explain the differential sensitivity of type II collagen protein to 17β-E2 in dedifferentiated chondrocytes where lower doses of 17β-E2 activate type II collagen protein production compared to primary RAC. This differential effect could also be explained by the fact that limitative quantities Sp1 and Sox-9 are present in dedifferentiated RAC, and therefore, high doses of 17β-E2 fail to mobilize supplementary quantities of such transcription factors to activate transcription more. Besides, Webb et al. [56] have demonstrated that the more differentiated a cell is, the more likely it will use the AF-1 domain of hERα66 to mediate estrogen responses, and dedifferentiated cells tend to depend more on AF-2 domain of hERα66. Nevertheless, from a transcriptional point of view, we observed similar results of hERα66 or hERα46 on COL2A1 transcriptional activity between primary and dedifferentiated chondrocytes. This is why further studies are now required to clarify the potential changes in hERα66/hERα46 ratio during chondrocyte dedifferentiation and OA process.
Our IP and Re-ChIP data showed that unliganded hERα66 is associated with Sp protein bound to Col2a1 and addition of 17β-E2 does not significantly alter the ERα66 promoter interactions. Previous studies have confirmed that hERα66 could interact with Sp proteins in the absence of ligand [32, 37]. Nevertheless, estrogens can be synthesized locally from androgens via aromatase [57] suggesting that the intracrine pathway contributes, beside the known endocrine one, to the estrogens production in articular chondrocytes. This hypothesis is supported by a study of Richette et al. [58] who showed that 17β-E2 is present in human synovial fluid from OA joints, at concentrations closely reflecting serum levels. We may postulate that estrogens produced by chondrocytes or synovial tissues, acting both as autocrine or paracrine regulators of ECM components synthesis, could have a profound effect on the development of OA, so that the increased incidence of OA in postmenopausal women might thus be explained by a decrease in local estrogen concentrations because 17β-E2 has been shown to have anti-erosion properties at physiological levels.
In summary, our data further corroborate that 17β-E2 and its receptor ERα66 are critical actors in COL2A1 transcriptional regulation, making them motivating targets in order to better understand the OA dedifferentiation process and facilitate the recovery of differentiated articular cartilage in osteoarthritic chondrocytes. Such insights are needed not only to implement our basic understanding of articular cartilage biology but also to help developing novel and improved strategies for cartilage repair and treatments for degenerative diseases such as OA. From this point of view, the use of this hormone and/or its nuclear receptor in the tissue engineering of articular cartilage could be a promising strategy in order to repair this tissue.
References
Goldwasser M, Astley T, Van der Rest M, Glorieux FH (1982) Analysis of the type of collagen present in osteoarthritic human cartilage. Clin Orthop Relat Res 167:296–302
Von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, Stoss H (1992) Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum 35:806–811
Ala-Kokko L, Kvist AP, Metsaranta M, Kivirikko KI, De Crombrugghe B, Prockop DJ, Vuorio E (1995) Conservation of the sizes of 53 introns and over 100 intronic sequences for the binding of common transcription factors in the human and mouse genes for type II procollagen (COL2A1). Biochem J 308(Pt 3):923–929
Ryan MC, Sieraski M, Sandell LJ (1990) The human type II procollagen gene: identification of an additional protein-coding domain and location of potential regulatory sequences in the promoter and first intron. Genomics 8:41–48
Ghayor C, Herrouin JF, Chadjichristos C, Ala-Kokko L, Takigawa M, Pujol JP, Galera P (2000) Regulation of human COL2A1 gene expression in chondrocytes. Identification of C-Krox-responsive elements and modulation by phenotype alteration. J Biol Chem 275:27421–27438
Ghayor C, Chadjichristos C, Herrouin JF, Ala-Kokko L, Suske G, Pujol JP, Galera P (2001) Sp3 represses the Sp1-mediated transactivation of the human COL2A1 gene in primary and de-differentiated chondrocytes. J Biol Chem 276:36881–36895
Lefebvre V, Li P, De Crombrugghe B (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J 17:5718–5733
Poree B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ, Mallein-Gerin F et al (2008) Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem 283:4850–4865
Bay-Jensen AC, Tabassi NC, Sondergaard LV, Andersen TL, Dagnaes-Hansen F, Garnero P, Kassem M, Delaisse JM (2009) The response to oestrogen deprivation of the cartilage collagen degradation marker, CTX-II, is unique compared with other markers of collagen turnover. Arthritis Res Ther 11:R9
Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C (2003) Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis 62:332–336
Sandmark H, Hogstedt C, Lewold S, Vingard E (1999) Osteoarthrosis of the knee in men and women in association with overweight, smoking, and hormone therapy. Ann Rheum Dis 58:151–155
Von Muhlen D, Morton D, Von Muhlen CA, Barrett-Connor E (2002) Postmenopausal estrogen and increased risk of clinical osteoarthritis at the hip, hand, and knee in older women. J Womens Health Gend Based Med 11:511–518
Le Bail J, Liagre B, Vergne P, Bertin P, Beneytout J, Habrioux G (2001) Aromatase in synovial cells from postmenopausal women. Steroids 66:749–757
Ham KD, Loeser RF, Lindgren BR, Carlson CS (2002) Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum 46:1956–1964
Fernihough JK, Richmond RS, Carlson CS, Cherpes T, Holly JM, Loeser RF (1999) Estrogen replacement therapy modulation of the insulin-like growth factor system in monkey knee joints. Arthritis Rheum 42:2103–2111
Yaeger PC, Masi TL, De Ortiz JL, Binette F, Tubo R, McPherson JM (1997) Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res 237:318–325
Renard E, Poree B, Chadjichristos C, Kypriotou M, Maneix L, Bigot N, Legendre F, Ollitrault D, De Crombrugghe B, Mallein-Gerin F et al (2012) Sox9/Sox6 and Sp1 are involved in the insulin-like growth factor-I-mediated upregulation of human type II collagen gene expression in articular chondrocytes. J Mol Med (Berlin, Germany) 90:649–666
Saggese G, Federico G, Cinquanta L (1993) In vitro effects of growth hormone and other hormones on chondrocytes and osteoblast-like cells. Acta Paediatr (Oslo, Norway) 82(Suppl 391):54–59
Dayani N, Corvol MT, Robel P, Eychenne B, Moncharmont B, Tsagris L, Rappaport R (1988) Estrogen receptors in cultured rabbit articular chondrocytes: influence of age. J Steroid Biochem 31:351–356
Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S (1999) Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage 7:560–566
Sniekers YH, Van Osch GJ, Ederveen AG, Inzunza J, Gustafsson JA, Van Leeuwen JP, Weinans H (2009) Development of osteoarthritic features in estrogen receptor knockout mice. Osteoarthritis Cartilage 17:1356–1361
O'Lone R, Frith MC, Karlsson EK, Hansen U (2004) Genomic targets of nuclear estrogen receptors. Mol Endocrinol (Baltimore, Md) 18:1859–1875
Galien R, Garcia T (1997) Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res 25:2424–2429
Li D, Mitchell D, Luo J, Yi Z, Cho SG, Guo J, Li X, Ning G, Wu X, Liu M (2007) Estrogen regulates KiSS1 gene expression through estrogen receptor alpha and SP protein complexes. Endocrinology 148:4821–4828
Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F (2000) Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J 19:4688–4700
Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H, Korach KS, Sonntag-Buck V, Gannon F (2001) ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol (Baltimore, Md) 15:2064–2077
Maneix L, Beauchef G, Servent A, Wegrowski Y, Maquart FX, Boujrad N, Flouriot G, Pujol JP, Boumediene K, Galera P et al (2008) 17Beta-oestradiol up-regulates the expression of a functional UDP-glucose dehydrogenase in articular chondrocytes: comparison with effects of cytokines and growth factors. Rheumatology (Oxford, England) 47:281–288
Chadjichristos C, Ghayor C, Kypriotou M, Martin G, Renard E, Ala-Kokko L, Suske G, De Crombrugghe B, Pujol JP, Galera P (2003) Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J Biol Chem 278:39762–39772
Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19:2499
Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, Papa MZ (2006) Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: involvement of transcription factor Sp1. J Endocrinol 191:605–612
Marino M, Galluzzo P, Ascenzi P (2006) Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7:497–508
Porter W, Saville B, Hoivik D, Safe S (1997) Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol (Baltimore, Md) 11:1569–1580
Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB (1998) Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem 273:10696–10701
Lu S, Jenster G, Epner DE (2000) Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol (Baltimore, Md) 14:753–760
Kim K, Thu N, Saville B, Safe S (2003) Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Mol Endocrinol (Baltimore, Md) 17:804–817
Chadjichristos C, Ghayor C, Herrouin JF, Ala-Kokko L, Suske G, Pujol JP, Galera P (2002) Down-regulation of human type II collagen gene expression by transforming growth factor-beta 1 (TGF-beta 1) in articular chondrocytes involves SP3/SP1 ratio. J Biol Chem 277:43903–43917
Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S (2000) Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor alpha and Sp3 proteins. J Biol Chem 275:22769–22779
Schultz JR, Petz LN, Nardulli AM (2003) Estrogen receptor alpha and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol 201:165–175
Higgins KJ, Liu S, Abdelrahim M, Vanderlaag K, Liu X, Porter W, Metz R, Safe S (2008) Vascular endothelial growth factor receptor-2 expression is down-regulated by 17beta-estradiol in MCF-7 breast cancer cells by estrogen receptor alpha/Sp proteins. Mol Endocrinol (Baltimore, Md) 22:388–402
Kypriotou M, Fossard-Demoor M, Chadjichristos C, Ghayor C, De Crombrugghe B, Pujol JP, Galera P (2003) SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol 22:119–129
Bi W, Deng JM, Zhang Z, Behringer RR, De Crombrugghe B (1999) Sox9 is required for cartilage formation. Nat Genet 22:85–89
Lafont JE, Talma S, Murphy CL (2007) Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum 56:3297–3306
Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bosl MR, Hess A, Surmann-Schmitt C, Von der Mark H et al (2010) SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development (Cambridge, England) 137:901–911
Savagner P, Krebsbach PH, Hatano O, Miyashita T, Liebman J, Yamada Y (1995) Collagen II promoter and enhancer interact synergistically through Sp1 and distinct nuclear factors. DNA Cell Biol 14:501–510
Leung KK, Ng LJ, Ho KK, Tam PP, Cheah KS (1998) Different cis-regulatory DNA elements mediate developmental stage- and tissue-specific expression of the human COL2A1 gene in transgenic mice. J Cell Biol 141:1291–1300
Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E et al (1998) Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol (Baltimore, Md) 12:1605–1618
Kobayashi Y, Kitamoto T, Masuhiro Y, Watanabe M, Kase T, Metzger D, Yanagisawa J, Kato S (2000) p300 mediates functional synergism between AF-1 and AF-2 of estrogen receptor alpha and beta by interacting directly with the N-terminal A/B domains. J Biol Chem 275:15645–15651
Kim MY, Hsiao SJ, Kraus WL (2001) A role for coactivators and histone acetylation in estrogen receptor alpha-mediated transcription initiation. EMBO J 20:6084–6094
Tsuda M, Takahashi S, Takahashi Y, Asahara H (2003) Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem 278:27224–27229
Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M, Ito T, Asahara H (2005) Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J Biol Chem 280:35203–35208
Achari Y, Lu T, Katzenellenbogen BS, Hart DA (2009) Distinct roles for AF-1 and -2 of ER-alpha in regulation of MMP-13 promoter activity. Biochim Biophys Acta 1792:211–220
Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A et al (2009) The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci U S A 106:2053–2058
Tzukerman MT, Esty A, Santiso-Mere D, Danielian P, Parker MG, Stein RB, Pike JW, McDonnell DP (1994) Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol (Baltimore, Md) 8:21–30
Berry M, Metzger D, Chambon P (1990) Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J 9:2811–2818
Jiang HP, Teng RY, Wang Q, Zhang X, Wang HH, Cao J, Teng LS (2008) Estrogen receptor alpha variant ERalpha46 mediates growth inhibition and apoptosis of human HT-29 colon adenocarcinoma cells in the presence of 17beta-oestradiol. Chin Med J 121:1025–1031
Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S et al (1999) The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol (Baltimore, Md) 13:1672–1685
Takeuchi S, Mukai N, Tateishi T, Miyakawa S (2007) Production of sex steroid hormones from DHEA in articular chondrocyte of rats. Am J Physiol Endocrinol Metab 293:E410–E415
Richette P, Laborde K, Boutron C, Bardin T, Corvol MT, Savouret JF (2007) Correlation between serum and synovial fluid estrogen concentrations: comment on the article by Sowers et al. Arthritis Rheum 56:698, author reply 698–699
Acknowledgments
This work was supported by the French Ministry of Research and Technology and is a part of a more global program on tissue engineering of articular cartilage of the laboratory supported by the French Agency of Research (ANR: Agence Nationale de la Recherche, Tecsan, PROMOCART) and the Regional Council of Lower-Normandy.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Safa Moslemi and Philippe Galéra contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 373 kb)
Rights and permissions
About this article
Cite this article
Maneix, L., Servent, A., Porée, B. et al. Up-regulation of type II collagen gene by 17β-estradiol in articular chondrocytes involves Sp1/3, Sox-9, and estrogen receptor α. J Mol Med 92, 1179–1200 (2014). https://doi.org/10.1007/s00109-014-1195-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1195-5