Abstract
In autosomal dominant polycystic kidney disease (ADPKD), renal cyst development and enlargement, as well as cell growth, are associated with alterations in several pathways, including cAMP and activator protein 1 (AP1) signalling. However, the precise mechanism by which these molecules stimulate cell proliferation is not yet fully understood. We now show by microarray analysis, luciferase assay, mutagenesis, and chromatin immunoprecipitation that CREB and AP1 contribute to increased expression of the amphiregulin gene, which codifies for an epidermal growth factor-like peptide, in ADPKD cystic cells, thereby promoting their cell growth. Increased amphiregulin (AR) expression was associated with abnormal cell proliferation in both PKD1-depleted and -mutated epithelial cells, as well as primary cystic cell lines isolated from ADPKD kidney tissues. Consistently, normal AR expression and proliferation were re-established in cystic cells by the expression of a mouse full-length PC1. Finally, we show that anti-AR antibodies and inhibitors of AP1 are able to reduce cell proliferation in cystic cells by reducing AR expression and EGFR activity. AR can therefore be considered as one of the key activators of the growth of human ADPKD cystic cells and thus a new potential therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a common renal disease caused by mutation of PKD1 or PKD2 gene [1]. PKD1 and PKD2 encode for polycystin-1 (PC1) and polycystin-2, respectively [2] which, independently or interacting as a complex, possess regulatory functions in the regulation of cell differentiation and proliferation [1]. In particular, mutated polycystins alter pathways featuring important players in the control of epithelial cell proliferation, such as adenosine 3′,5′-cyclic monophosphate (cAMP), the mammalian target of rapamycin (m-Tor), and the epidermal growth factor receptor (EGFR) [1], thereby leading to increased cell proliferation and ultimately causing development and enlargement of renal cysts.
Increased cAMP levels stimulate trans-epithelial chloride and fluid secretion and promote proliferation of human cyst epithelial cells [3]. Consistently, treatment with molecules able to reduce cAMP levels causes a reduction of cell proliferation in PKD1-mutated cystic cells [4]. In addition, abnormal expression of epidermal growth factor (EGF) and EGFR has been demonstrated in both autosomal-recessive and autosomal-dominant PKD [5, 6]. Furthermore, multiple EGF-related growth factors, including amphiregulin (AR) and heparin-binding EGF, are abnormally expressed in murine ARPKD supporting a role of these molecules in the pathogenesis of cystic kidney disease [5]. Nevertheless, the specific pathway by which PKD1 mutation leads to increased expression of EGF-like peptides in ADPKD kidney cells has not yet been elucidated.
Here, we show mechanisms that lead from PC1 depletion to increased transcription of the gene for AR, an EGF-like peptide that promotes the growth of most normal and transformed epithelial cells [7]. AR promoter hyperactivity in PC1-mutated cells results from the activation of cAMP response element-binding transcription factor (CREB) and activator protein 1 (AP1). Finally, we show the inhibitory effects of anti-AR antibodies and inhibitors of AP1 on cell proliferation of cystic cells, indicating AR as one of the key activators of ADPKD cystic cell proliferation.
Material and methods
Reagents
Protease inhibitors, Cl-IB-MECA, curcumin, and all material for cell culture were purchased from Sigma, Italy. Rabbit polyclonal anti-β-actin, anti-PC1 (7E12), anti-Ha, anti-c-jun, anti-calnexin, and monoclonal anti-AR antibodies were from Santa Cruz, Italy. Anti-AR polyclonal antibody was from Abcam (Cambridge, UK). Anti-EGFR, anti-pEGFR, anti-CREB, and anti-pCREB antibodies were from Cell Signaling Technology (Euroclone, Italy). Enhanced chemiluminescent substrates for Western blotting (SuperSignalDura or SuperSignalFemto) and HRP-conjugated goat anti-rabbit and anti-mouse antibodies were from Pierce (Euroclone, Italy).
Cell lines and tissues
HEK293 cells expressing PKD1 siRNA were generated in our laboratory [8]. Human kidney cell lines, one derived from a normal (4/5) and two from a cystic kidney (9.7 and 9.12) carrying the Q2556X PKD1 mutation were generated by others [9], as well as the homozygous (Pkd1 −/−) and heterozygous (Pkd1 +/−) Pkd1-gene-deleted mouse cell lines [10]. Kidney samples were obtained from two non-ADPKD adult kidneys with normal histology and from the kidneys of four ADPKD patients, respectively. These presented typical clinical manifestations and end-stage renal disease [4]. The study protocol was in line with the 1975 Declaration of Helsinki. Primary cell cultures were produced according to published protocols [11, 12].
RT-PCR analysis and mutagenesis
RNA was reverted with the ImProm-II Reverse Transcriptase (Promega, Italy) with random primers. Amphiregulin and β-actin were co-amplified with Dream Taq polymerase (Fermentas, Italy) with AR specific primers (F5′AGAGTTGAACAGGTAGTTAAGCCCC3′; R5′ GTCGAAGTTTCTTTCGTTCCTCAG3′) [13] and β-actin primers (Sigma, Italy). 1μL of cDNA mixture was amplified by an initial incubation at 94°C for 4 min followed by 28 cycles of 94°C for 30 s, 54°C for 30 s, 72°C for 50 s, and a final extension at 72°C for 4 min. Reverse transcription polymerase chain reaction (RT-PCR) values were calculated as the ratio between AR and β-actin band intensities. Quantitative real-time RT-PCR was performed by TaqMan protocol (Applied Biosystems, Italy) as described in Electronic supplementary material.
Mutagenesis of AR promoter was performed by PCR using AR-pGL2C plasmid [14] through primers AR-pGL2C-mutF (5′CCGGGCTCGAGTAAACGTCATGGGCT3′ and AR-pGL2C-mutR (5′AGCCCATGACGTTTACTCGAGCCCGG3′) mutated on CRE consensus site. The inserted bases (underlined) are located at the beginning of CRE, –274 and –273 nt from the AR translation start site [15]. AR-pGL2-C plasmid was amplified with pGL2F (5′TACTAACATACGCTCTCCATC3′) and AR-pGL2C-mutR and with AR-pGL2C-mutF and pGL2R (5′ATATGTGCATCTGTAAAAGC3′). pGL2F and pGL2R were located upstream and downstream of insertion site of the AR promoter in the pGL2-C vector. Amplicons were purified by PCR Clean-Up System (Promega Italy), then diluted to 1:100, and then re-amplified, using pGL2F and pGL2R primers to reconstruct the AR promoter with the inserted mutations. The purified PCR product was digested and re-cloned in pGL2-C. Correct base substitutions were confirmed by sequencing (ABI Prism 3100 sequencer, Applied Biosystems, Italy).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed following published protocols [16]. Cells were transfected overnight with AR-PGL2C, AR-PGL2C-ΔCRE, and AR-pGL2C-mut plasmids using TurboFect in vitro Transfection Reagent (Fermentas, Italy). After cross-linking, cells were resuspended in sodium dodecyl sulfate (SDS) lysis buffer, and 100 μL of each sample were pre-cleared with 60 μL of protein A-agarose beads. The 10 μL amount was removed from the supernatant (input), and the remainder was used directly for overnight immunoprecipitation with 2 μg of anti-c-jun and anti-calnexin (irrelevant antibody). After incubation with protein A-agarose beads, immunocomplexes were collected and washed. Input and immunoprecipitated plasmids were eluted, and cross-linking reactions were reversed by incubation at 65°C. Finally, samples were digested with proteinase K, and plasmid DNA was purified by PCR Clean-Up System. PCR analysis of input (positive control) and immunoprecipitated plasmids was performed (25 cycles) with pGL2F and pGL2R primers.
AR gene silencing and proliferation assays
AR gene was silenced using a primer cocktail (ON-TARGETplus SMARTpool siRNA) according to the manufacturer’s protocol (Dharmacon, Italy). AR siRNA specific and scramble sequences were diluted to a final concentration of 10 nM and used for 48 h cell transfection.
For cell counting, cells (25,000 cells/ml) were plated overnight in 24-well plates in 10% fetal bovine serum (FBS)-supplemented medium, starved for 24 h in Dulbecco’s modified Eagle’s medium (DMEM) with 0.4% bovine serum albumin (BSA), and cultured for further 24 h in DMEM containing 1% FBS and different substances, as indicated. Cell proliferation was calculated by direct cell counting (Burker chamber) after trypan blue staining [4, 8].
Western blotting
Disrupted tissues and cultured cells were lysed in a 1% Triton X-100 solution containing a cocktail of protease inhibitors and processed for immunoblots as described [17]. Total proteins (25 μg) were electrophoresed in 10% SDS-polyacrylamide gel and blotted to PVDF (for AR blotting) and nitrocellulose filters for other proteins (Euroclone, Italy). Blocked membranes were probed overnight at 4°C with the primary antibody and then for 2 h with the secondary antibody. Finally, proteins were visualized using the chemiluminescence system. Band intensity was detected by X-ray film scanning with the Model GS-700 Imaging Densitometer (BIO-RAD, Italy). Quantitative phosphorylation and protein levels were calculated as the ratio between phosphorylated and un-phosphorylated protein and between protein and β-actin, respectively [4].
Luciferase assay
After 24 h of culture in six-well plates, cells were transiently co-transfected with 6 μg of promoter construct and 3 μg of β-galactosidase plasmid. CRE-Luc and 7x-AP1-Luc plasmids contain binding elements for CREB and AP1, respectively [18, 19]. Six hours post-transfection, cells were incubated for an additional 20 h in medium–0.4% BSA (serum free) or 1% FBS. After cell lysis, luciferase activity was analyzed following the Promega protocol with 20/20 n luminometer (Turner Biosystems, USA). Data were expressed as relative firefly luciferase units normalized for the β-galactosidase units and as n-fold change with respect to control cells [4].
Statistical analysis
Analysis of data was performed using Student’s t test (unpaired analysis). Differences were considered significant at a value of p < 0.05. All data are reported as mean ± SD of at least three independent experiments.
Results
Amphiregulin gene expression is upregulated in PKD1-mutated kidney cells and ADPKD kidney tissues
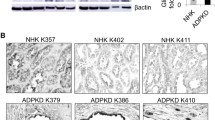
To identify genes that may contribute to ADPKD, we performed a high-density oligonucleotide microarray analysis, comparing the gene expression pattern of wild-type HEK293 cells with those depleted in PC1 protein by siRNA silencing [8]. Our study revealed an altered gene expression profile in many genes related to/dependent from PKD1 and involved in cell growth, including AR, FGF1, HGF, and CREB. In particular, we discovered that the AR gene was upregulated more than ninefold in PC1-depleted HEK293 cells, as compared with wild-type cells (Table 1). Consistently, RT-PCR analysis of AR mRNA showed an increased expression of the AR gene in PC1-depleted cells (Fig. 1a). Similar results were observed in 9.7 and 9.12 PKD1-mutated kidney cystic cells, which showed higher AR expression at both mRNA and protein levels with respect to 4/5 normal kidney cells (Fig. 1b). Increased AR mRNA levels were also observed in kidney tissues (Fig. 1c, d) and in primary cystic cultures (Fig. 1e, f) of various ADPKD patients, as compared with healthy controls. These data were confirmed also by immunohistochemistry in further cystic tissues. This analysis showed a marked AR-positive staining confined to epithelial cells of early tubular and enlarged cysts of ADPKD samples (Fig. 1g). Furthermore, immunofluorescence showed a more marked and punctuated detection of AR in 9.7 and 9.12 cystic cells as compared with 4/5 control cells (Fig. 1h). Upregulation of AR gene appears, therefore, to be a typical feature of both cystic cells and tissues in ADPKD.
Increased amphiregulin expression in PKD1-deficient cells and ADPKD tissues. a RT-PCR analysis of AR and β-actin mRNA from three PKD1-downregulated HEK293pSsiPKD1 clones (a1, a2, and b3) and wild-type HEK293pS cells (wt), stably transfected with vector expressing PKD1 siRNA and scramble RNA sequences [8]. b RT-PCR (left) and Western blot (right) analysis of AR and β-actin gene showing AR upregulation in PKD1-mutated 9.7 and 9.12 kidney cystic cells, as compared with 4/5 normal tubular kidney cells. In 1B on the right, the PC1 content in normal (4/5) and in cystic (9.7 and 9.12) cells was also shown. Anti-PC1 N-ter antibody recognized full-length (PC1-FL) and N-terminal fragment (NTF) only in 4/5 normal tubular kidney cells. c Analysis of AR mRNA by RT-PCR in normal (wt 1–2) and ADPKD (PK 1–4) tissues. Bars show the mean ± SD, calculated as ratio between the optical density (OD) of AR and β-actin RNA bands—0.63 ± 0.28 in ADPKD tissues (n = 4) vs. 0.077 ± 0.01 in normal kidney tissues (n = 2); **p < 0.01. d AR expression was quantified by real-time quantitative RT-PCR in normal and ADPKD kidney tissues. RNA levels relative to those of actin RNA were measured by ΔΔCt values (see Electronic supplementary material) and shown as fold increase (ADPKD vs. wt). Values were expressed as mean ± SD—1.69 ± 0.09 in control kidneys vs. 7.07 ± 2.21 in ADPKD tissues; ***p < 0.001. Data are representative of three independent experiments in duplicate. e Analysis of AR mRNA by RT-PCR in normal (wt 1–2) and ADPKD (PK 1–4) primary cells. Normal and ADPKD primary cell lines were obtained from the same kidney tissues numbered in the point c. Bars indicate the mean ± SD—0.43 ± 0.05 in primary cystic vs. 0.12 ± 0.04 in normal tubular cells; ***p < 0.001. Data are representative of three independent experiments. f Real-time quantitative RT-PCR in normal and ADPKD primary cells. Bars, calculated as fold increase (ADPKD vs. control cells), show the mean ± SD of three independent experiments in duplicate. The values were 1.07 ± 0.13 in control cells vs. 3.13 ± 0.8 in ADPKD primary cystic cells, ***p < 0.001. g Representative images of immunohistochemistry performed with anti-AR polyclonal antibody in normal and ADPKD kidney tissues. Pictures were acquired with ×20 and ×40 (inset) magnification. Wt (3–4) are different control kidneys and PK (5–6) are polycystic kidneys from two patients different from those reported in c. The numbering of new normal and polycystic tissues follows that of previous tissues shown in c. Results were confirmed in other four ADPKD kidneys (see Electronic supplementary material Fig. 1). Arrows indicate higher AR positivity in flat than cubic cells of early cystic tubules (tub). h Immunofluorescence detection of AR in normal 4/5, and 9.7, and 9.12 cystic cells. Arrows show in 4/5 control cells an AR prevalent plasma membrane distribution which becomes markedly punctuated in cystic cells. Pictures were acquired with ×63 magnification and with five times longer exposure for 4/5 control cells. See Electronic supplementary material for detailed methods
Increased AR promoter activity is CREB-dependent in PKD1-mutated cells
Since the presence of a CREB consensus site has been identified in AR promoter [14] and the response to cAMP is known to be involved in ADPKD cyst development [3, 4], we investigated the possible role of CREB, a downstream effector of cAMP, on AR expression in ADPKD cystic cells and tissues.
We observed a significant increase in CREB phosphorylation in both 9.7 and 9.12 cystic cells and ADPKD tissues, as compared with normal 4/5 cells and tissues, respectively (Fig. 2a, b). At a functional level, increased CREB activity was confirmed by transfection of renal cells with a CREB-responsive luciferase reporter plasmid. Luciferase activity was increased roughly 16-fold in cystic, with respect to control, cells (Fig. 2c). Hence, it is feasible that abnormally increased CREB activity is implicated in increased AR expression in cystic cells.
Increased CREB activation and AR promoter activity in PKD1-mutated cells. a Western blot analysis of CREB phosphorylation in normal and ADPKD cells. Ratios between phospho- and unphospho-CREB in 9.7 and 9.12 cystic cells (1.05 ± 0.12 and 1.63 ± 0.08, respectively) and 4/5 control cells (0.6 ± 0.09) are shown as mean ± SD of three independent experiments, ***p < 0.001 and *p < 0.05. b CREB activation, calculated as described in a, in ADPKD (2.99 ± 1.08) and normal tissues (1.55 ± 0.31), **p < 0.01. c CREB activation measured by luciferase activity using a plasmid with CREB-specific promoter sequences in 9.7 and 9.12 cystic cells and in 4/5 normal cells. Luciferase activity was higher in 9.7 and 9.12 cells (2.72 ± 0.23 and 2.85 ± 0.25, respectively) than in normal cells (0.18 ± 0.026); ***p < 0.001. Data expressed as mean ± SD are representative of three different experiments in duplicate. d Luciferase activity of AR promoter after 24 h transfection with AR-pGL2C plasmid. Inset: sequences and nucleotide position of CRE and ΔCRE sites in AR promoter are indicated. Bars show the mean ± SD of the ratio between luciferase and β-galactosidase counts (0.024 ± 0.004 in 4/5 cells vs. 0.57 ± 0.15 and 0.52 ± 0.14 in 9.7 and 9.12 cystic cells, respectively; ***p < 0.001). AR promoter activity decreases in cystic cells if CRE is lost. e Promoter activity (calculated as fold increase between cystic and control cells) was 23.36 ± 1.85 and 21.45 ± 2.48, in AR-pGL2C-transfected 9.7 and 9.12 cystic cells vs. 8.43 ± 0.42 and 8.98 ± 1.16 in AR-pGL2C-ΔCRE-transfected 9.7 and 9.12 cells. Bars represent the mean ± SD of three independent experiments in duplicate; ***p < 0.001. f Promoter activity (luciferase/β-gal) was 0.39 ± 0.11 and 0.31 ± 0.05 in AR-pGL2-C-transfected heterozygous (+/−) and homozygous (−/−) mouse Pkd1-KO cells vs. 0.09 ± 0.038 and 0.12 ± 0.083 in AR-pGL2C-ΔCRE-transfected cells. Data are shown as mean ± SD of three independent experiments in duplicate, **p < 0.01 and *p < 0.05
To confirm the role of CREB in AR gene upregulation in cystic cells, we analyzed AR promoter activity using two luciferase reporter plasmids: AR-pGL2-C, containing a cAMP-responsive element (CRE), and AR-pGL2-C-ΔCRE, with a three-base deletion in the CRE sequence (inset of Fig. 2d) of the AR promoter sequence [14]. After transfection with AR-pGL2-C plasmid, AR promoter activity was markedly increased in 9.7 and 9.12 cells, as compared with 4/5 cells (Fig. 2d), but this increase was clearly reduced after AR-pGL2-C-ΔCRE transfection (Fig. 2e). The reduction of AR promoter activity due to loss of CRE was also observed in homozygous and heterozygous Pkd1 knock-out mouse kidney cells (Fig. 2f). AR gene overexpression is, therefore, modulated by CREB activation in ADPKD cells.
Consistently, treatment with Cl-IB-MECA, a specific A3 adenosine receptor agonist that reduces cAMP levels in 9.7 and 9.12 cystic cells [4], also reduced AR promoter activity in AR-pGL2C-transfected cystic cells (Fig. 3a). However, reduction of AR promoter activity by Cl-IB-MECA was not observed in cells transfected with AR-pGL2-C-ΔCRE, which lacks CRE (Fig. 3b). Notably, Cl-IB-MECA also significantly decreased endogenous AR protein levels in 9.7 and 9.12 cystic cells (Fig. 3c). Increased AR expression in ADPKD cystic cells is, therefore, CREB- and cAMP-dependent.
Cl-IB-MECA treatment caused a reduction in both AR promoter activity and AR protein levels in ADPKD cystic cells. a 9.7 and 9.12 cells treated for 24 h with 100 nM Cl-IB-MECA showed lower AR promoter activity than untreated cells. The values, expressed as luciferase/β-gal arbitrary units, were 0.506 ± 0.12 and 0.46 ± 0.067 in 9.7 and 9.12 untreated cells, respectively, vs. 0.294 ± 0.045 and 0.297 ± 0.07 in 9.7 and 9.12 Cl-IB-MECA-treated cells, respectively. Data are reported as mean ± SD of three independent experiments in duplicate; *p < 0.05 and **p < 0.01. b The Cl-IB-MECA treatment of AR-pGL2-CΔCRE transfected cells caused no changes in AR promoter activity. c Western blot analysis of AR in cells treated with 100 nM Cl-IB-MECA for 24 h. Protein levels are 0.775 ± 0.03 and 0.606 ± 0.08 in untreated 9.7 and 9.12 cells, respectively, vs. 0.501 ± 0.009 and 0.446 ± 0.01 in Cl-IB-MECA-treated 9.7 and 9.12 cells, respectively; **p < 0.01 and *p < 0.05. Bars represent the mean ± SD of AR/β-actin band intensity ratio, calculated by a densitometer in three independent experiments
AP1 contributes to increased AR promoter activity in PKD1-mutated cells
Despite the loss of CRE function, AR promoter activity was, on the whole, still higher in cystic than in control cells (Fig. 2e), indicating the involvement of other factors. Hence, we analyzed the AR-pGL2-C-ΔCRE plasmid using the transcription element search system database and thereby identified a putative element for Jun (a member of the AP1 transcription factor family) overlapping the CRE sequence. We therefore analyzed the activity of AP1 in cystic and normal cells. Luciferase activity was found higher in 9.7 and 9.12 cystic cells transfected with a plasmid containing a 7× repeated AP1 element than in 4/5 control cells (Fig. 4a). Furthermore, treatment of cells transfected with the AR-pGL2C plasmid with 20 μM curcumin, a specific AP1 inhibitor [20], significantly decreased the AR promoter activity in cystic, with respect to control, cells (Fig. 4b). AP1 may, therefore, contribute to the increased activity of AR promoter in cystic cells, possibly by binding to CRE.
The enhanced promoter activity of AR in ADPKD cystic cells is associated with increased AP1 activation. a AP1 activity measured as luciferase/β-gal counts using a 7× AP1 consensus plasmid in 9.7 and 9.12 cystic and normal 4/5 cells. The mean of values ±SD of three independent experiments in duplicate was 0.19 ± 0.031 in 4/5 cells vs. 1.23 ± 0.48 and 1.89 ± 0.58 in 9.7 and 9.12 cells, respectively; ***p < 0.001. b Treatment of the cells with 20 μM curcumin. Values from three independent experiments were calculated as the ratio between treated and untreated cells (1.25 ± 0.55 in 4/5 cells vs. 0.17 ± 0.029 and 0.37 ± 0.21 in 9.7 and 9.12 cells, respectively; *p < 0.05). c The mutation of CRE/AP1 like element reduced the activity of AR promoter, as compared with CRE deleted site in cystic cells. Inset: Nucleotide positions of CRE in the AR promoter, the AP1 core (bold letters) in AR-pGL2C-ΔCRE (ΔCRE) and the substituted bases (boxed) in AR-pGL2C-mut (CRE-mut) plasmids are indicated. Values were calculated as fold increase in the ratio between cystic versus control cells (9.89 ± 3.2 in 9.7 and 9.72 ± 4.1 in 9.12 cells, respectively, transfected with AR-pGL2-C-ΔCRE vs. 3.19 ± 2.88 in 9.7 and 2.38 ± 2.45 in 9.12 cells, respectively, transfected with AR-pGL2C-mutated plasmid; *p < 0.05). Data are expressed as mean ± SD of four independent experiments in duplicate. d Chip analysis in AR-pGL2-C, AR-pGL2-C-ΔCRE, and AR-pGL2-C-mut transfected 4/5, 9.7, and 9.12 cells. Input was the positive control obtained by PCR amplification of total lysates from cells transfected with different plasmids. ‘Anti c-jun’ and ‘irrelevant’ represent PCR amplification of lysates immunoprecipitated with anti-Jun and anti-calnexin antibodies, respectively. ‘NC’ is the negative control. The wild-type and mutated CRE sequence in the different constructs are illustrated in the inset, and the AP1 core sequence is in bold. The arrows indicate the F and R primer position located up- and downstream of the AR promoter in the pGL2C vector
We therefore investigated the putative AP1 binding to CRE/ΔCRE sequences in the AR promoter by mutagenesis of CRE and subsequent ChIP. Accordingly, the first two bases (TG) of the CRE sequence in the AR-pGL2C plasmid were substituted with AA (inset of Fig. 4c). Interestingly, in 9.7 and 9.12 cells transfected with the CREB/AP1 mutated plasmid (AR-pGL2C-mut), luciferase activity was lower than in the same cells transfected with AR-pGL2-C-ΔCRE (Fig. 4c). Moreover, ChIP analysis of cells transfected with AR-pGL2C, AR-pGL2-C-ΔCRE, and AR-pGL2C-mut plasmids (inset of Fig. 4d) and immunoprecipitated using anti-Jun antibody showed PCR fragments in cells transfected with AR-pGL2C and AR-pGL2-C-ΔCRE (Fig. 4d) and none in cells transfected with AR-pGL2C-mut plasmid (Fig. 4d). Both wild-type and CRE-deleted versions are, therefore, recognized by Jun, which thereby contributes to increased AR promoter activity in ADPKD cystic cells.
AR gene expression is modulated by PC1
Since the upregulation of amphiregulin was observed only in ADPKD cystic cells and tissues, this may be a direct effect of PKD1 gene mutation. Indeed, a significant reduction of AR promoter activity was observed in cystic cells transfected with full-length Pkd1 mouse cDNA as compared with those transfected with the empty vector (Fig. 5a). Consistently, expression of mouse PC1 led to a considerable reduction in AR protein levels in both 9.7 and 9.12 cystic cells (Fig. 5c, d). PC1 controls, therefore, amphiregulin gene expression.
The expression of wild-type PC1 inhibits AR upregulation in ADPKD cells. a Cells were co-transfected with mouse Pkd1 cDNA or empty vector and AR-pGL2C for 24 h. AR luciferase activity was 3.76 ± 0.86 in 9.7 and 3.45 ± 0.87 in 9.12 cells transfected with empty vector vs. 1.45 ± 0.92 in 9.7 and 1.71 ± 0.25 in 9.12 cells transfected with wild-type PC1; *p < 0.05 and **p < 0.01, respectively. No difference was observed in 4/5 cells. Approximately 20% is the efficiency of cell transfection with the mPkd1 construct (b), as previously reported [8]. Positions of HA tag and cleavage site originating the PC1 C terminal fragment (CTF) are indicated. c Western blot analysis of mouse PC1, AR, and β-actin in empty vector- and pkd1 cDNA-transfected cells. Mouse wild-type PC1 and CTF-mPC1 were detected by anti-HA antibody. d AR expression in cells transfected with empty vector was 1.08 ± 0.13 in 9.7 and 1.07 ± 0.06 in 9.12 cystic cells vs. 0.65 ± 0.13 in 4/5 control cells; *p < 0.05, **p < 0.01. In 9.7 and 9.12 cells, mPkd1 transfection reduced AR protein expression as compared with empty vector-transfected cells (0.67 ± 0.15 and 0.66 ± 0.20 in 9.7 and 9.12 cells, respectively; °p < 0.05)
Increased EGFR activity is associated with AR overexpression in ADPKD cystic cells
Since AR growth factor, a ligand of EGFR, was overexpressed in ADPKD cells and tissues, it is conceivable that EGF receptor activation is greater in cystic than in normal cells. Accordingly, the phosphorylated form of EGFR was found to be higher in 9.7 and 9.12 than in 4/5 cells (Fig. 6a). In addition, the AR gene silencing that was shown to cause a reduction in both AR mRNA (Electronic supplementary material Fig. 2A) and protein levels (Fig. 6b and Electronic supplementary material Fig. 2B) in AR-siRNA transfected cystic cells also induced a significant reduction in EGFR phosphorylation in 9.7 and 9.12 but not in 4/5 cells (Fig. 6c). Likewise, treatment with specific anti-AR antibodies only caused a reduction in EGFR phosphorylation in 9.7 and 9.12 cystic cells but not in 4/5 cells (Fig. 6d). Thus, amphiregulin overexpression stimulates the activation of EGF receptor in ADPKD cystic cells.
The increased EGFR activation in ADPKD cystic cells is AR-dependent. a EGFR protein phosphorylation was analyzed by Western blot, using antibodies for total and phosphorylated forms of EGFR. The ratio between phosphorylated and unphosphorylated form of the receptor was 0.42 ± 0.27 in 4/5 cells vs. 2.5 ± 1.09 and 1.95 ± 0.47 in 9.7 and 9.12 cells, respectively; *p < 0.05; **p < 0.01. b Cells, transfected with scramble oligonucleotides or specific anti-AR siRNAs for 48 h, were analyzed for AR protein content. The values of the ratio AR/β-actin were 0.55 ± 0.08 in 4/5- vs. 1.15 ± 0.20 and 0.922 ± 0.05 in 9.7 and 9.12-scramble transfected cells; **p < 0.01. The ratio AR/β-actin was 1.15 ± 0.20 and 0.922 ± 0.05 in 9.7 and 9.12 scramble transfected cells, respectively, vs. 0.66 ± 0.21 and 0.38 ± 0.09 in 9.7 and 9.12 AR-siRNA transfected cells, respectively; °p < 0.05, °°°p < 0.001. c. EGFR phosphorylation in AR silenced cells. The values, obtained as the ratio between AR siRNA-treated/untreated cells, were 1.003 ± 0.08 in 4/5 control cells vs. 0.679 ± 0.01 and 0.47 ± 0.24 in 9.7 and 9.12 cystic cells, respectively; *p < 0.05, **p < 0.01. d EGFR activity was quantified after cell treatment with unrelated or anti-AR antibodies (2 μg/mL) for 24 h. In cystic cells treated with unrelated antibodies ratios between phosphorylated and unphosphorylated form of the receptor were higher than in control cells (1.56 ± 0.32 in 9.7 and 2.25 ± 0.35 in 9.12 cystic cells vs. 0.37 ± 0.028 in 4/5 control cells; **p < 0.05; ***p < 0.001). The treatment with anti-AR antibodies caused a reduction of EGFR activity in cystic cells but not in control cells. Phosphorylated to unphosphorylated ratios were 0.50 ± 0.14 in 4/5, 0.75 ± 0.21 in 9.7, and 0.65 ± 0.28 in 9.12 anti-AR-treated cells; °p < 0.05, °°p < 0.01 vs. unrelated antibodies treated 9.7 and 9.12 cells, respectively. Values are expressed as mean ± SD of three different experiments
AR upregulation contributes to increased cell growth in PKD1-mutated cystic cells
As expected, treatment with cetuximab, an EGFR-blocking antibody, reduced cell proliferation in cystic cells to a greater extent than in control cells (Fig. 7a). Interestingly, treatment with a specific anti-AR polyclonal antibody also caused a reduction in cystic cell proliferation with respect to their untreated counterparts or those treated with an irrelevant antibody (Fig. 7b). Comparable reductions were also obtained using a monoclonal anti-AR antibody (data not shown), which also reduced cell growth in two different human primary cystic cell cultures but not in normal cells (Fig. 7c). Moreover, the treatment with anti-AR antibodies reduced cell proliferation in both homozygous and heterozygous mouse Pkd1 knock-out cell lines (Fig. 7d). In addition, cell cycle analysis showed that the anti-AR treatment increased G1 phase and reduced S and G2 + M phases in 9.7 and 9.12 cystic but not in normal cells (see percentage values in Fig. 7e). These changes were also observed in anti-AR-treated mouse heterozygous Pkd1 +/− and homozygous Pkd1 −/− cells (Fig. 7e). Consistently, 9.7 and 9.12 cystic cells treated with anti-AR antibody shoved a significant reduction of 3H-thymidine incorporation compared with untreated cells (Fig. 7f). The reduction in cell proliferation by anti-AR treatment was not associated to increased apoptosis (Fig. 7g). These results are consistent with those obtained by siRNA-mediated AR gene downregulation in cystic cells (Fig. 7h) and strongly support the implication of AR in increased ADPKD cystic cell proliferation.
AR downregulation reduces the proliferation of ADPKD cystic cells. a Cells were treated with 200 μg/mL of cetuximab for 24 h and directly counted in a Burker chamber. Values calculated as percentage ratio between treated versus untreated cells were 88 ± 12% in 4/5 cells vs. 70 ± 5% in 9.7 and 9.12 cystic cells; *p < 0.05. b Cell medium alone or mixed with 2 μg/mL of anti-AR and irrelevant (anti-NFAT) antibodies was added to the cells for 24 h. Cell counts were 274,000 ± 28,000 and 277,000 ± 30,000 in untreated vs. 159000 ± 60000 and 180,000 ± 47,000 in 9.7 and 9.12 cells treated with anti-AR, respectively; ***p < 0.001. No significant variations were observed in 4/5 cells. c Two different ADPKD primary cystic cells cultured in medium with 1% FBS grew more than primary normal kidney cells (*p < 0.05). Treatment with anti-AR antibody reduced the proliferation of cystic cells (57,000 ± 13,000 and 112,000 ± 59,000 in untreated vs. 27,000 ± 10,000 and 40,000 ± 11,000 in anti-AR treated cystic cells, respectively; °°p < 0.01). d Anti-AR antibody reduced cell proliferation in mouse pkd1-KO homozygous and heterozygous kidney cells (292,000 ± 17,000 and 430,000 ± 39,000 in untreated heterozygous and homozygous pkd1-KO cells, respectively, vs. 194,000 ± 26,000 and 360,000 ± 39,000 in those treated with anti-AR, respectively; ***p < 0.001 and **p < 0.01). e Cell cycle analysis showed in human cystic cells treated with anti-AR polyclonal antibody, an increase in G1 phase (p < 0.001 in both 9.7 and 9.12) and a decrease in both S (p < 0.01 and p < 0.001 in 9.7 and 9.12, respectively) and G2 + M phases (p < 0.001 in both 9.7 and 9.12), compared with untreated cells. Similar results were observed in anti-AR treated mouse kidney Pkd1-KO heterozygous and homozygous cells, as compared with untreated cells. For G1 phase, p < 0.001 in both heterozygous and homozygous treated vs. untreated cells. For S phase, p < 0.01 and p < 0.001 in heterozygous and homozygous treated vs. untreated cells, respectively. For G2 + M phase, p < 0.001 in both heterozygous and homozygous treated vs. untreated cells. Data represent the mean ± SD of three independent experiments in duplicate. f 3H-thymidine incorporation after 24 h of culture was lower in 9.7 and 9.12 anti-AR antibody treated than in untreated cystic cells (987 ± 101 and 951 ± 60 cpm in 9.7 and 9.12 anti-AR treated cells vs. 1,572 ± 339 cpm and 1,260 ± 155 cpm in 9.7 and 9.12 untreated cells). Data shown are the mean ± SD of two independent experiments in triplicate (**p < 0.01). g Anti-AR treatment did not change apoptosis because Caspase 3 activity is not modified by the antibody in normal (4/5) and cystic (9.7 and 9.12) cells. h AR silencing and proliferation of 9.7 and 9.12 cystic cells. Proliferation values were expressed as percentage of AR-silenced with respect to non-silenced cells (110 ± 19% in 4/5 control cells vs. 79 ± 10% and 83 ± 4.9% in 9.7 and 9.12 cells, respectively; ***p < 0.001). i Cell growth after 24 h treatment with the AP1 inhibitor curcumin (20 μM). Cells were 124,000 ± 17,000 and 130,000 ± 8,000 in 9.7 and 9.12 untreated cells, respectively, vs. 63,000 ± 13,000 and 81,000 ± 6,000 in 9.7 and 9.12 curcumin-treated cells, respectively; **p < 0.01 and ***p < 0.001. l Expression of wild-type PC1 decreased cell growth in cystic cells. Cells (see legend of Fig. 5) were transfected with empty vector or full-length mouse Pkd1 cDNA and cultured for a further 24 h in medium with 0.4% BSA. Cell number, calculated as percentage of Pkd1 cDNA-transfected with respect to untransfected cells, was 87 ± 8.5% in 4/5 cells versus 78 ± 5.6% and 78.5 ± 7.5% in 9.7 and 9.12 cells, respectively; **p < 0.01 and *p < 0.05. Data represent the mean ± SD of at least three independent experiments in triplicate. Detailed methods are described in Electronic supplementary material
Interestingly, treatment with curcumin, an AP1 inhibitor, also reduced cell proliferation to a greater extent in cystic than in normal cells (Fig. 7i). On the other hand, we have previously reported that Cl-IB-MECA, by decreasing cAMP levels [4] and probably CREB activity, reduced 9.7 and 9.12 cystic cell proliferation [4], as well as reducing AR promoter activity (Fig. 3). In addition, reintroduction of a functional PC1 protein to 9.7, 9.12, and 4/5 cells (Fig. 5c) reduced cystic cell growth (Fig. 7j), which is consistent with a reduction in AR expression (Fig. 5c, d). Functional PC1 was shown, therefore, to be responsible for the control of cell proliferation in kidney cells by modulating AR expression via CREB and AP1 signalling.
Discussion
The present study shows that loss of PC1 expression stimulates proliferation of ADPKD cystic cells through upregulation of the AR gene. This occurs through two main signalling pathways, one driven by CREB and the second involving AP1 transcription factors. Increased AR expression potentiates the activation of EGFR, which is an important mediator of proliferative abnormalities observed in ADPKD cyst formation [21, 22]. These results are consistent with previous observations in EGF-like proteins, including AR, reported in polycystic kidneys [23].
In particular, we provide evidence for increased expression of the AR gene at the mRNA and protein levels in ADPKD cystic cells and tissues. AR immunohistochemical detection in ADPKD tissues was in fact more marked in flattened cyst lining cells than in cubic cells of cystic tubules, while it was faint in normal kidneys (Fig. 1g). This likely associates increased AR expression to cell dedifferentiation and cyst expansion. In addition, AR immunofluorescence in cystic PKD1-mutated cells was marked and punctuated, possibly compatible with an increased turnover of vesicular AR–EGFR complex (Fig. 1h). We also show that upregulation of the AR gene in cystic cells depends on the activation of CREB, which we found at particularly high levels in cystic cells and tissues. Interestingly, a CRE site in the AR promoter [24, 25] appears to be critical for its enhanced activity in cystic cells. This observation is supported by two findings, the first being that deletion of CRE causes a strong reduction in AR promoter activity of cystic cells. This reduction was also shown in mouse Pkd1(−/−) null cells, as well as in Pkd1(+/−) heterozygous cells which are likely PKD1 haploinsufficient. The second point is that cAMP reduction in cystic cells caused by Cl-IB-MECA [4] induces a decrease in AR protein levels and in AR promoter activity when CRE is present but not when it is deleted. This is significant because, although the elevation of cAMP signalling is well-known in ADPKD [26] and cAMP-dependent activation of the AR promoter has been reported in other cell types [24, 27], this is the first time that an association between cAMP levels, CREB activation, and AR expression in cystic cells has been reported.
As the activity of the AR promoter is still high in cystic cells after the CRE deletion, other promoting factors are implicated in binding to the CRE region. Indeed, we showed that AP1, whose binding site overlaps with CRE and ΔCRE sequences, contributes to the increased activity of AR promoter in cystic cells; AP1 activity is indeed increased in cystic cells, and consistently, curcumin, an AP1 inhibitor, strongly reduces the activity of their AR promoter. Furthermore, the mutation of CRE and the AP1-like consensus site in these cells reduces AR promoter activity more than the loss of CRE in the ΔCRE sequence, which still maintains the AP1 binding site. Moreover, we showed that AP1 may bind to CRE and ΔCRE sequences through the fact that Jun, a component of AP1, was able to bind both sequences, but not the mutated CRE/AP1 site (Fig. 4d). This is in line with previous observations showing that Jun is able to recognize CRE sequences [28, 29], as homodimer Jun/Jun or heterodimer Jun/ATF2, and that AP1 and CREB are part of the same superfamily [28]. The core “TGAC” sequence recognized by Jun/ATF2 heterodimer is, in fact, identical in AP1 and CREB consensus sites [30]; it is present in AR-pGL2C and AR-pGL2C-ΔCRE but not in the AR-pGL2C-mut construct. This observation suggests a new mechanism for the altered activities of c-Jun and ATF2 in the renal cystic tissue of ADPKD patients [31, 32].
As previously reported, the AR peptide may function in an autocrine–paracrine manner by EGFR activation, thereby driving cell proliferation in ADPKD cysts and malignant cells [23, 27]. We found that phosphorylated levels of EGFR, which are increased in cystic cells, are blunted by AR gene silencing and treatment with anti-AR antibodies, thereby providing evidence for a role of AR in EGFR activation. Consistently, either treatment with anti-AR antibodies or depletion of the AR gene induces a decrease in cell proliferation of primary and transformed cystic cells. In addition, the treatment with anti-AR antibodies caused a reduction of cell proliferation also in both Pkd1(−/−) null and Pkd1(+/−) heterozygous mouse cells. Similar results observed in Pkd1 homozygous and heterozygous knock-out cells are likely due to PKD1 haploinsufficiency of Pkd1(+/−) cells. In fact, it is known that polycystin-1 deficiency in human cell lines was associated with increased sensitivity to IGF-1 as well as a permissive effect of cAMP on cell growth [33].
We have previously reported that a reduction in cAMP levels, and possibly in CREB activity, by Cl-IB-MECA induces a decrease in cystic cell proliferation [4]. Interestingly, inhibition of AP1 by curcumin also induces a reduction in the growth of cystic cells. Notably, curcumin treatment has been already reported to improve renal histology and reduce the proliferation index in ADPKD mice [34, 35]. Therefore, a similar inhibition of either AR expression or function could be important to the pharmacological control of cystic cell proliferation.
Upregulation of AR expression in ADPKD cells is associated with the loss of PC1 function. Indeed, PC1 protein is shown to control AR expression in kidney cells as exogenous expression of wild-type PC1 reduces AR promoter activity and protein levels in cystic cells. Consistently, the expression of functional exogenous PC1 reduces cell proliferation to a greater extent in cystic than in normal cells, thereby emphasizing, as reported by others [36], the role of PC1 in the control of proliferation in kidney cells.
As a whole, these findings suggest that loss of PC1 expression causes upregulation of the AR gene via CREB and AP1 signalling, ultimately leading to abnormal cell proliferation (Fig. 8). This indicates new avenues of research, suggesting multi-target compounds for the treatment of ADPKD.
References
Harris PC, Torres VE (2009) Polycystic kidney disease. Annu Rev Med 60:321–337
Meijer E, de Jong PE, Peters DJ, Gansevoort RT (2008) Better understanding of ADPKD results in potential new treatment options: ready for the cure? J Nephrol 21(2):133–138
Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM Jr, Grantham JJ (2004) Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 66(3):964–973
Aguiari G, Varani K, Bogo M, Mangolini A, Vincenzi F, Durante C, Gessi S, Sacchetto V, Catizone L, Harris P et al (2009) Deficiency of polycystic kidney disease-1 gene (PKD1) expression increases A(3) adenosine receptors in human renal cells: implications for cAMP-dependent signalling and proliferation of PKD1-mutated cystic cells. Biochim Biophys Acta 1792(6):531–540
MacRae Dell K, Nemo R, Sweeney WE Jr, Avner ED (2004) EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int 65(6):2018–2029
Du J, Wilson PD (1995) Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 269:C487–C495
Ciardiello F, Tortora G (1998) Interactions between the epidermal growth factor receptor and type I protein kinase A: biological significance and therapeutic implications. Clin Cancer Res 4(4):821–828
Aguiari G, Trimi V, Bogo M, Mangolini A, Szabadkai G, Pinton P, Witzgall R, Harris PC, Borea PA, Rizzuto R et al (2008) Novel role for polycystin-1 in modulating cell proliferation through calcium oscillations in kidney cells. Cell Prolif 41(3):554–573
Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J (2006) Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17:1015–1025
Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119(Pt 7):1383–1395
Qi W, Johnson DW, Vesey DA, Pollock CA, Chen X (2007) Isolation, propagation and characterization of primary tubule cell culture from human kidney. Nephrology 12:155–159
Loghman-Adham M, Nauli SM, Soto CE, Kariuki B, Zhou J (2003) Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am J Physiol Renal Physiol 285(3):F397–F412
Freimann S, Ben-Ami I, Dantes A, Armon L, Ben Ya’cov-Klein A, Ron-El R, Amsterdam A (2005) Differential expression of genes coding for EGF-like factors and ADAMTS1 following gonadotropin stimulation in normal and transformed human granulosa cells. Biochem Biophys Res Commun 333(3):935–943
Lee SB, Huang K, Palmer R, Truong VB, Herzlinger D, Kolquist KA, Wong J, Paulding C, Yoon SK et al (1999) The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell 98(5):663–673
Plowman GD, Whitney GS, Neubauer MG, Green JM, McDonald VL, Todaro GJ, Shoyab M (1990) Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci U S A 87(13):4905–4909
Lambertini E, Tavanti E, Torreggiani E, Penolazzi L, Gambari R, Piva R (2008) ERalpha and AP-1 interact in vivo with a specific sequence of the F promoter of the human ERalpha gene in osteoblasts. J Cell Physiol 216(1):101–110
Aguiari G, Campanella M, Manzati E, Pinton P, Banzi M, Moretti S, Piva R, Rizzuto R, del Senno L (2003) Expression of polycystin-1 C-terminal fragment enhances the ATP-induced Ca2+ release in human kidney cells. Biochem Biophys Res Commun 301(3):657–664
Watanabe H, Smith MJ, Heilig E, Beglopoulos V, Kelleher RJ 3rd, Shen J (2009) Indirect regulation of presenilins in CREB-mediated transcription. J Biol Chem 284(20):13705–13713
Islam MR, Jimenez T, Pelham C, Rodova M, Puri S, Magenheimer BS, Maser RL, Widmann C, Calvet JP (2010) MAP/ERK kinase kinase 1 (MEKK1) mediates transcriptional repression by interacting with polycystic kidney disease-1 (PKD1) promoter-bound p53 tumor suppressor protein. J Biol Chem 285(50):38818–38831
Hanazawa S, Takeshita A, Amano S, Semba T, Nirazuka T, Katoh H, Kitano S (1993) Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem 268(13):9526–9532
PD Wilson (2004) Polycystic kidney disease. N Engl J Med. 350(2):151–164
Zheleznova NN, Wilson PD, Staruschenko A (2011) Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells. Biochim Biophys Acta 1812:1301–1313
Wilson PD (2004) A plethora of epidermal growth factor-like proteins in polycystic kidneys. Kidney Int 65(6):2441–2452
Du B, Altorki NK, Kopelovich L, Subbaramaiah K, Dannenberg AJ (2005) Tobacco smoke stimulates the transcription of amphiregulin in human oral epithelial cells: evidence of a cyclic AMP-responsive element binding protein-dependent mechanism. Cancer Res 65(13):5982–5988
Shao J, Evers BM, Sheng H (2004) Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem 279(14):14287–14293
Wang X, Ward CJ, Harris PC, Torres VE (2010) Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int 77(2):129–140
Johansson CC, Yndestad A, Enserink JM, Ree AH, Aukrust P, Taskén K (2004) The epidermal growth factor-like growth factor amphiregulin is strongly induced by the adenosine 3′,5′-monophosphate pathway in various cell types. Endocrinology 145(11):5177–5184
Hai T, Curran T (1991) Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A 88(9):3720–3724
Matsuo N, Tanaka S, Gordon MK, Koch M, Yoshioka H, Ramirez F (2006) CREB-AP1 protein complexes regulate transcription of the collagen XXIV gene (Col24a1) in osteoblasts. J Biol Chem 281(9):5445–5452
Falvo JV, Parekh BS, Lin CH, Fraenkel E, Maniatis T (2000) Assembly of a functional beta interferon enhanceosome is dependent on ATF-2-c-jun heterodimer orientation. Mol Cell Biol 20(13):4814–4825
Parnell SC, Magenheimer BS, Maser RL, Zien CA, Frischauf AM, Calvet JP (2002) Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem 277(22):19566–19572
Le NH, van der Wal A, van der Bent P, Lantinga-van Leeuwen IS, Breuning MH, van Dam H, de Heer E, Peters DJ (2005) Increased activity of activator protein-1 transcription factor components ATF2, c-Jun, and c-Fos in human and mouse autosomal dominant polycystic kidney disease. J Am Soc Nephrol 16(9):2724–2731
Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O’Hare MJ, Ong AC (2007) Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72(2):157–165
Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ (2011) Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways. in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300(5):F1193–F1202
Gao J, Zhou H, Lei T, Zhou L, Li W, Li X, Yang B (2011) Curcumin inhibits renal cyst formation and enlargement in vitro by regulating intracellular signaling pathways. Eur J Pharmacol 654(1):92–99
Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG (2002) PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109(2):157–168
Acknowledgments
We thank Dr. Hirotaka Watanabe (Center for Neurologic Diseases, Louis Pasteur, Boston, USA) and Prof. James P. Calvet (Kidney Institute, University of Kansas, USA) for the CRE-Luc and 7×-AP1-Luc plasmids, respectively. We are grateful to Prof. Sean Lee and Prof. Kjetil Tasken (Biotechnology Center of Oslo, University of Oslo, Norway) for the AR-pGL2C and AR-pGL2C-ΔCRE plasmids, respectively. We also thank Dr. A. Canella (Dep. of Biochemistry and Molecular Biology, University of Ferrara) for cell cycle analysis, Dr. K. Dong for technical support on mouse Pkd1 knock-out cell lines, and Dr. V. Iannoni for carrying out some experiments. Finally, we thank Prof. P. Pinton for the access to imaging instruments and Dr. F. Vincenzi for 3H-thymidine incorporation analysis.
Work supported by grants from CaRiFe, CaRiCe, Italian MIUR COFIN 2008, and Regione Emilia Romagna (Ricerca Regione-Università) 2007–2009. The English was edited by Dr. Anna Forster.
Disclosure statement
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
(PDF 506 kb)
Rights and permissions
About this article
Cite this article
Aguiari, G., Bizzarri, F., Bonon, A. et al. Polycystin-1 regulates amphiregulin expression through CREB and AP1 signalling: implications in ADPKD cell proliferation. J Mol Med 90, 1267–1282 (2012). https://doi.org/10.1007/s00109-012-0902-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0902-3