Abstract
The role of mitochondrial dysfunction in the development of insulin resistance and type 2 diabetes remains controversial. In order to specifically define the relationship between insulin receptor (InsR) signaling, insulin resistance, hyperglycemia, hyperlipidemia and mitochondrial function, we analyzed mitochondrial performance of insulin-sensitive, slow-oxidative muscle in four different mouse models. In obese but normoglycemic ob/ob mice as well as in obese but diabetic mice under high-fat diet, mitochondrial performance remained unchanged even though intramyocellular diacylglycerols (DAGs), triacylglycerols (TAGs), and ceramides accumulated. In contrast, in muscle-specific InsR knockout (MIRKO) and streptozotocin (STZ)-treated hypoinsulinemic, hyperglycemic mice, levels of mitochondrial respiratory chain complexes and mitochondrial function were markedly reduced. In STZ, but not in MIRKO mice, this was caused by reduced transcription of mitochondrial genes mediated via decreased PGC-1α expression. We conclude that mitochondrial dysfunction is not causally involved in the pathogenesis of obesity-associated insulin resistance under normoglycemic conditions. However, obesity-associated type 2 diabetes and accumulation of DAGs or TAGs is not associated with impaired mitochondrial function. In contrast, chronic hypoinsulinemia and hyperglycemia as seen in STZ-treated mice as well as InsR deficiency in muscle of MIRKO mice lead to mitochondrial dysfunction. We postulate that decreased mitochondrial mass and/or performance in skeletal muscle of non-diabetic, obese or type 2 diabetic, obese patients observed in clinical studies must be explained by genetic predisposition, physical inactivity, or other still unknown factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite intensive research, the pathways leading to insulin resistance in muscle and liver, which precede and finally result in overt type 2 diabetes, have not been firmly established yet. Circulating metabolic factors and/or genetic predisposition are most likely involved. Ectopic lipids and their metabolites accumulate in liver and muscle due to an imbalance of fatty acid delivery vs. oxidation or triglyceride synthesis [1]. These metabolites are thought to be responsible for alterations in the phosphorylation state of proteins involved in signaling pathways from the insulin receptor (InsR) to the glucose uptake machinery [2, 3]. Mitochondrial dysfunction was observed in muscle of diabetic patients and has been postulated to impair fatty acid oxidation, thus being primarily responsible for the accumulation of such toxic lipid metabolites. Indeed, a reduced in vivo rate of mitochondrial ATP synthesis has already been shown in skeletal muscle of prediabetic, insulin-resistant offspring of patients with type 2 diabetes [4, 5]. In addition, a concerted downregulation of nuclear genes encoding mitochondrial proteins, probably coordinated by peroxisomal proliferator activator receptor γ coactivator-1 (PGC-1) family members, has been found in muscle of type 2 diabetic patients [6, 7]. Thus, mitochondrial dysfunction in muscle was claimed to be either a primary cause or a consequence of insulin resistance and/or diabetes in humans, in the latter case probably initiating a vicious cycle [8, 9].
However, muscle-specific depletion of mtDNA, causing complete absence of the respiratory chain, does not lead to diabetes, but rather improves glucose tolerance [10]. Also, deletion of the apoptosis inducing factor (AIF) in muscle or liver, leading to a moderately compromised oxidative phosphorylation system, results in improved insulin sensitivity in mice [11]. Very recently, respiratory chain dysfunction following an iron-deficient diet was shown to protect against high fat diet-induced insulin resistance in muscle [12]. Furthermore, patients with mutations in mtDNA or nuclear genes encoding mitochondrial proteins are prone to develop β-cell failure and type 1 diabetes, but rarely present with type 2 diabetes [13]. These findings rather argue against mitochondrial dysfunction being the cause to develop insulin resistance and/or type 2 diabetes.
Therefore, we studied mitochondrial performance in slow-oxidative muscle in four well-established mouse models each showing a distinct combination of factors possibly responsible for mitochondrial dysfunction: (1) obese, hyperinsulinemic, but normoglycemic ob/ob mice as model for obesity; (2) obese, hyperinsulinemic, hyperglycemic, hyperlipidemic, insulin-resistant mice under high fat/high sucrose diet (HFD) as model for obesity associated type 2 diabetes; (3) mice lacking the InsR in muscle (MIRKO), displaying an isolated muscle insulin resistance with otherwise unaltered metabolic conditions; and (4) mice injected with streptozotocin (STZ) to destroy pancreatic β cells with severe hypoinsulinemia and hyperglycemia, but an intact InsR. We used mice exposed to these conditions for 5–7 months in order to study their long-term effects and studied slow-oxidative muscles since these muscles have been shown to be the prominent insulin-sensitive muscle in mice [14].
Materials and methods
Animals
Animal studies were approved by local government authorities (Bezirksregierung Köln and Landesamt für Naturschutz, Recklinghausen). In all models, male mice on a C57BL/6 background were analyzed at the age of 5–7 months. MIRKO mice (5 months) were generated as described previously [15]. Ob/ob mice (7 months) were obtained from Janvier Laboratory (Le Genest-St-Isle). In the HFD model, 3-week-old mice were fed a high fat, high sucrose diet (Altromin, Lage, Germany, #105712, 35.3% fat, 19.4% disaccharide, 19% protein) or a normal diet (Altromin, #TPF-1314, 5% fat, 4.8% disaccharide, 23% protein) for 6 months. In STZ mice, 65 mg/kg of streptozotocin was injected i.p. into 3-month-old mice on five consecutive days while controls were mock-injected with citrate buffer, and the animals were killed at 6 months of age.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed after 16 h of fasting by injecting i.p. 2 g/kg glucose or 0.75 U/kg insulin, respectively, and measuring consecutively glucose in tail vein blood using a glucometer.
Tissue preparation
Mice were sacrificed and slow-oxidative M. soleus and red and white M. gastrocnemius were separately dissected from both hind limbs; a part was shock frozen in liquid nitrogen and stored at −80°C, and the other part of M. soleus was used immediately for high resolution respirometry [16]. Blood was collected from the left ventricular cavity and serum was obtained by centrifugation. Serum insulin levels were determined by ELISA, and serum triglyceride and cholesterol levels were analyzed by routine clinical chemistry.
Western blots
Slow-oxidative muscle was homogenized and Western blots were performed as described previously [17]. The following antibodies were used: anti-complex I (Ndufa9), anti-complex II (Sdha), anti-complex III (Uqcrc2), anti-complex IV (mt-COXI subunit, Mtco1), anti-complex V (F1 beta subunit, Atp5b) from Molecular Probes/Invitrogen, anti-carbonylated groups and anti-beta-tubulin (Sigma), anti-MnSOD and anti-Cu/ZnSOD (Stressgen/Biotrend), anti-mouse-HRP, and anti-rabbit-HRP (Jackson Immunoresearch/Dianova).
For carbonylation blots, homogenates were first treated with equal volumes of 24% SDS and 2 vol of 20 mM 2,4-DNPH (dinitrophenylhydrazine) in 10% trifluoroacetic acid for 30 min in the dark, and neutralized with 2 M Tris, 30% glycerol, and 19% beta-mercaptoethanol buffer [18]. Homogenates containing 3 μg of protein were submitted to standard SDS–PAGE.
High resolution respirometry
Mitochondrial respiratory chain function was analyzed in slow-oxidative soleus muscle in situ as described previously [16]. Fibers were recovered after polarography for determination of dry weight and results were expressed as nanomoles of oxygen per minute per milligram of dry weight. Mitochondrial membrane integrity was verified by cytochrome c release test and determination of the respiratory control ratio (RCR) which was routinely >5.
Measurement of mitochondrial enzyme activities
Carnitine palmitoyl transferase activities (CPT I and II) were determined by a modified radiochemical assay reported by [19], while the tricarboxylic acid (TCA) cycle enzymes were assayed by state-of-the-art analyses [20].
Malondialdehyde (MDA) colorimetric assay
The assay was performed on slow-oxidative soleus muscle as described previously [21].
Nicotinamide nucleotide transhydrogenase (Nnt) mutation PCR analysis
PCR analysis was performed using forward and reverse primers Exon12_L1_R3, Exon6_L1_R4, and Exon12_L1_Exon6_L4 as described in [22].
Real-time PCR
Two micrograms of total RNA was reversely transcribed by one-step RT (RT–PCR Master Mix, Eurogentec). Real-time PCR was performed using TaqMan Universal PCR Master Mix on an ABI Prism 7700 sequence detector (Applied Biosystems). Specific primers and probes were purchased from Applied Biosystems. Calculations were done by a comparative method (2−ΔΔCt). Results were normalized to mRNAs to those “housekeeping genes”, which remained unchanged according to absolute Ct values: Hprt was used for ob/ob and HFD, Tbp for STZ-treated, and beta-2 microglobulin B2m for MIRKO mice.
Lipid extraction and thin layer chromatography analysis
The white part of M. gastrocnemius was homogenized in water using the Precellys 24 homogenizer (Peqlab, Erlangen, Germany) at 6,500 rpm for 30 s. The protein content of the homogenate was routinely determined using bicinchoninic acid. Lipids were extracted as previously described [23]. The lipid extract was applied to 20 × 10 cm high performance thin layer chromatography (HPTLC) Silica Gel 60 plates (Merck, Darmstadt, Germany), which were pre-washed twice with chloroform/methanol 1:1 and air-dried for 30 min. For quantification of triacylglycerols and cardiolipins, each lane of the TLC plate was loaded with the equivalent of 330 μg and 7.5 mg wet weight of muscle tissue, respectively. The TLC solvent system used for detection of triacylglycerols was hexane/toluene 1:1, followed by hexane/diethyl ether/glacial acetic acid 80:20:1. Glycerophospholipids were separated using chloroform/methanol/glacial acetic acid 65:28:8. For quantification of cholesterol, diacylglycerols, and free fatty acids, the equivalent of 15 mg of muscle tissue was applied to 20 × 20 cm TLC plates, which were developed in hexane/diethyl ether/formic acid 30:50:1. Quantitative analytical TLC determination was performed as previously described [23].
Liquid chromatography–tandem mass spectrometry analysis
Sphingolipid levels in skeletal muscle tissue were determined by liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC–ESI–MS/MS) using a previously described procedure [24].
PGC1-α levels and acetylation
After subsequent washing with relaxation buffer (0.1 M KCl, 5 mM MgCl2, 5 mM EGTA, 5 mM pyrophosphate, pH 6.8), red muscle was homogenized (0.25 M sucrose, 50 mM KCl, 5 mM MgCl2, 5 mM EGTA, 1 mM pyrophosphate, protease inhibitor, pH 6.8). Cell nuclei were isolated using an iodixanol gradient and lysed in RIPA buffer (50 mM Hepes, 1% Triton X-100, 50 mM NaCl, 0.1 M NaF, 10 mM EDTA, 10 mM Na4VO3, 0.1% SDS, protease inhibitors, pH 7.4). For immunoprecipitation, 500 μg of nuclear protein was incubated with 10 μl of anti-PGC1-α antibody (Santa Cruz, USA) and Protein A-Agarose. Then 100 μg of immunoprecipitated nuclear protein was used for SDS–PAGE, proteins were blotted on PVDF membranes (Bio-Rad, Hercules, USA), and Western blots were probed with a pan-acetyl-antibody (Santa Cruz). To quantify changes in optical density, AIDA software was used (Raytest).
Statistics
O’Brien’s OLS (ordinary least squares) test [25] was used to compare Western blot signals of complex I, III, and IV subunits. In all other cases, the two tailed Student’s t test was used; results are expressed as means ± standard deviation (SD).
Results
Mouse models
Obesity is strongly involved in the pathogenesis of type 2 diabetes; thus, we studied ob/ob mice with a twofold increased body weight and highly elevated insulin levels (Table 1). Despite their severe obesity, the 7-month-old ob/ob mice we used were not diabetic, as reported previously [15, 26]. In contrast, HFD mice were overweight and hyperinsulinemic, but also presented with hyperglycemia and hyperlipidemia, and were glucose intolerant and insulin resistant (Table 1 and Fig. 1a, b). In order to distinguish between this model of type 2 diabetes and one of its preceding steps, muscle insulin resistance, we analyzed mitochondrial performance in muscle-specific InsR knockout (MIRKO) mice being euglycemic (Table 1) and insulin sensitive [14] (data not shown). STZ-treated animals had lower body weight and serum insulin concentration was reduced by almost 80% compared to untreated littermates, resulting in severe hyperglycemia (Table 1), glucose intolerance, and insulin resistance (Fig. 1c, d).
Severely impaired glucose tolerance and insulin resistance in HFD and STZ mice. a Glucose tolerance test and b insulin tolerance test from HFD mice (black triangle) with respective controls (white circle). c Glucose tolerance test and d insulin tolerance test from STZ mice (black triangle) with respective controls (white circle). Gray filled triangles indicate blood glucose levels >600 mg/dl, the upper detection level of the system used, thus making the calculation of mean values impossible. Results in (b) and (d) are expressed as means normalized to t = 0 min values ± SD, n = 3–6, *p < 0.05, **p < 0.01, when analyzed by two-tailed Student’s t test
Since in C57BL/6 mice previously a mutation in nicotinamide nucleotide transhydrogenase (Nnt) has been described which could affect the mitochondrial defense against oxidative stress [22] and glucose tolerance under HFD conditions [27], we tested all mouse strains used concerning this mutation. MIRKO mice, STZ-treated C57BL/6 mice as well as HFD fed C57BL/6N mice did not carry the Nnt mutation (data not shown). However, ob/ob mice were only available on a C57BL/6J background carrying the Nnt mutation (data not shown).
Obesity, but not muscle InsR ablation (MIRKO) or STZ treatment, leads to accumulation of intramyocellular lipids (IMCLs)
Type 2 diabetes is frequently associated with increased levels of IMCLs, and lipotoxicity has been postulated to be the main cause of insulin resistance [8, 9]. To assess the relationship between elevated IMCLs and mitochondrial function, muscle lipid profiles were analyzed. We found increased levels of diacylglycerols (DAGs) and triacylglycerols (TAGs) in muscle of HFD mice (Fig. 2a, b). In ob/ob mice, elevated intramuscular levels of TAGs as well as DAGs have been reported recently [28]. Lipid levels were similar in muscle of STZ mice compared to controls (Fig. 2c, d). In MIRKO mice 1,2-DAG was significantly decreased, but cholesterol, free fatty acids, and TAGs remained unchanged (Fig. 2e, f). In order to confirm that accumulation of these lipids in obese mice is indeed sufficient to modulate muscle metabolism, we measured activities of the mitochondrial inner membrane fatty acid transporters. CPT-II (carnitine palmitoyl transferase II) was significantly elevated in ob/ob and HFD mice, whereas MIRKO mice showed decreased and STZ mice normal CPT-II activity (Fig. 2g–j). CPT-I activities changed in the same directions, but differences did not reach significance. In conclusion, the increased intramyocellular lipid levels observed in HFD and ob/ob mice obviously trigger an elevated lipid transport capacity into mitochondria, thus adapting to the high lipid load.
Lipid levels and CPT I/II activity in muscle of HFD, STZ-treated, and MIRKO mice. a Thin layer chromatography of white gastrocnemius muscle lipids from HFD mice and respective controls with b quantitative analysis. c Thin layer chromatography of white gastrocnemius muscle lipids from STZ mice and respective controls with d quantitative analysis. e Thin layer chromatography of white gastrocnemius muscle lipids from MIRKO mice and respective controls with f quantitative analysis. Cho cholesterol, FFA free fatty acids, TG triglycerides, CL cardiolipin. g–j Enzymatic activities of carnitine palmitoyl transferase (CPT) I and II of red gastrocnemius muscle determined by 14C-carnitine conversion to 14C-palmitoylcarnitine from g ob/ob mice, h HFD mice, i STZ mice, h MIRKO mice, and respective controls. Results are expressed as means ± SD, n = 4–5, *p < 0.05, **p < 0.01
Unchanged mitochondrial performance in slow-oxidative muscle of ob/ob and HFD mice
Oxygen flux supported by the complex I substrates pyruvate, glutamate + malate, and the complex II substrate succinate were unchanged in 7-month-old ob/ob mice (Fig. 3a). Since levels of individual subunits determined by Western blots largely represent levels of assembled oxidative phosphorylation complexes [29], we analyzed five representative subunits. In muscle of ob/ob mice, all respiratory chain complexes showed a tendency to increase (Fig. 3b, c). Since complexes I, III, and IV are strongly interdependent and assembled into supercomplexes or respirasomes [30], it is reasonable to assume that their steady-state levels change in parallel. Using O’Brien’s OLS test, we therefore assessed coordinated changes of these complexes and found a significant increase of complex I, III, and IV (p = 0.014). The tricarboxylic acid (TCA) cycle enzymes citrate synthase (CS) and isocitrate dehydrogenase (IDH) also tended to increase, and fumarase activity was significantly elevated in ob/ob mice (Fig. 3d). We conclude that hyperlipidemia and enhanced IMCLs [28] in ob/ob mice do not decrease, but rather increase activity and levels of mitochondrial enzymes and respiratory chain complexes.
Normal mitochondrial performance in muscle of ob/ob and HFD mice. a High resolution respirometry/oxidation rates in saponin skinned fibers of M. soleus from ob/ob mice and respective controls. b Steady-state levels of proteins representing the five respiratory chain complexes (I–V) determined by Western blotting of red gastrocnemius muscle from ob/ob mice with c quantification after normalization to beta-tubulin. d Activity of TCA cycle enzymes of red gastrocnemius muscle from ob/ob mice. e High resolution respirometry/oxidation rates in saponin skinned fibers of M. soleus from HFD mice and respective controls. f Steady-state levels of proteins representing the five respiratory chain complexes (I–V) determined by Western blotting of red gastrocnemius muscle from HFD mice with g quantification after normalization to beta-tubulin. e Activity of TCA cycle enzymes of red gastrocnemius muscle from HFD mice. Results were normalized to controls and expressed as means ± SD, n = 4–5, *p < 0.05, **p < 0.01 using two-tailed Student’s t test. Levels of complex I, III, and IV, assembled as supercomplex were compared to controls using O’Brien’s OLS test, # p < 0.05. pyr pyruvate, mal malate, glu glutamate, succ succinate, TMPD artificial complex IV substrate, CS citrate synthase, IDH isocitrate dehydrogenase
After 6 months of HFD, neither the oxidation of complex I or complex II substrates nor of the complex IV substrate TMPD was changed (Fig. 3e). Western blots showed a significant decrease of complex II in HFD mice, but the levels of subunits of the other four complexes were identical (Fig. 3f, g). Using O’Brien’s OLS test, no significant coordinated changes of complex I, III, and IV were found (p = 0.762). Enzyme activities of CS, IDH as well as fumarase were even slightly elevated in HFD mice (Fig. 3h). However, although HFD mice presented with hyperlipidemia, increased IMCLs, hyperglycemia and high insulin levels, performance, and levels of respiratory chain complexes were similar to controls. Thus, even the metabolic environment of long-lasting, overt type 2 diabetes does not cause decreased mitochondrial performance in mice.
Decreased respiratory chain performance in STZ and MIRKO mice
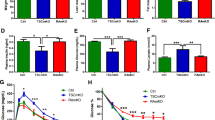
Since mitochondrial impairment could not be detected in these two mouse models of obesity and type 2 diabetes, we decided to further investigate the relation between insulin, the InsR, and mitochondrial function, using STZ-treated and MIRKO mice. STZ treatment resulted in a significantly reduced mitochondrial oxidation capacity through complex I and II, but not IV (Fig. 4a). The steady state level of complex III was significantly reduced and levels of complex I, II, and IV subunits decreased upon STZ treatment (Fig. 4b, c), which was found to be significant (O’Brien’s OLS test, p = 0.007). Levels of complex V, the ATP synthase as well as activities of CS, IDH, and fumarase tended to decrease (Fig. 4b–d). Thus, low insulin and high blood glucose levels are accompanied by a reduced respiratory chain performance, obviously due to a coordinated decrease of mitochondrial proteins involved in energy metabolism.
Decreased mitochondrial performance in muscle of STZ and MIRKO mice. a High resolution respirometry/oxidation rates in saponin skinned fibers of M. soleus from ob/ob mice and respective controls. b Steady-state levels of proteins representing the five respiratory chain complexes (I–V) determined by Western blotting of red gastrocnemius muscle from ob/ob mice with c quantification after normalization to beta-tubulin. d Activity of TCA cycle enzymes of red gastrocnemius muscle from ob/ob mice. e High resolution respirometry/oxidation rates in saponin skinned fibers of M. soleus from HFD mice and respective controls. f Steady-state levels of proteins representing the five respiratory chain complexes (I–V) determined by Western blotting of red gastrocnemius muscle from HFD mice with g quantification after normalization to beta-tubulin. e Activity of TCA cycle enzymes of red gastrocnemius muscle from HFD mice. Results were normalized to controls and expressed as means ± SD, n = 4–5, *p < 0.05, **p < 0.01, ***p < 0.001 using two-tailed Student’s t test. Levels of complex I, III, and IV, assembled as supercomplex, were compared to controls using O’Brien’s OLS test, # p < 0.05, ## p < 0.01. pyr pyruvate, mal malate, glu glutamate, succ succinate, TMPD artificial complex IV substrate, CS citrate synthase, IDH isocitrate dehydrogenase
In MIRKO mice, we likewise found a significant reduction of mitochondrial oxidation capacity through complexes I, II, and IV (Fig. 4e). Levels of complex I, III, and IV subunits were significantly decreased (Fig. 4f, g) (O’Brien’s OLS test, p = 0.038). CS and IDH activity were slightly reduced and fumarase activity was significantly reduced (Fig. 4h). In conclusion, InsR ablation also leads to an attenuated mitochondrial oxygen flux in situ as well as to decreased levels of respiratory chain complexes.
In order to test if the observed changes were specific for the respiratory chain and TCA cycle enzymes, we also assessed levels of HSP-60, a high abundance chaperone and marker of the mitochondrial matrix. This was found unchanged in all models (data not shown), suggesting a normal mitochondrial “content”, which is strongly supported by similar levels of cardiolipin, a mitochondrial inner membrane lipid (Fig. 2a–f).
No evidence for oxidative stress contributing to decreased mitochondrial performance
Oxidative stress has been discussed to contribute to decreased mitochondrial activity in type 2 and also in type 1 diabetic muscle [31]. To study whether oxidative stress was indeed increased, we performed Western blots detecting carbonylated proteins (oxyblots) as well as levels of key defense enzymes against reactive oxygen species (ROS). Furthermore, oxidative stress was analyzed using a malondialdehyde (MDA) colorimetric assay. We found similar degrees of protein carbonylation in MIRKO, ob/ob, HFD, and STZ mice (Fig. 5a–h). When ROS are increased, cells generally upregulate the scavenger enzymes cytosolic Cu/ZnSOD (SOD-1) and mitochondrial MnSOD (SOD-2). However, Western blots showed normal or even slightly reduced levels of Cu/ZnSOD and MnSOD levels in our mouse models (Fig. 5i–l), and MDA assays revealed no significant changes in muscle of the different genotypes (data not shown). These data exclude increased ROS as the cause for decreased mitochondrial performance in MIRKO as well as in STZ-treated mice.
No evidence for increased oxidative stress in muscle of all mouse models. Slow-oxidative muscle homogenates from a, b ob/ob, c, d HFD, e, f STZ, and g, h MIRKO mice analyzed using a, c, e, g Western blot with anti-DNP antibody and b, d, f, h acrylamide gels run in parallel stained with Coomassie blue. I–l Western blots of slow-oxidative muscle revealing steady-state levels of MnSOD and Cu/ZnSOD with beta-tubulin as loading control from i ob/ob, j HFD, k STZ, and l MIRKO mice. C control, O ob/ob, H HFD, S STZ, M MIRKO mice
Reduced expression of mitochondrial genes is responsible for decreased OXPHOS complexes in STZ-treated but not in MIRKO mice
To test if decreased respiratory chain subunits in MIRKO and STZ mice were due to decreased gene expression, real-time PCR was used to quantify mRNA levels of these subunits as well as PGC-1α, a master co-activator regulating many mitochondrial genes. Furthermore, mRNA of PPARγ and CPT enzymes were analyzed. Expression of subunits of complex I, III, IV, and V was significantly reduced in STZ mice, but unchanged in ob/ob and HFD mice (Fig. 6a–d; upper panels). In MIRKO mice, mRNA of the complex IV subunit Cox4i1 was increased, but levels of all other mRNAs tested were unchanged. PGC-1α and PPARγ mRNA levels were decreased in STZ treated mice, but unaltered in ob/ob, HFD, and MIRKO mice. Furthermore, CPT-I and CPT-II mRNA levels were elevated in ob/ob and in HFD mice, in accordance with changes in enzymatic activity (Fig. 2g, h), but decreased in STZ mice (Fig. 6a–d; lower panels). Recently, it was reported that heme oxygenase expression is low in insulin-resistant liver of ob/ob mice as well as mice lacking InsR substrate-1 (IRS-1) and IRS-2 proteins in liver [32]. Downregulation of heme oxygenase gene expression was proposed to be responsible for a selective depletion of the heme-containing complexes III and IV, but not I and II. Thus, we measured heme oxygenase mRNA by real-time PCR in all our models, but could not find any differences (data not shown), excluding this as a possible mechanism for mitochondrial dysfunction in muscle of MIRKO and STZ-treated mice.
Reduced expression of mitochondrial genes in muscle of STZ mice. qPCR analysis of tibialis cranialis muscle from a ob/ob, b HFD, c STZ, d MIRKO showing mRNA levels of representative subunits from respiratory chain complexes Co I–Co V, the transcriptional coactivator PGC-1α, one of its target transcription factors PPARγ, and key enzymes of the mitochondrial fatty acid oxidation, CPT-I and CPT-II. Results were normalized to controls and expressed as means ± SD, n = 4–5, *p < 0.05, **p < 0.01, two-tailed Student’s t test
These results suggest that a coordinated downregulation of mitochondrial gene expression, probably induced by decreased PGC-1α-mediated transcription, is responsible for the suppressed mitochondrial respiratory chain complex levels in STZ, but not in MIRKO mice. Consequently, we analyzed nuclear extracts from muscle of STZ-treated mice and found a 40% decrease (p < 0.05) of PGC-1α protein levels (Fig. 7a, b). However, acetylation of the protein, which strongly influences its coactivation activity [33], was unchanged (Fig. 7c, d).
Decreased level but unchanged acetylation of PGC-1α in muscle of STZ-treated mice. a Western blots of nuclear extracts from red gastrocnemius muscle probed for PGC-1α with b densitometric evaluation. c Western blots of proteins after immunoprecipitation (IP) of PGC-1α with d densitometric evaluation
Increased autophagy in muscle of STZ mice
Ultimately, we analyzed autophagy, a degradation process important for cellular maintenance, degrading portions of the cytoplasm containing proteins as well as organelles [34]. Since InsR signaling has been shown to inhibit autophagy [35], we addressed the question whether this process is induced in muscle of MIRKO mice or by low insulin levels (STZ). The LC3 protein is involved in the execution of the autophagic process and serves as a convenient readout since the activated phosphatidylethanolamine-conjugated, membrane-bound LC3 isoform II migrates faster [36]. Indeed, we found more membrane-bound LC3 in STZ mice (Fig. 8d), but not in MIRKO, HFD, and ob/ob animals (Fig. 8a–c). Thus, upregulated autophagy in muscle of STZ-treated mice might be a response to extremely low insulin levels. However, absence of the InsR in MIRKO did not induce autophagy, indicating that deficiency of InsR signaling alone is not sufficient to trigger this process.
Elevated glucosylceramide levels in MIRKO mice
In addition to 1,2-DAG and other toxic IMCLs, ceramides were also reported to accumulate and impair InsR signaling via enhanced serine phosphorylation of IRS-1 in type 2 diabetic muscle [3]. Levels of all ceramide species analyzed were markedly increased in HFD mice (Fig. 9a) and were shown to be increased in muscle of ob/ob mice recently [28]. However, also in MIRKO mice, we found a significant increase in levels of 16:0, 20:0, and 24:1 ceramides (Fig. 9b), excluding an important contribution of augmented ceramide levels to mitochondrial dysfunction.
Elevated levels of all ceramides in HFD mice and increased levels of all glucosylceramides in MIRKO mice. Liquid chromatography–tandem mass spectrometry (LC–ESI–MS/MS) of ceramide levels from a HFD and b MIRKO mice and glucosylceramide levels from c HFD and d MIRKO mice. Results are means ± SD of three analytical replicates of three biological replicates. Data were normalized to controls and expressed as means ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Student’s t test
Ceramides and glucosylceramides do not represent a significant proportion in isolated mitochondria compared to muscle homogenates. Liquid chromatography–tandem mass spectrometry (LC–ESI–MS/MS) of a ceramide and b glucosylceramide levels from isolated mitochondria of muscle tissue compared to total muscle homogenate. Results are means ± SD of three analytical replicates, *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Student’s t test
Finally, glucosylceramides are not only important constituents of the plasma membrane domains containing the InsR [3] and have been reported to be increased in muscle and liver of obese rodent models [37], but have also been found in both mitochondrial membranes [38] where they might disturb oxidative phosphorylation [39]. In MIRKO muscle, all glucosylceramides were significantly increased, while they remained unchanged or even decreased under HFD (Fig. 8c, d). To investigate if a significant proportion of this lipid class is present in muscle mitochondria, we analyzed highly purified organelles. Indeed, 18:0, 24:0, and 24:1 ceramides were found in relatively high concentrations (Fig. 10a), while glucosylceramides, although detectable, were present in much lower concentrations (Fig. 10b). Taking into account that about 20% of total protein is mitochondrial protein in slow-oxidative muscle [40], most of the glucosylceramides found in muscle homogenates are thus constituents of non-mitochondrial membranes. Therefore, increased glucosylceramides may be a consequence of, but likely not a cause for, mitochondrial dysfunction.
Discussion
Mitochondrial dysfunction in human muscle was postulated to be a key factor in the pathogenesis of insulin resistance and type 2 diabetes mellitus [2]. In contrast, several other studies revealed unchanged mitochondrial ATP production or respiration in patients with type 2 diabetes [41–43]. Moreover, data obtained in rodents suggested that insulin resistance induced by high-fat diet (HFD) rather increased PGC-1α protein levels, activated fatty acid oxidation enzymes, CPT-I, TCA cycle enzymes including CS, and enhanced ADP-stimulated mitochondrial respiration [44, 45]. Taken together, the role of mitochondrial function in the pathogenesis of insulin resistance and type 2 diabetes as well as the impact of InsR signaling itself on mitochondrial performance are still largely unknown.
In order to analyze a possible connection between InsR signaling, insulin resistance, type 2 diabetes, and mitochondrial function, we analyzed slow-oxidative red muscle of four different mouse models with (1) isolated muscle insulin resistance (MIRKO), (2) HFD-induced obesity, insulin resistance, and diabetes, (3) obesity in the absence of diabetes (ob/ob mice), and (4) hypoinsulinemia and hyperglycemia following STZ treatment. We find that obesity-associated type 2 diabetes in HFD mice does not lead to any impairment of mitochondrial performance, even in the presence of elevated levels of DAGs, TAGs, and ceramides, which have been postulated to cause insulin resistance [2, 3]. Thus, insulin resistance or intramyocellular lipid accumulation alone is neither cause nor consequence of mitochondrial dysfunction in mouse skeletal muscle. Accordingly, ob/ob mice showed unchanged mitochondrial respiration and even increased levels of respiratory chain proteins. Therefore, we postulate that decreased mitochondrial mass and/or performance in non-diabetic/obese or in type 2 diabetic/obese patients observed in clinical studies have to be explained by genetic predisposition, physical inactivity, or other still unknown factors and are probably not causally involved in disease development or progression.
Oxidative stress has been discussed to contribute to decreased mitochondrial activity in type 2 and also in type 1 diabetic muscle [31]. However, analysis of oxidative stress using two independent methods revealed no difference between the different genotypes and their respective controls. Even in the presence of the Nnt mutation in the ob/ob mouse strain, neither increased oxidative stress nor reduced mitochondrial activity was found. These data exclude increased ROS as a cause of decreased mitochondrial performance.
Previously, skeletal muscle of mice on a HFD was shown to have reduced complex I levels, CS activities, and PGC-1α mRNA levels as well as reduced mitochondrial area judged by electron microscopy [31]. The discrepancy between these data and our study is likely due to the different muscle types used: Most of the studies reported in the literature, which examine mitochondrial function in insulin resistant human subjects or animals, including Bonnard’s data [31], use M. vastus lateralis or M. gastrocnemius. These muscles mainly consist of fast-glycolytic, white fibers, which however are hardly responsive to insulin [14, 46] and thus cannot contribute significantly to muscle insulin resistance. The decreased mitochondrial content reported in these muscles may simply be due to physical inactivity after HFD-induced obesity. In contrast to these data, we analyzed slow-oxidative red muscle tissue rich in mitochondria, which is a permanently active, postural muscle capable of using lipids as fuel [47, 48]. Although this muscle type only represents a minor fraction of total muscle mass in mice, it is the most insulin sensitive, maybe even the only insulin sensitive type of muscle [14, 46].
In STZ-treated mice with severely increased glucose and very low insulin levels, decreased mRNA levels for mitochondrial proteins are probably responsible for impaired respiration in skeletal muscle, supporting the functional significance of previously published micro-array data [49]. Furthermore, short-term insulin infusion was shown to directly stimulate mitochondrial oxidative phosphorylation in human skeletal muscle [50]. Furthermore, mitochondrial dysfunction was reported in patients with type 1 diabetes [51]. In order to dissect whether there is a direct relationship between the InsR and mitochondrial function in the presence of normal serum parameters and unimpaired glucose tolerance, we analyzed mitochondrial performance in MIRKO mice. Interestingly, muscles from these mice showed reduced mitochondrial performance and respiratory chain complex levels, consistent with a very recent study analyzing in vivo mitochondrial function by MRS from patients harboring missense mutations in the InsR gene [52]. Thus, intact InsR signaling is mandatory to maintain normal respiratory chain function.
In patients with type 2 diabetes as well as in obesity, aging plays an important role concerning disease progression. Here, mice were analyzed with 6 months of age. Even though we could not show any difference in mitochondrial function in respect to glucose metabolism and InsR signaling, these factors could gain more importance with older age. Further studies will be needed to address the influence of aging on glucose metabolism and mitochondrial performance.
In summary, the present study reveals several new findings: (1) In mice, mitochondrial dysfunction is not a consequence of obesity-associated insulin resistance under normoglycemic conditions; (2) obesity-associated type 2 diabetes as well as DAG or TAG accumulation is not associated with impaired mitochondrial function; and (3) severe hypoinsulinemia and long-term hyperglycemia in STZ-treated mice or complete absence of the InsR in MIRKO mice cause mitochondrial dysfunction due to decreased respiratory chain complex expression via two distinct molecular mechanisms.
References
Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP (2005) A potential link between muscle peroxisome proliferator-activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 1:133–144
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Holland WL, Summers SA (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29:381–402
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671
Petersen KF, Dufour S, Shulman GI (2005) Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2:e233
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E et al (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Szendroedi J, Roden M (2009) Ectopic lipids and organ function. Curr Opin Lipidol 20:50–56
Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK (2009) Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801:266–271
Wredenberg A, Freyer C, Sandstrom ME, Katz A, Wibom R, Westerblad H, Larsson NG (2006) Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun 350:202–207
Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G et al (2007) Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131:476–491
Han DH, Hancock CR, Jung SR, Higashida K, Kim SH, Holloszy JO (2011) Deficiency of the mitochondrial electron transport chain in muscle does not cause insulin resistance. PLoS One 6:e19739
Schiff M, Loublier S, Coulibaly A, Benit P, de Ogier B, Rustin P (2009) Mitochondria and diabetes mellitus: untangling a conflictive relationship? J Inherit Metab Dis 32:684–698
Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2:559–569
Wade JM, Juneja P, MacKay AW, Graham J, Havel PJ, Tecott LH, Goulding EH (2008) Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology 149:955–961
Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3:965–976
Franko A, Mayer S, Thiel G, Mercy L, Arnould T, Hornig-Do HT, Wiesner RJ, Goffart S (2008) CREB-1alpha is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol Cell Biol 28:2446–2459
Kleist-Retzow JC, Hornig-Do HT, Schauen M, Eckertz S, Dinh TA, Stassen F, Lottmann N, Bust M, Galunska B, Wielckens K et al (2007) Impaired mitochondrial Ca2+ homeostasis in respiratory chain-deficient cells but efficient compensation of energetic disadvantage by enhanced anaerobic glycolysis due to low ATP steady state levels. Exp Cell Res 313:3076–3089
Kerner J, Parland WK, Minkler PE, Hoppel CL (2008) Rat liver mitochondrial carnitine palmitoyltransferase-I, hepatic carnitine, and malonyl-CoA: effect of starvation. Arch Physiol Biochem 114:161–170
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Hornig-Do HT, von Kleist-Retzow JC, Lanz K, Wickenhauser C, Kudin AP, Kunz WS, Wiesner RJ, Schauen M (2007) Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol 127:1084–1093
Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ (2006) Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet 15:1187–1194
Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Bronneke HS, Brodesser S, Hampel B, Schauss AC et al (2010) Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA 107:6028–6033
Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH Jr (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res 50:1692–1707
O’Brien PC (1984) Procedures for comparing samples with multiple endpoints. Biometrics 40:1079–1087
Lindstrom P (2007) The physiology of obese-hyperglycemic mice [ob/ob mice]. Sci World J 7:666–685
Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD (2006) Deletion of nicotinamide nucleotide transhydrogenase: a new quantitative trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55:2153–2156
Turpin SM, Ryall JG, Southgate R, Darby I, Hevener AL, Febbraio MA, Kemp BE, Lynch GS, Watt MJ (2009) Examination of ‘lipotoxicity’ in skeletal muscle of high-fat fed and ob/ob mice. J Physiol 587:1593–1605
Schagger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19:1777–1783
Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32:529–539
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800
Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF (2009) Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15:1307–1311
Canto C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20:98–105
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518
Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Aten J, Kuipers F, Serlie MJ, Wennekes T et al (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56:1341–1349
Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J (2001) Occurrence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett 488:160–164
Strasberg P (1986) Cerebrosides and psychosine disrupt mitochondrial functions. Biochem Cell Biol 64:485–489
Ljubicic V, Hood DA (2008) Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am J Physiol Endocrinol Metab 295:E195–E204
Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA (2008) Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57:1166–1175
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796
Rabol R, Hojberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F (2009) Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 94:1372–1378
Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ (2007) Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56:2085–2092
Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO (2008) High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105:7815–7820
Bonen A, Holloway GP, Tandon NN, Han XX, McFarlan JT, Glatz JF, Luiken JJ (2009) Cardiac and skeletal muscle fatty acid transport and transporters, triacylglycerol and fatty acid oxidation in lean and Zucker diabetic fatty (ZDF) rats. Am J Physiol 297:R1202–R1212
Ogata T, Yamasaki Y (1997) Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248:214–223
Gollnick PD, Sjodin B, Karlsson J, Jansson E, Saltin B (1974) Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch 348:247–255
Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, Kahn CR (2004) Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA 101:16525–16530
Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS (2003) Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001
Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS (2007) Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 56:2683–2689
Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S et al (2011) Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest 121:2457–2461
Acknowledgments
We acknowledge Puk Andreasen and Benjamin August (Taconic Europe, Lille Skensved, Denmark) for sharing data on blood sugar values of old ob/ob mice and Dr. Martin Hellmich (Institute of Medical Statistics, Informatics and Epidemiology, University of Cologne) for advice with statistical analyses. Prof. Jens Brüning (Department of Mouse Genetics and Metabolism, University of Cologne) provided MCK-Cre and InsR-loxP mice and Maria Bust, Alexander Müller, and Olivia Kawaletz provided excellent technical assistance. This work was funded by Köln Fortune (WK, MS, RJW), Center for Molecular Medicine Cologne (JCvKR), and Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (WK, MS, RJW).
Disclosure statement
The authors declare that there is no conflict of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Franko, A., von Kleist-Retzow, J.C., Böse, M. et al. Complete failure of insulin-transmitted signaling, but not obesity-induced insulin resistance, impairs respiratory chain function in muscle. J Mol Med 90, 1145–1160 (2012). https://doi.org/10.1007/s00109-012-0887-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0887-y