Abstract
Human anion exchanger 2 (AE2) is a plasma membrane protein that regulates intracellular pH and cell volume. AE2 contributes to transepithelial transport of chloride and bicarbonate in normal colon and other epithelial tissues. We now report that AE2 overexpression in colon cancer cells is correlated with expression of the nuclear proliferation marker, Ki67. Survival analysis of 24 patients with colon cancer in early stage or 33 patients with tubular adenocarcinoma demonstrated that expression of AE2 is correlated with poor prognosis. Cellular and molecular experiments indicated that AE2 expression promoted proliferation of colon cancer cells. In addition, we found that transcription factor EGR1 underlies AE2 upregulation and the AE2 sequester p16INK4a (P16) in the cytoplasm of colon cancer cells. Cytoplasmic P16 enhanced ERK phosphorylation and promoted proliferation of colon cancer cells. Gastrin inhibited proliferation of colon cancer cells by suppressing expression of EGR1 and AE2 and by blocking ERK phosphorylation. Taken together, our data describe a novel EGR1/AE2/P16/P-ERK signaling pathway in colon carcinogenesis, with implications for pathologic prognosis and for novel therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon cancer is a major global public health problem with rapidly rising incidence. Current estimates of annual deaths from colon cancer in China range from 60,000 to 90,000. Our molecular understanding of the genetic and epigenetic changes that drive the transformation of normal colonic epithelial cells and the progression of colon cancer has progressed rapidly. Examples include mutation or deletion of APC, MCC, and KRAS genes. These genetic changes disrupt or alter multiple signaling pathways such as Wnt/β-catenin, Notch, Hedgehog, epidermal growth factor receptor, Ras, and PI3K/Akt [1].
Mitogen-activated protein kinases (MAPK) transmit cell-proliferation signals from plasma membrane to nucleus. Among the MAPKs, ERK may be the most important for stimulation of cell proliferation [2, 3]. Intestinal epithelial differentiation is dependent on activation of the ERK MAPK pathway, and increasing evidence also suggests involvement of the ERK pathway in the pathogenesis and progression of human colon cancer [4]. Although the ERK MAPK cascade may have potential as a biomarker or as a therapeutic target for prevention or treatment of colon cancer, the molecular mechanisms underlying aberrant activation of the ERK pathway remain largely undefined.
The SLC4 bicarbonate transporter gene family includes the Na+-independent electroneutral anion exchangers AE1/SLC4A1, AE2/SLC4A2, and AE3/SLC4A3 [5, 6]. AE1 is expressed predominantly in erythrocytes and renal type A intercalated cells; AE2 is widely expressed, but is most abundant in parietal cell of stomach and in choroid plexus [7]; and AE3 is predominantly expressed in brain and heart. All members of AE polypeptides have three structural domains. An N-terminal cytoplasmic domain is followed by a transmembrane domain and completed by a short C-terminal cytoplasmic domain. The AEs mediate the exchange of Cl−/HCO −3 to regulate intracellular pH and cell volume. Although the ion transport functions of AEs have been extensively studied, AE expression and function in tumorigenesis remain poorly characterized. We previously found that aberrant expression of AE1 and AE2 is associated with gastric carcinogenesis [8], and others reported that AE2 was overexpressed in human hepatocellular carcinoma cells [9, 10]. However, the role of AE polypeptides in malignant transformation of the cells remains unclear.
We now report that AE2 was overexpressed in colon cancer in a largely cytoplasmic distribution, and under regulation of the transcription factor EGR1. AE2 interacted with tumor suppressor P16 in the cytoplasmic region of colon cancer cells, leading to activation of a novel EGR1/AE2/P16/P-ERK signaling pathway. Gastrin inhibited EGR1 expression, leading to downregulation of AE2 and P16, ERK dephosphorylation, and growth inhibition of colon cancer cells.
Materials and methods
Antibodies and reagents
For immunoblotting, the antibodies below were used in this study: anti-AE2 C-terminal antibody [11], anti-cyclin D1 (sc-8396, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-P-ERK (Cell Signaling Technology, Danvers, MA, USA), anti-ERK (Cell Signaling), anti-β-catenin (sc-59737, Santa Cruz), anti-P16 (sc-81613, Santa Cruz), anti-CCKBR (sc-33221, Santa Cruz), and anti-EGR1 (sc-189, Santa Cruz). For immunohistochemistry, antibodies anti-SLC4A2 (HPA019339, Sigma), anti-Ki67 (RMA-0129, Maixin_Bio, Fuzhou, China), anti-P16 (BA0266, Boster, Wuhan, China), and anti-EGR1 (sc-189, Santa Cruz) were used in this study. For immunofluorescence assay, anti-SLC4A2 (HPA019339, Sigma) and anti-P16 antibodies (sc-81613, Santa Cruz) were used in this study. Gastrin was obtained from ChinaPeptides, China. H2O2 was purchased from Sinopharm Chemical Reagent Company, China. U0126, proglumide, and 5-Fluorouracil (5-Fu) were obtained from Sigma, USA. PD98059 was purchased from Beyotime Institute of Biotechnology, China. EGR1-siRNA (sc-44203) was purchased from Santa Cruz Biotechnology, CA. AE2-siRNA was designed and synthesized by Shanghai GenePharma, China.
Tissue samples
One fresh colon tumor sample was obtained by surgical resection from Shanghai Shidong Hospital in China. The fresh colon tumor tissue and the adjacent normal-appearing non-tumor tissue were used to test AE2 expression by RT-PCR. Paraffin-embedded colon cancer specimens resected from 202 patients (89 mucinous adenocarcinoma, 112 tubular adenocarcinoma, and 1 carcinoid tumor) were obtained from Zhenjiang First People’s Hospital, China. Clinical protocols were approved by the Shanghai Jiao Tong University Ethics Committee.
Immunohistochemistry
Deparaffinized sections were treated with 3% H2O2 and subjected to antigen retrieval by citric acid (pH 6.0). After overnight incubation with primary antibody at 4°C, the sections were incubated 15 min at room temperature with horseradish peroxidase-labeled polymer conjugated with secondary antibody (MaxVision™ Kits) and incubated 3 min with diaminobenzidine. All sections were evaluated by two independent observers (Song and Zeng) unaware of the disease outcome. For Ki67 expression, distinct staining was considered to be positive, regardless of the staining intensity. For AE2, P16, and EGR1, less than 10% expression was considered to represent “loss” (−), and >10% expression were designated (+).
Cell culture and reagent treatment
The following cell lines were used: human colon cancer cell lines SW1116, HT29, SW480, HCT116, LS174T, and Colo205; and human embryonic kidney HEK293T cells (Cell Bank of Shanghai Institutes for Biological Sciences, Shanghai, China). Cells were maintained at 37°C in Dulbecco’s modified Eagles medium or RPMI1640 (Gibco) containing 10% fetal bovine serum (Hyclone), 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Invitrogen) in 5% CO2. SW1116 cells were treated with drugs (gastrin, H2O2, U0126, PD98059, proglumide) and examined by immunoblotting.
Plasmid construction
To construct pEGFP-C1-AE2, a 3.7-kb EcoR I-Spe I fragment from PXT7-AE2a [12] was inserted in-frame into pEGFP-C1 plasmid with DNA polymerase I (Klenow) fragment (M0210, New England Biolabs). pGL3-AE2, a human AE2 promoter reporter construct (−769/−64 relative to the initiator codon ATG), was generated by PCR and cloned into the pGL3-Basic vector (Promega, Madison, WI, USA). pEGFP-P16 and pSIREN-P16 plasmids were previously described [13].
RNA extraction and RT-PCR
RNA was extracted from fresh colonic cancer tissue and fresh adjacent tissue with TRIZOL (15596-026, Invitrogen) following the manufacturer’s instructions. Reverse transcription was performed with ReverTra Ace-α-(FSK100, TOYOBO). The amplified fragments were resolved by 1% agarose gel electrophoresis with ethidium bromide staining. The primers used for quantitative PCR were as follows: AE2 forward (5′-GTGGACGGCGGTGAGAACA-3′) and AE2 reverse (5′-CGTGAGTGACAGAGCGGACAG-3′) yielded an amplification product 607 nt in length. GAPDH forward (5′-GAAGGTGAAGGTCGGAGTC-3′), GAPDH reverse (5′-GAAGATGGTGATGGGATTTC-3′) yielded a product 226 nt in length.
Immunoblotting
Cell lysates equalized for protein content were loaded onto an 8–12% acrylamide gel, electrophoresed, and transferred onto nitrocellulose membranes. After blocking with 5% nonfat milk for 1 h, primary incubation was performed overnight at 4°C. Blots were incubated with peroxidase-conjugated secondary antibodies for 1 h at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence reagent.
Luciferase assay
SW1116 cells were transiently transfected with the luciferase vector pGL3-AE2 or with empty vector (pGL3). A pRL-TK reporter construct was co-transfected in the cells to normalize for transfection efficiency. Luciferase activity measured by luminometer (Berthold Detection Systems, Pforzheim, Germany) was normalized for transfection efficiency by Renilla luciferase, using a dual luciferase assay kit.
Cell fractionation
Cell fractionation was as previously described [14].
Immunoprecipitation
SW1116 cells were lysed in immunoprecipitation (IP) buffer (20 mM HEPES, pH 7.5, 50 mM KCl, 1 mM EDTA, 1% NP40, 0.2 mM PMSF) and lysates were incubated overnight at 4°C with 1 μg anti-AE2 (AE21-S, Alpha diagnostic Intl Inc, San Antonio, USA) or 1 μg of rabbit IgG. Immune complexes were captured on protein A/G plus-agarose (sc-2003, Santa Cruz) at 4°C for 2 h, washed in PBS (pH 7.4), and analyzed by immunoblotting with anti-P16 antibody (sc-81613, Santa Cruz).
Immunofluorescence assay
Cells were washed twice with PBS, fixed with cold acetone for 15 min, and treated with 0.2% Triton X-100 for 4 min or with 0.5% Triton X-100 for 10 min. After 3 washes with PBS, permeabilized cells were blocked with 5% BSA for 20 min. Cells were incubated with primary antibody for 1 h at 37°C, and then with fluorochrome-conjugated secondary antibody for 1 h at room temperature. After washed three times with PBS, cells were stained with DAPI for 5 min. Following three more PBS washes, cells were observed by fluorescence microscopy.
Manual cell counting
Exponentially growing cells of SW1116 were plated into 12-well plates containing 1 ml medium per well, and medium was changed daily. Cells were digested and suspended in 1 ml medium, mixed well, and 10 μl was removed for cell counting by hemocytometer. The rate of growth inhibition (%) was calculated as = (B − A)/B × 100, where A is the cell numbers of treated cells and B is the cell numbers of untreated control cells.
Clonogenic assay
SW1116 cells (600 per well) were plated (in triplicate) in 6-well plates. After 24 h, cells were treated up to 10 days with 10−7 M gastrin. Colonies were fixed with 10% buffered formalin, stained with 0.1% crystal violet, counted, and normalized to the number of control colonies.
Statistical analysis
Experimental data were analyzed with SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). Comparisons of proportions were evaluated using a chi-square test. Student’s two-tailed t test was used to compare data between two groups. Values are expressed as mean ± SD. Survival was analyzed by conducting a standard Kaplan–Meier analysis with a log-rank test. Statistical differences with P values less than 0.05 were considered to be significant.
Results
Overexpression of AE2 contributed to progression of colon cancer
One fresh sample of colon cancer tissue with adjacent normal tissue (obtained from Shanghai Shidong Hospital) was used to detect expression of AE2 mRNA. Figure 1a shows that AE2 mRNA was more highly expressed in colon cancer tissue than in adjacent normal-appearing tissue (Fig. 1a (a), top right corner). Immunohistochemistry showed that the AE2 protein was strongly expressed on both plasma membrane and in a cytoplasmic distribution in cancerous tissue as compared with adjacent normal areas (Fig. 1a (a–c)). AE2 expression was detected histologically in 69.3% of paraffin-embedded specimens (140/202 cases), but in only 38.9% (44/113 cases) in adjacent normal (para-cancer) tissues (Fig. 1b). In AE2-positive sections, AE2 staining intensity was also stronger in cancerous than in adjacent normal tissues.
Overexpression of AE2 was correlated with cell proliferation in colon cancer. (a) AE2 mRNA was analyzed by RT-PCR (a, upper right corner: 1 adjacent normal tissue, 2 colon cancer tissue; upper bands AE2, lower bands GAPDH). AE2 protein was analyzed by immunohistochemistry. The arrowed profiles in (a) were enlarged in (b, black arrow) and (c, red arrow). Original magnifications ×40 in a and ×400 in b and c. (b) AE2 expression in para-cancer and colon cancer tissues was statistically analyzed. (c) Co-localization of AE2 and Ki67 in colon cancer tissues detected by immunohistochemistry. The arrowed profiles in (a) were enlarged in (c, black arrow) and (e, red arrow). The arrowed profiles in (b) were enlarged in (d, black arrow) and (f, red arrow). Original magnifications ×200 in a and b, ×1,000 in c, d, e, and f. (d) Association of histochemically detected expression of AE2 and Ki67 in colon cancer sections. (e) Growth curves of SW1116 cells transfected with siRNA-AE2 or siRNA-NC (NSC). Inset immunoblot analysis of AE2 and cyclin D1; *P < 0.05 (n = 3). (f) Growth curves of SW1116 cells transfected with pEGFP-AE2 or pEGFP-C1 empty vector (control). Inset immunoblot analysis of AE2 and cyclin D1; *P < 0.05 (n = 3)
AE2 expression in colon cancer sections was compared with expression of nuclear antigen Ki67, a proliferation marker in cycling cells. As shown in Fig. 1c, d, AE2 and Ki67 were both expressed in the same cells in tubular adenocarcinoma. Statistical analysis demonstrated that the AE2 expression significantly correlated with expression of Ki67. Knockdown of AE2 by AE2-targeted siRNA remarkably inhibited the growth of SW1116 cells (Fig. 1e). To further determine the role of AE2 in proliferation of colon cancer cells, AE2 cDNA was transfected into SW1116 cells. Figure 1f shows that overexpression of AE2 significantly promoted proliferation of SW1116 cells. Clinicopathological analysis of tubular adenocarcinomas with AE2 expression demonstrated that the AE2 expression was correlated with bigger tumor size and poor differentiation state (Table 1).
The P16/P-ERK is involved in AE2-promoted proliferation of colon cancer cells
We found previously that the AE1 C-terminus interacts with tumor suppressor P16 to sequester and inactivate it in the cytoplasm of gastric cancer cells [14, 15]. The 60% amino acid identity between the C-terminal cytoplasmic tails of AE1 and AE2 suggests that P16 may also bind to the AE2 C-terminal region. To test this, co-IP experiments was performed and the results confirmed interaction of P16 and AE2 proteins (Fig. 2a). To explore how AE2 and P16 contribute to proliferation of colon cancer cells, AE2 was overexpressed in SW1116 cells and the expression levels of β-catenin and ERK were tested. The Wnt/β-catenin signaling pathway has been strongly linked to the pathogenesis and progression of colon cancer [16, 17], and we hypothesized that the components of this signaling pathway may be regulated in part by AE2. In addition, AE2 expression correlated with MEK/MAPK pathway activity during mouse oocyte meiosis [18]. Indeed, AE2 overexpression elevated the levels of cyclin D1, P16, and phosphorylated ERK, but did not affect expression (Fig. 2b) or localization (data not shown) of β-catenin. Relative expression of P-ERK and P16 (normalized to β-actin) was statistically analyzed in Fig. 2c. The increased P16 was accumulated in the post-nuclear cytoplasmic fraction of SW1116 cells (Fig. 2d).
Interaction of AE2 and P16 in cytoplasm was associated with elevated P-ERK abundance in colon cancer cells. (a) Interaction of AE2 with P16 in SW1116 cells. Cell lysates were subjected to immunoprecipitation with antibodies to AE2 or with rabbit serum. Immunoprecipitates were then immunoblotted with antibody to P16. (b) Immunoblot detection of cyclin D1, β-catenin, ERK, P-ERK, and P16 proteins in SW1116 cells 24 h after transfection with pEGFP-AE2 or with pEGFP-C1 empty vector. (c) Densitometric scanning ratios of β-actin-normalized expression of P-ERK and P16 protein expression. Results were expressed as mean ± SD. *P < 0.05 as compared with empty vector (n = 3). (d) Nuclear and post-nuclear fractions of SW1116 cells were separated 24 h post-transfection with pEGFP-AE2 or pEGFP-C1 empty vector, then assessed for expression of P16 and Lamin B by immunoblot
Cytoplasmic P16 altered membrane trafficking of AE2 in colon cancer cells, correlating with poor patient prognosis
To explore the role of cytoplasmic P16 in colon cancer cells, P16 was knocked down by P16-targeted shRNA in SW1116 cells. Figure 3a shows that pSIREN-P16 vector transfection strongly suppressed P16 expression in three independent experiments. Reduction of P16 levels promoted AE2 trafficking to the SW1116 cell plasma membrane (Fig. 3b).
Coexpression of AE2 and P16 is correlated with poor prognosis of colon cancer patients through ERK pathway. (a) Downregulation of P16 protein in SW1116 cells by pSIREN-P16 but not by pSIREN-NC vector (NSC). Densitometric P16 protein expression was normalized to vinculin expression. Results were expressed as mean ± SD. *P < 0.05 as compared with NSC (n = 3). (b) Immunofluorescence assay of AE2 (red) in SW1116 cells transfected with pSIREN-P16 or pSIREN-NC empty vector (NSC). Blue, DAPI-stained nuclei (original magnification ×400). (c) P16 expression was analyzed by immunohistochemistry in adjacent normal (a, para-cancer) and colon cancer tissues (b, original magnification ×100) and statistically analyzed (c). The arrowed regions were enlarged in lower right corner (original magnification ×1,000). (d) Correlation analysis of AE2 and cytoplasmic P16 in colon cancer specimens. (e) Twenty-four patients were followed up for survival to assess AE2 expression as a prognostic factor. Kaplan–Meier survival curves and log-rank test comparing patient groups with colon cancer segregated according to histologic detection of cytoplasmic AE2 expression. AE2-positive cases showed a significantly lower survival rate (63.6%) compared with AE2-negative patients (100%; log rank = 4.121, P = 0.042). (f) Thirty-three patients with tubular colon adenocarcinoma were followed up for survival to assess AE2 expression as a prognostic factor. Kaplan–Meier survival curves and log-rank test comparing patient groups with colon cancer segregated according to histologic detection of cytoplasmic AE2 expression. AE2-positive cases showed a significantly lower survival rate (61.1%) compared with AE2-negative patients (100%; log rank = 4.699, P = 0.030). (g) Immunofluorescence assay of P16 (green) in HEK293T and SW1116 cells transfected with pEGFP-P16 vector. Blue, DAPI-stained nuclei (original magnification ×400). (h) Immunoblot detection of cyclin D1, ERK, P-ERK, and P16 in HEK293T and SW1116 cells transfected with pEGFP-P16 or pEGFP-C1 empty vector. (i) Immunoblot measurement of cyclin D1, ERK, P-ERK, and P16 in SW1116 cells transfected with pSIREN-P16 or pSIREN-NC vector (NSC). pSIREN-NC is scrambled-sequence control
To further clarify the relationship between the AE2 and P16, expression of P16 was assessed by immunohistochemistry in 198 colon cancer specimens and 110 para-cancer tissues. P16 staining was detected in 181 of the 198 colon cancer specimens, a significantly higher proportion than in para-cancer tissues (86/110; Fig. 3c). A cytoplasmic distribution of P16 was observed in 171 specimens, and cytoplasmic P16 was closely correlated with AE2 expression (Fig. 3d). Kaplan–Meier survival analysis demonstrated significant differences in prognosis between 11 patients with AE2-positive tumors and 13 patients with AE2-negative tumors (with all tumor sizes below median value). Patients (63.6%) with AE2-positive cancers was alive after the follow-up survey, whereas 100% of AE2-negative patients survived (log rank = 4.121, P = 0.042, Fig. 3e). Significant differences in prognosis of 33 patients with tubular adenocarcinoma were also observed as a function of tumor AE2 expression (Fig. 3f).
The cytoplasmic localization of P16 may be important for understanding the role of P16 in carcinogenesis. Therefore, HEK293T and SW1116 cells were transfected with P16 expression constructs. After 24 h, P16 was expressed in the nucleus in HEK293T cells but in cytoplasmic regions in SW1116 cells (Fig. 3g). P16 downregulated cyclin D1 expression, but did not change ERK level in HEK293T cells. These data are consistent with nuclear P16 acting as a tumor suppressor in normal HEK293T cells. In contrast, cytoplasmic P16 in SW1116 cells likely promotes carcinogenesis through increasing ERK phosphorylation and expression of cyclin D1 (Fig. 3h, i).
EGR1 induced the overexpression of AE2 in colonic cancer cells
We applied bioinformatics analysis to identify candidate transcription factors responsible for AE2 expression in colon cancer cells [19]. EGR1 was defined in both bioinformatics analysis and luciferase promoter assays as a transcription factor which upregulates AE2 promoter activity (Fig. 4a, b). EGR1 immunohistochemistry in the same colon cancer specimen used for histological detection of AE2 demonstrated strong EGR1 expression in 90% of cancer specimens (Fig. 4c). Knockdown of EGR1 decreased expression of both AE2 and P-ERK (Fig. 4d, e). Although EGR1-targeted siRNA did not affect P16 expression, reduction of EGR1 promoted the translocation of P16 from cytoplasm to the nucleus (Fig. 4f). These results suggest a novel signaling pathway linking EGR1, AE2, P16, and P-ERK in colon cancer cells.
AE2 expression is regulated by EGR1. (a) Schematic diagram of human AE2 proximal promoter reigion, containing three GC boxes and an EGR1 binding site. The numbers indicate the position relative to the ATG (+1). (b) SW1116 cells were transiently co-transfected with pGL3-AE2 luciferase reporter vector and pcDNA3.0-EGR1 or pcDNA3.0-NC vector (control). AE2 promoter activity was evaluated by luciferase assay. (c) Immunohistochemistry staining of EGR1 in colon cancer and para-cancer tissues (original magnification ×400). (d) Immunoblotting analysis of AE2, ERK, P-ERK, and P16 expressions in SW1116 cells transfected with EGR1-siRNA or NC-siRNA (NSC). (e) Densitometric, vinculin-normalized relative expression of EGR1, AE2, and P-ERK proteins. Results are expressed as mean ± SD from three independent experiments. (f) Immunofluorescence assay of P16 (green) in SW1116 cells and cells transfected with EGR1-siRNA or NC-siRNA. Blue, DAPI-stained nuclei (original magnification ×400)
Gastrin inhibits growth of colon cancer cells by inhibiting the EGR1/AE2/P16/P-ERK signal pathway
We previously reported that gastrin inhibited the growth of gastric cancer cells [13]. The present results show that gastrin inhibited the growth and clone formation of colon cancer SW1116 cells after 10 days’ treatment, accompanied by downregulation of AE2 and cyclin D1 (Fig. 5a, b). Gastrin also suppressed the expression of EGR1 and P16, and blocked the H2O2-induced phosphorylation of ERK [20] (Fig. 5c). The results demonstrated that gastrin inhibits proliferation of colon cancer cells, at least partially through suppression of the EGR1/AE2/P16/P-ERK pathway, a result confirmed by treatment of SW1116 cells with ERK inhibitors (U0126 or PD98059) or with 5-Fu (Fig. 5d, e), an agent in clinical use for treatment of colon cancer. The results are consistent with the possibility that 5-Fu acts at least partially through inhibition of AE2 expression. Co-treatment of SW1116 cells with gastrin and proglumide, an antagonist of gastrin receptor (Cholecystokinin B receptor, CCKBR), abolished the action of gastrin on upregulation of AE2, suggesting that gastrin inhibits AE2 expression through CCKBR-dependent pathways (Fig. 5f). Four of six tested colon cancer cell lines express CCKBR as detected by immunoblot (Fig. 5g), suggesting the possibility that the gastrin can be used for inhibition of colon cancer. Figure 6 illustrates the proposed EGR1/AE2/P16/P-ERK signaling pathway in a colon cancer cell.
Gastrin suppresses growth and clone formation of SW1116 cells by inhibiting the EGR1/AE2/P16/P-ERK pathway. (a) SW1116 cell number was assessed following growth in the absence or presence of 10−7 M gastrin for periods of 3, 5, 7, and 10 days. Cell counts are expressed as values normalized to those of untreated cells (upper panel); *P < 0.05 (n = 3). Expression of AE2 and cyclin D1 was detected by immunoblot (lower panel). (b) Effect of 10 days’ treatment of SW1116 cells with 10−7 M gastrin was assessed by clonogenic assay using crystal violet staining (upper panel, left) and phase contrast microscopy (upper panel, right). Number of clones per dish after 10 days in the absence or presence of 10−7 M gastrin (lower panel); *P < 0.05 (n = 3). (c) Immunoblot analysis of AE2, EGR1, and P16 in SW1116 cells treated without or with 10−7 M gastrin for 24 h (left panel). Immunoblot analysis of ERK and P-ERK in SW1116 cells treated with 100 μM H2O2 in the absence or presence of 10−7 M gastrin for 24 h (right panel). (d) Cell growth of SW1116 cells treated with ERK inhibitors (30 μM U0126 or 50 μM PD98059). Inset immunoblot analysis of P-ERK; *P < 0.05 (n = 3). (e) Immunoblot analysis of AE2 in SW1116 cells treated with 0.25 or 0.5 mM 5-Fluorouracil (5-Fu) for 24 h. (f) Immunoblot detection of AE2 expression in SW1116 cells treated with 10−7 M gastrin in the absence or presence of the cholecystokinin B receptor (CCKBR) antagonist proglumide (10−4 M). (g) Immunoblot detection of CCKBR expression in colon cancer cell lines
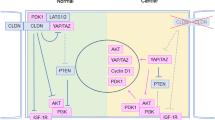
Diagram of a proposed novel EGR1/AE2/P16/P-ERK signal pathway in colon cancer cells. EGR1 transcriptionally upregulates AE2 expression. AE2 polypeptide interacts with and sequesters P16 in the cancer cell cytoplasm. Cytoplasmic P16 promotes phosphorylation of ERK, which can translocate into the nucleus, and activate cyclin D1 to promote cell proliferation. Gastrin acts through CCKBR at the plasma membrane to decrease EGR1 expression. Blockade of the EGR1/AE2/P16/P-ERK signaling pathway inhibits growth of colon cancer cells
Discussion
EGR1 is a transcription factor implicated in tumor progression and apoptosis in response to diverse stimuli mediated by EGR1 target genes such as TNF-α, p53, Rb, and Bax [21, 22]. Studies have found that EGR1 is constitutively expressed at a relatively high level in colon carcinoma cell lines and upregulated at the mRNA level in early-onset colorectal cancers [23–25]. However, no information is available about EGR1 expression or signaling in primary colon cancers. Our data presented here demonstrate that EGR1 was highly expressed in 90% of advanced colon cancer tissues, as compared with para-cancer tissues. The data suggest that EGR1 contributes to proliferation of colon cancer cells in part by promoting AE2 expression. Clinicopathological analysis demonstrated that AE2 expression was correlated with bigger tumor size, with poor tumor differentiation state, and with shortened patient survival time.
AE2-mediated Cl−/HCO −3 exchange supports anion secretion in colon [7, 26–28]. However, regulation of AE2 polypeptide expression and potential additional functional roles in normal colon epithelium remain unclear. As for EGR1, we found that expression of AE2 protein was also increased in colon cancer tissues, and especially in tubular adenocarcinoma. Molecular experiments indicated that AE2 expression was regulated by EGR1. The elevated levels of AE2 polypeptide were largely in a cytoplasmic distribution, suggesting reduced AE2 trafficking to the plasma membrane, and the possibility that the cytoplasmic accumulation of AE2 might initiate aberrant signaling. Isochronous expression of AE2 with Ki67 and cyclin D1 indicated that AE2 polypeptide could also serve as a marker of cell proliferation in colon cancer. So far, very little is known about the role of AE2 in cell proliferation and tumorigenesis. Zhou et al. found the movement of AE2 between the plasma membrane and cytoplasm during oocyte cell cycle progression, with loss of AE2 from the plasma membrane during meiosis [18]. In addition, AE2 was noted to be increased in hepatic cancer and decreased in gastric cancer, suggesting potentially varied roles for AE2 in tumorigenesis [8–10], although unlinked to any known signaling pathway.
Our data demonstrated that AE2 can contribute to carcinogenesis through cytoplasmic sequestration of P16 in colon cancer cells. P16 is an important factor linked to senescence and tumor suppression, commonly believed to act in the nucleus. However, P16 protein is cytoplasmically localized in several cancer cell types, including cervical, gastric, breast, lung, and prostate [29–33]. We previously reported that binding of cytoplasmic P16 to the AE1 C terminus is related to gastric carcinogenesis [14]. In the present paper, we show that AE2 and P16 also interact and are co-expressed in cytoplasmic regions of colon cancer cells.
How might the cytoplasmic interaction of AE2 and P16 promote proliferation of colon cancer cells? We show for the first time that expression of AE2 and P16 in cytoplasm in colon cancer cells activated the ERK signaling pathway, which may be a crucial early event in AE2-associated colon cancer. Early research on colon carcinogenesis focused attention on abnormal activation of the APC/β-catenin signal pathway. We found that β-catenin was not affected by AE2 expression, further supporting a role for AE2-associated cell proliferation through activation of the ERK pathway. ERK is the best characterized component of the MAPK signaling pathway, which plays a critical role in regulation of cell proliferation [34]. Recent reports have shown that ERK addiction drives survival of certain intestinal epithelial cell lines and intestinal tumorigenesis in Apcmin/+ mice [35] suggest that ERK signaling can be more important than APC deficiency in malignant transformation of colonic epithelium. We have thus far no evidence of a direct interaction between P16 and ERK, and further investigations are needed to elucidate the relationship between P16 and enhanced ERK phosphorylation. P16 induced upregulation of cyclin D1 and phosphorylation of ERK in colon cancer cells but not in HEK293T cells, suggesting an indirect activation of the ERK pathway by P16.
The gastrointestinal hormone gastrin binds to CCKBR to stimulate acid secretion under physiological conditions. Intriguingly, gastrin suppresses the growth of both gastric cancer and colon cancer cells through up- or downregulation of AE2. In gastric cancer cells, gastrin downregulates the interdependent expression of AE1 and P16, and removes the block for AE2 trafficking to the plasma membrane (unpublished). In colon cancer, gastrin directly suppresses expression of EGR1, AE2, and P16 and inhibits ERK phosphorylation. Taken together, the data presented in this paper provide insight into a new signaling pathway with implications for a potentially novel therapeutic approach to the treatment of colon cancer.
References
Saif MW, Chu E (2010) Biology of colorectal cancer. Cancer J 16:196–201. doi:10.1097/PPO.0b013e3181e076af
Plotnikov A, Chuderland D, Karamansha Y, Livnah O, Seger R (2011) Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation. Mol Cell Biol 31:3515–3530. doi:10.1128/MCB.05424-11
Kang YH, Yang IJ, Shin HM (2011) Herbal formula HMC05 prevents human aortic smooth muscle cell migration and proliferation by inhibiting the ERK1/2 MAPK signaling cascade. J Nat Med. doi:10.1007/s11418-011-0573-3
She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, Solit DB, Rosen N (2010) 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 18:39–51. doi:10.1016/j.ccr.2010.05.023
Alper SL (2006) Molecular physiology of SLC4 anion exchangers. Exp Physiol 91:153–161. doi:10.1113/expphysiol.2005.031765
Alper SL (2009) Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212:1672–1683. doi:10.1242/jeb.029454
Rossmann H, Alper SL, Nader M, Wang Z, Gregor M, Seidler U (2000) Three 5′-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann NY Acad Sci 915:81–91
Yang Y, Wu PP, Wu J, Shen WW, Wu YL, Fu AF, Zheng L, Jin XL, Fu GH (2008) Expression of anion exchanger 2 in human gastric cancer. Exp Oncol 30:81–87
Wu TT, Hsieh YH, Wu CC, Tsai JH, Hsieh YS, Huang CY, Liu JY (2006) Overexpression of anion exchanger 2 in human hepatocellular carcinoma. Chin J Physiol 49:192–198
Hwang JM, Kao SH, Hsieh YH, Li KL, Wang PH, Hsu LS, Liu JY (2009) Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mol Cell Biochem 327:135–144. doi:10.1007/s11010-009-0051-3
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T et al (2004) Mice with a targeted disruption of the AE2 Cl−/HCO3− exchanger are achlorhydric. J Biol Chem 279:30531–30539. doi:10.1074/jbc.M403779200
Frische S, Zolotarev AS, Kim YH, Praetorius J, Alper S, Nielsen S, Wall SM (2004) AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Am J Physiol Renal Physiol 286:F1163–F1170. doi:10.1152/ajprenal.00409.2003
Tian H, Zhang N, Suo WH, Wang T, Song LJ, Wu J, Liu Q, Shen WW, Fu GH (2010) Gastrin suppresses the interdependent expression of p16 and anion exchanger 1 favoring growth inhibition of gastric cancer cells. Int J Cancer 127:1462–1474. doi:10.1002/ijc.25124
Shen WW, Wu J, Cai L, Liu BY, Gao Y, Chen GQ, Fu GH (2007) Expression of anion exchanger 1 sequestrates p16 in the cytoplasm in gastric and colonic adenocarcinoma. Neoplasia 9:812–819
Fu GH, Wang Y, Xi YH, Shen WW, Pan XY, Shen WZ, Jiang XS, Chen GQ (2005) Direct interaction and cooperative role of tumor suppressor p16 with band 3 (AE1). FEBS Lett 579:2105–2110. doi:10.1016/j.febslet.2005.02.063
Naik S, Dothager RS, Marasa J, Lewis CL, Piwnica-Worms D (2009) Vascular endothelial growth factor receptor-1 is synthetic lethal to aberrant {beta}-catenin activation in colon cancer. Clin Cancer Res 15:7529–7537. doi:10.1158/1078-0432.CCR-09-0336
Munne A, Fabre M, Marinoso ML, Gallen M, Real FX (1999) Nuclear beta-catenin in colorectal tumors: to freeze or not to freeze? Colon cancer team at IMAS. J Histochem Cytochem 47:1089–1094
Zhou C, Tiberi M, Liang B, Alper SL, Baltz JM (2009) HCO3(−)/Cl(−) exchange inactivation and reactivation during mouse oocyte meiosis correlates with MEK/MAPK-regulated Ae2 plasma membrane localization. PLoS One 4:e7417. doi:10.1371/journal.pone.0007417
Vares G, Uehara Y, Ono T, Nakajima T, Wang B, Taki K, Matsumoto T, Oghiso Y, Tanaka K, Ichinohe K et al (2011) Transcription factor-recognition sequences potentially involved in modulation of gene expression after exposure to low-dose-rate gamma-rays in the mouse liver. J Radiat Res (Tokyo) 52:249–256
Vindis C, Seguelas MH, Lanier S, Parini A, Cambon C (2001) Dopamine induces ERK activation in renal epithelial cells through H2O2 produced by monoamine oxidase. Kidney Int 59:76–86. doi:10.1046/j.1523-1755.2001.00468.x
Baron V, Adamson ED, Calogero A, Ragona G, Mercola D (2006) The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther 13:115–124. doi:10.1038/sj.cgt.7700896
Zagurovskaya M, Shareef MM, Das A, Reeves A, Gupta S, Sudol M, Bedford MT, Prichard J, Mohiuddin M, Ahmed MM (2009) EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene 28:1121–1131. doi:10.1038/onc.2008.461
Hong Y, Ho KS, Eu KW, Cheah PY (2007) A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clin Cancer Res 13:1107–1114. doi:10.1158/1078-0432.CCR-06-1633
Wilson AJ, Chueh AC, Togel L, Corner GA, Ahmed N, Goel S, Byun DS, Nasser S, Houston MA, Jhawer M et al (2010) Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res 70:609–620. doi:10.1158/0008-5472.CAN-09-2327
Mahalingam D, Natoni A, Keane M, Samali A, Szegezdi E (2010) Early growth response-1 is a regulator of DR5-induced apoptosis in colon cancer cells. Br J Cancer 102:754–764. doi:10.1038/sj.bjc.6605545
Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE (2010) AE2 Cl−/HCO3− exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol 298:G493–G503. doi:10.1152/ajpgi.00178.2009
Currid A, Ortega B, Valverde MA (2004) Chloride secretion in a morphologically differentiated human colonic cell line that expresses the epithelial Na+ channel. J Physiol 555:241–250. doi:10.1113/jphysiol.2003.059295
Rajendran VM, Binder HJ (2000) Characterization and molecular localization of anion transporters in colonic epithelial cells. Ann NY Acad Sci 915:15–29
Pientong C, Ekalaksananan T, Swadpanich U, Kongyingyoes B, Kritpetcharat O, Yuenyao P, Ruckait N (2003) Immunocytochemical detection of p16INK4a protein in scraped cervical cells. Acta Cytol 47:616–623
Liu Q, Song LJ, Xu WQ, Zhao L, Zheng L, Yan ZW, Fu GH (2009) Expression of cytoplasmic p16 and anion exchanger 1 is associated with the invasion and absence of lymph metastasis in gastric carcinoma. Mol Med Report 2:169–174. doi:10.3892/mmr_00000079
Emig R, Magener A, Ehemann V, Meyer A, Stilgenbauer F, Volkmann M, Wallwiener D, Sinn HP (1998) Aberrant cytoplasmic expression of the p16 protein in breast cancer is associated with accelerated tumour proliferation. Br J Cancer 78:1661–1668
Feng XL, Li L, Gao YN, Zhang JJ, Xiao T, Ying JM, Gao JD, Sun YT, Cheng SJ (2011) Overexpression of c-erbB-2 and loss of p16 have molecular diagnostic relevance but no prognostic value in lung cancer. Med Oncol 28:336–341. doi:10.1007/s12032-010-9452-0
Kudahetti SC, Fisher G, Ambroisine L, Prowse D, Kattan MW, Foster CS, Moller H, Oliver T, Fletcher A, Cooper C et al (2010) Immunohistochemistry for p16, but not Rb or p21, is an independent predictor of prognosis in conservatively treated, clinically localised prostate cancer. Pathology 42:519–523. doi:10.3109/00313025.2010.508788
Pintus G, Tadolini B, Posadino AM, Sanna B, Debidda M, Carru C, Deiana L, Ventura C (2003) PKC/Raf/MEK/ERK signaling pathway modulates native-LDL-induced E2F-1 gene expression and endothelial cell proliferation. Cardiovasc Res 59:934–944
Fenton JI, Hursting SD, Perkins SN, Hord NG (2006) Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis 27:1507–1515. doi:10.1093/carcin/bgl018
Acknowledgments
The authors thank China Zhenjiang First People’s Hospital for providing the 202 paraffin-embedded colon cancer specimens. pcDNA3.0-EGR1 construct were kindly provided by Ian de Belle (Laval University, Canada). This work was supported in part by the National Natural Science Foundation of China (NO30570697; NO30770960), National High Technology Research and Development Program of China (863 Program; NO2008AA02Z120), Shanghai Natural Science Foundation (NO11ZR1419700).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ling-Jun Song and Rui-Jun Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Song, LJ., Liu, RJ., Zeng, Z. et al. Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK. J Mol Med 90, 707–718 (2012). https://doi.org/10.1007/s00109-011-0851-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0851-2