Abstract
The mitogen-activated protein kinase (MAPK) and PI3K pathways are regulated by extensive crosstalk, occurring at different levels. In tumors, transactivation of the alternate pathway is a frequent “escape” mechanism, suggesting that combined inhibition of both pathways may achieve synergistic antitumor activity. Here we show that, in the M14 melanoma model, simultaneous inhibition of both MEK and mammalian target of rapamycin (mTOR) achieves synergistic effects at suboptimal concentrations, but becomes frankly antagonistic in the presence of relatively high concentrations of MEK inhibitors. This observation led to the identification of a novel crosstalk mechanism, by which either pharmacologic or genetic inhibition of constitutive MEK signaling restores phosphatase and tensin homolog (PTEN) expression, both in vitro and in vivo, and inhibits downstream signaling through AKT and mTOR, thus bypassing the need for double pathway blockade. This appears to be a general regulatory mechanism and is mediated by multiple mechanisms, such as MAPK-dependent c-Jun and miR-25 regulation. Finally, PTEN upregulation appears to be a major effector of MEK inhibitors’ antitumor activity, as cancer cells in which PTEN is inactivated are consistently more resistant to the growth inhibitory and anti-angiogenic effects of MEK blockade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Our knowledge of signal transduction pathways has recently evolved from the classical notion of “linear” signaling pathways to the much more complex vision of “signaling networks,” in which every component is closely intertwined with an array of different players in a complex signaling circuitry [1, 2]. In this context, even highly specific interference with a single signaling component may actually lead to unexpected, and sometimes “undesired” from a therapeutic perspective, functional outputs. The RAF/MEK/ERK and the PI3K/phosphatase and tensin homolog (PTEN)/AKT/mammalian target of rapamycin (mTOR) signaling pathways are two major regulators of cell growth, metabolism, survival, and angiogenesis [3]; these two pathways are interconnected through “vertical” and “lateral” feedback loops operating at multiple levels along the signaling cascades, and their dysregulation through genetic and epigenetic mechanisms may cooperate in the transformation and progression of experimental and human tumors [4]. Compensatory signaling through the RAS/mitogen-activated protein kinase (MAPK) pathway may mediate resistance to selective PI3K/mTOR axis inhibition and vice versa, making combined inhibition of the RAF/MEK/ERK and PI3K/AKT/mTOR an attractive therapeutic strategy [4–6].
In malignant melanoma, uncontrolled MAPK pathway activation is nearly ubiquitous and occurs most commonly through gain-of-function mutations involving codon 600 of the BRAF kinase (V600EBRAF in up to 70% of cases) [7]. BRAF mutations result in the aberrant activation of ERK which, in turn, provides an essential tumor growth and maintenance signal, by fostering proliferation, survival, chemoresistance, and the autocrine production of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF). Most interestingly, BRAF-mutated tumors appear to be exquisitely sensitive to selective BRAF and/or MEK inhibitors [8]. However, BRAF mutational status does not completely explain sensitivity to MEK inhibitors ([9] and Ricciardi MR, 2010 manuscript submitted) and MAPK activation does not account for all aspects of melanoma progression. Activation of the PI3K/PTEN/AKT/mTOR pathway, on the other end, occurs in 5–10% of melanomas by virtue of PTEN mutations, receptor–ligand interactions, or NRAS-activating mutations [10]. In melanoma and other cancers, the RAF/MEK/ERK and PI3K/PTEN/AKT/mTOR pathways have partially overlapping functions and cooperate to promote resistance to apoptosis and tumor progression [4, 11].
Here we set out to investigate the therapeutic potential of pharmacologic modulation of the MEK/ERK and PI3K/AKT/mTOR pathways and discovered a novel crosstalk pathway that, through ERK-dependent upregulation of c-Jun and miR-25, leads to suppression of PTEN expression. Moreover, we identify PTEN as a crucial mediator of the growth inhibitory and anti-angiogenic effects of MEK inhibition. These findings shed new light on the molecular mechanisms of crosstalk between the RAF/MEK/ERK and PI3K/PTEN/AKT/mTOR pathways and may be helpful in selecting appropriate cellular contexts for the design of rational therapeutic strategies based on single- or combined pathway blockade.
Experimental procedures
Pathway inhibitors, cell cultures, and in vitro treatments
PD0325901 [N-((R)-2,3-dihydroxy-propoxy)-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide] was obtained from Pfizer Global Research and Development (Ann Arbor, MI, USA). Temsirolimus (CCI-779) was obtained from Wyeth-Ayerst Research (Pearl River, NY, USA). The MEK inhibitor GSK1120212B was kindly provided by Glaxo Smith Kline (Verona, Italy). Cell lines were routinely maintained in RPMI 1640 or DMEM medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics in a humidified atmosphere with 5% CO2 at 37°C. Cell culture reagents were purchased from Invitrogen (Milan, Italy). Melanoma CSC were generated in vitro as previously reported for lung cancer [12]. Cell proliferation in response to different treatments was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO, USA). The concentration of drug(s) causing 50% reduction in cell viability (IC50) was calculated according to the Chou–Talalay method using the Calcusyn software.
Western blot analysis and reverse transcription PCR
For western blotting, equal amounts of total cell lysate (35 μg of proteins), prepared as described previously [13], were fractionated by SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Amersham). Membranes were probed with primary antibody, and the signal was detected using peroxidase-conjugated antimouse or antirabbit secondary antibodies (Cell Signaling Technology Inc., Beverly, MA, USA). The enhanced chemiluminescence system (Amersham, Arlington Heights, IL) was used for detection (see “Electronic supplementary material” section for a detailed list of antibodies employed). In vivo experiments were conducted as described in [9], and lysates for western blot analysis were prepared from frozen tumor tissue. The levels of PTEN mRNA were determined by reverse transcription PCR (RT-PCR) as described previously [14].
Immunofluorescence
Cells grown on coverslips were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min, permeabilized in PBS containing 0.5% Triton X-100 for 10 min, and blocked with 3% BSA in PBS for 1 h. Coverslips were incubated overnight with primary antibodies. Coverslips were then washed three times in PBS and then incubated with one of the following secondary antibodies for 1 h at a dilution of 1:200: goat antirabbit Alexa Fluor 488 (A11034, Invitrogen) and goat antimouse Alexa Fluor 594 (A11032, Invitrogen). After washing, the nuclei were counterstained with DAPI. Cells were then washed twice with PBS and observed under Zeiss Axiovert 200 M fluorescence microscope. Specific fields were photographed with a digital camera equipped with Zeiss Axiovision acquisition software.
Luciferase reporter assay
The plasmids encoding PTEN promoter luciferase reporter vector or 10× PF-1-luc were reported previously [14]. To study PTEN promoter activity, 1 × 105 cells/well (24-well plate) were seeded in triplicate and 24 h later transfected using lipofectamine with an internal control PEQ-176 plasmid (0.1 μg) and the luciferase reporter vector 10× PF-1-luc (0.4 μg) carrying the PTEN promoter (nucleotides 1348–1527 of the PTEN gene) or 10× synthetic construct that contained the PF-1sequence of the PTEN promoter repeated 10 times, respectively. The luciferase activity of each sample was normalized to β-galactosidase activity to calculate the relative luciferase activity.
Plasmids and transfection experiments
M14 cells were infected with retroviral vectors encoding DN-MEK1 (MEKLIDA) or an empty retroviral vector (pLXSN) [15]. ERK2/MEK1 and ERK2/MEK1 LA (kindly provided by Melanie H. Cobb, University of Texas Southwestern Medical Center, Dallas, TX, USA) and wild-type c-Jun (kindly provided by Ze'ev Ronai, Sanford-Burnham Medical Research Institute, La Jolla, CA, USA) expression vectors have been previously reported [15–17]. The GFP-PTEN C124S mutant expression construct encodes for a mutant PTEN(C124S) protein, lacking both lipid and protein phosphatase activities [18]. Transient or stable transfections of plasmids were performed using Lipofectamine (Invitrogen, Carlsbad, CA, USA). For RNA interference experiments, cells were transfected with 100 nM of siJUN smart pool (Dharmacon, Chicago, IL, USA) in 35-mm petri dishes using Lipofectamine 2000 reagent (Lipofectamine 2000, Invitrogen) for 72 h.
Immunohistochemistry
For morphological and immunohistochemical analysis, tumor sections were placed in 10% buffered formalin for 24 h, dehydrated and embedded in paraffin. Immunohistochemistry was performed on 4-μm-thick sections as reported [19]. Semiquantitative evaluation of immunoreactions was performed at ×200 magnification in at least 10 randomly selected fields in double blind, with an interobserver variability less than 5%, using a grading system in arbitrary units as follows: 0, absent; 0.5, weakly positive <50%; 1, weakly positive >50% or moderately positive <50%; 2, moderately positive >50% or strongly positive <50%; and 3, strongly positive >50%.
Statistical analysis
Differences between treatment groups were analyzed with a two-tailed Student’s t test for paired samples. Synergism, additivity, and antagonism were assessed by isobologram analysis with a fixed ratio experimental design using the Chou–Talalay method [20]. Results were analyzed with the Calcusyn software (Biosoft, Cambridge, UK), and combination indexes (CI) were appropriately derived. CI values <0.9, >0.9 < 1.2, and >1.2 indicate synergism, additive effect, and antagonism, respectively.
Results
Effects of combined MEK and mTOR inhibition in melanoma
Since both MEK (PD0325901) and mTOR (temsirolimus) inhibitors partially offset VEGF production and cell growth in melanoma models [9], we investigated whether their simultaneous blockade would achieve synergistic inhibitory effects. In the M14 model, both PD0325901 and temsirolimus partially inhibited VEGF production under normoxic and hypoxic conditions, but their combination did not increase VEGF inhibition over each agent alone (Fig. 1a), particularly at relatively high PD0325901 concentrations (100 nM). Similar results were obtained in terms of cell growth inhibition (Fig. 1b); conservative isobologram analysis confirmed that the growth inhibitory effects of the combination were in the additive/synergistic range (CI 0.2 to 0.8) up to a fraction affected of 0.7 and became frankly antagonistic (CI 1.2 to 5.7) thereafter (Fig. 1c), suggesting that the two agents may indirectly compete for the same intracellular target(s). Indeed, in addition to inhibiting phosphorylation of canonical and/or putative targets along the MAPK cascade (ERK, elongation initiation factor 4E—eIF4E, p70S6K Thr421/Ser424), PD0325901 also inhibited targets along the PI3K/AKT/mTOR cascade, such as AKT, p70S6K Ser371, and Ser389, and initiation factor 4E binding protein 1 (4E-BP1, Fig. 1d, e), particularly at relatively high concentrations (100 nM); conversely, mTOR inhibition by temsirolimus induced rebound ERK phosphorylation, as previously described (see also Fig. S2C).
Effects of combined MEK and mTOR inhibition in M14 cells. a M14 cells were treated with PD0325901 (PD) or temsirolimus (CCI), either alone or in combination, under normoxic (black bars) or hypoxic (grey bars) conditions. VEGF release in conditioned medium after 24 h was evaluated by ELISA. Results are expressed as pg of VEGF/106 cells and represent the average ± SD of three independent experiments. b M14 cells were treated with PD0325901 (PD) and temsirolimus (CCI), alone or in combination using a fixed ratio (1:50). Cell growth and viability were assessed by MTT assay after 72 h. Results represent the average ± SEM of three independent experiments and are expressed percentage of growth inhibition relative to untreated control. c CI were calculated by conservative isobologram analysis for experimental data shown in panel B. CI are plotted against the fraction affected; continuous line represents calculated simulations, while solid squares represent actual experimental data points for the combination. d, e M14 cells were treated with PD0325901 for 24 h, lysed, and analyzed by western blotting using antibodies specific for the phosphorylated or total forms of the indicated proteins. Western blot with antibodies specific for β-actin or Hsp70 are shown as protein loading and blotting control. Densitometric analysis of western blot data is shown in bottom panels; results represent the average ± SEM of three independent experiments and are expressed as the OD ratio of specific antibody/β-actin for each individual sample
MEK inhibition induces PTEN expression
Gene expression profiling of M14 cells exposed to PD0325901 [9] revealed a marked (>3-fold) upregulation of the expression of the tumor suppressor PTEN (Fig. 2a). PD0325901-induced increase in PTEN mRNA levels was confirmed qualitatively by RT-PCR and quantitatively by real-time PCR analysis (Fig. 2b, c). Western blot analysis revealed a dose- and time-dependent upregulation of PTEN protein levels in response to PD0325901 in M14 cells (Fig. 2d and Fig. S1A) and in two out of four patient-derived melanoma stem cell clones (clones 3 and B; Fig. S1B, C). Immunofluorescence experiments revealed that MEK inhibition-induced PTEN was functionally active, as demonstrated by the striking decrease in phosphatidylinositol (3,4,5)-trisphosphate (PIP3) levels in M14 cells treated with 100 nM PD0325901 (Fig. 2e). Most importantly, PTEN upregulation was observed in vivo, in M14-derived tumors growing in immunocompromised mice subjected to oral PD0325901 treatment (Fig. 3, see also ref. [9]) and was accompanied by a striking decrease in phosphorylated AKT levels (Fig. 3b, bottom panel). Similar results in terms of PTEN induction and inhibition of AKT phosphorylation were also obtained with different MEK inhibitors (PD98059 and GSK1120212B; Fig. S2A, B), but not with temsirolimus (Fig. S2C).
MEK inhibition induces functional PTEN expression in vitro. a M14 cells were exposed to 10 nM PD0325901 for 6 h, and gene expression profiles were analyzed using the Affymetrix U133 Plus 2.0 Gene Chip. Supervised analysis displaying differences in the expression of the indicated transcripts are shown using color codes. Average results from two indipendent experiments are shown. b M14 cells were exposed to PD0325901 (10 nM) for 24 h; equal amounts of mRNA were then subjected to RT-PCR, using PTEN-specific primers. Expression of β-actin was used as an internal standard for RNA loading. Results of one experiment representative of three independent experiments performed with superimposable results are shown. c M14 cells were treated as in b and PTEN mRNA quantified by quantitative real-time PCR. Results represent the average ± SEM of three independent experiments and are expressed as PTEN mRNA abundance relative to untreated control. d M14 cell were exposed to PD0325901 at the indicated concentrations for 24 h, lysed, and subjected to western blot analysis using antibodies specific for the indicated proteins. Densitometric analysis is shown in the bottom panel; results represent the average ± SEM of three independent experiments and are expressed as the OD ratio of specific antibody/β-actin for each individual sample. e M14 cells were exposed to PD0325901 (100 nM) for 24 h; PTEN and PIP3 levels were then assessed by immunofluorescence using specific antibodies. Microphotographs were taken at ×63 magnification. Results from one experiment representative of at least three independent experiments performed with superimposable results are shown
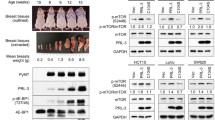
MEK inhibition induces functional PTEN expression in vivo. a M14-derived tumors growing in immunocompromised mice and subjected to oral PD0325901 treatment (25 mg/kg/day) or vehicle control [9] were assessed by western blotting using antibodies specific for the phosphorylated or total forms of the indicated proteins. Densitometric analysis is shown in the right panel; results represent the average ± SEM of two independent experiments and are expressed as the OD ratio of specific antibody/β-actin for each individual sample. b Effects of PD on p-ERK1/2, PTEN, and pAKT in M14 tumor xenografts. On the left, serial paraffin-embedded sections of M14 xenografts obtained from tumor-bearing mice untreated (vehicle) and treated with PD at 25 and 50 mg/kg/day, stained with hematoxylin–eosin (H-E) or immunostained with anti-phospho-p44/42 MAP kinase (p-ERK1/2), anti-PTEN, or anti-phospho-AKT specific antibodies (scale bars 50 μm.) On the right, semiquantitative evaluation of immunostainings. Student’s t test *p < 0.05,**p < 0.001
PD0325901-induced PTEN upregulation correlates with c-Jun downregulation
Recent evidence indicates that c-Jun promotes cell survival by negatively regulating PTEN expression [21]; we thus evaluated whether PD0325901-mediated induction of PTEN expression might be related to c-Jun modulation. As shown in Fig. 4a and Fig. S1D, PD0325901 dose and time dependently reduced c-Jun protein levels in M14 cells, in parallel to PTEN induction. Concomitant c-Jun downregulation and PTEN induction, accompanied by inhibition of AKT phosphorylation downstream of PTEN, were also observed in other melanoma cell lines and short-term primary cultures, regardless of their BRAF mutational status, as well as in breast cancer (MDA-MB-231 and BT474) and myeloid leukemia (OCI-AML3) cell lines (Fig. 4b–g). In other cancer cell lines [Miapaca (pancreatic), HT29 (colorectal), H1299 and H1975 (lung)], MEK inhibition produced qualitatively similar effects on c-Jun downregulation and PTEN induction, but a more variable response in terms of AKT phosphorylation (data not shown).
MEK inhibition-induced modulation of c-Jun, PTEN, and phosphorylated AKT. a M14 cells were treated with PD0325901 at the indicated concentrations for 24 h. Protein samples were analyzed by western blotting using PTEN- and c-Jun-specific antibodies. b–g Short-term melanoma primary cultures and tumor cell lines of different histological origin were treated with PD0325901 at the indicated concentrations for 24 h. Protein samples were analyzed by western blotting using the indicated antibodies. Densitometric analysis is shown in bottom panels; results represent the average ± SEM of three independent experiments and are expressed as the OD ratio of specific antibody/β-actin for each individual sample
Causal relationship between ERK activity, c-Jun expression, and PTEN suppression
In order to confirm that PD0325901-induced effects on c-Jun and PTEN were indeed specifically due to modulation of MEK/ERK activity, we overexpressed a dominant negative MEK1 construct (DN-MEK, [15]) in M14 cells, thereby efficiently abrogating ERK phosphorylation and resulting in concomitant c-Jun downregulation and PTEN upregulation (Fig. 5a); conversely, enforced expression of a constitutively active ERK2/MEK1 construct [17] in NIH-3T3 cells resulted in activation of p90 ribosomal S6 kinase (p90RSK) downstream of ERK, increased c-Jun, and decreased PTEN expression (Fig. 5b). Furthermore, transient transfection of NIH-3T3 cells with a wild-type c-Jun construct [16] also resulted in strongly reduced PTEN expression and increased p70S6K T389 phosphorylation downstream (Fig. 5c). Conversely, in M14 cells, either stable expression of a c-Jun-directed shRNA or transient transfection with multiple independent shRNAs effectively reduced c-Jun protein expression, resulting in increased PTEN expression (Fig. 5d–f); increased PTEN expression was functionally relevant, as evidenced by reduced PIP3 levels (Fig. 5g) and consequent suppression of AKT and p70S6K posphorylation (Fig. 5d, e). Finally, transient transfection of M14 cells with a synthetic promoter-luciferase reporter construct containing a 10× variant activator protein 1 (PF-1) site, responsible for c-Jun-mediated suppression of PTEN transcription [14], resulted in a dose-dependent increase in luciferase activity upon PD0325901 exposure (Fig. 5g), suggesting that MEK inhibition relieves c-Jun-mediated transcriptional repression. In parallel to c-Jun protein downregulation, PD0325901 dose dependently inhibited cAMP response element-binding (CREB), glycogen synthase kinase 3 (GSK3α, β), and c-Jun N-terminal kinase (JNK) phosphorylation (Fig. 6a), suggesting that MEK inhibition may affect both c-Jun transcription and protein stability.
ERK-dependent c-Jun modulation controls PTEN expression. a M14 cells were infected with either an empty retroviral vector control (pLXSN, vector) or a retrovirus encoding a dominant negative MEK1 construct (DN-MEK). Protein samples were analyzed by western blotting using antibodies specific for the indicated proteins. Results of one experiment representative of three independent experiments performed with superimposable results are shown. b NIH-3T3 cells were transfected with vectors encoding constitutively active ERK2/MEK1 fusion proteins. After 72 h, protein samples were analyzed by western blotting using the indicated antibodies. Anti-c-myc antibodies were used to reveal c-myc-tagged ERK2/MEK1 constructs. Results of one experiment representative of three independent experiments performed with superimposable results are shown. c NIH-3T3 cells were transfected with a FLAG-tagged c-Jun construct. After 72 h, protein samples were analyzed by western blotting using the indicated antibodies. Anti-FLAG antibodies were used to reveal FLAG-tagged c-Jun constructs. Results of one experiment representative of three independent experiments performed with superimposable results are shown. d, e M14 cells were stably transfected with a vector encoding c-Jun shRNA (clones 1 and 2) (d) or transiently transfected with multiple independent shRNAs directed against c-Jun (e). Protein samples were analyzed by western blotting using the indicated antibodies. Results of one experiment representative of three independent experiments performed with superimposable results are shown. f M14 cells were transiently transfected with multiple indepndent shRNAs directed against c-Jun; PTEN and PIP3 levels were then assessed by immunofluorescence using specific antibodies. Microphotographs were taken at ×63 magnification. Results from one experiment representative of at least three independent experiments performed with superimposable results are shown. g M14 cells were transiently transfected with a luciferase reporter construct containing the multimerized 10× PF1-PTEN. Cells were then exposed to the indicated PD0325901 concentrations for 24 h, and luciferase activity was measured by luminometer. Results are expressed as luciferase activity fold induction relative to untreated control cells and represent the average ± SD of three indipendent experiments. β-galactosidase activity was used to standardize mesurements. *p < 0.01 by two-tailed Student’s t test
PD0325901 inhibits c-Jun expression by multiple mechanisms and modulates miR-25 expression. a M14 cells were exposed to PD0325901 at the indicated concentrations for 24 h. Protein samples were then analyzed by western blotting using the indicated antibodies. Results of one experiment representative of three independent experiments performed with superimposable results are shown. b, c The presence of miR-106b and miR-25 was detected by real-time PCR in M14 cells exposed to PD0325901 at the indicated concentrations for 24 h. Results were evaluated as ΔΔct of miRNAs tested relative to RNU19 and expressed as the ratio assuming the levels in untreated control cells as 1.0. Average values ± SE from triplicates of one experiment representative of three independent experiments performed with superimposable results are shown. d, e M14 cells were treated with either an miR-25-specific antagomiR (d) or a miR-25 mimic (e) for 24 h; PTEN expression was then assessed by western blotting. Densitometric analysis is shown in the bottom panels; results represent the average ± SEM of three independent experiments and are expressed as the OD ratio of PTEN/β-actin for each individual sample
ERK activity controls miR-25 expression
In addition to trancriptional mechanisms, such as the one depicted above, PTEN expression levels have recently been shown to be tightly controlled by biologically active RNAs, such as the PTENP1 pseudogene and the miR-106b~25 microRNA cluster [22, 23]. We therefore investigated whether MEK inhibition regulates miR-106b and/or miR-25 expression in M14 cells. Under experimental conditions in which MEK inhibition by either PD0325901 or GSK1120212B induces upregulation of PTEN protein levels (Fig. 6c, inset), miR-25 expression was profoundly and selectively downregulated (>80% reduction at 24 h, Fig. 6c), while expression of miR-106b was relatively less affected (<40% reduction at 24 h, Fig. 6b). Modulation of miR-25 expression was functionally relevant, as inhibition of its activity in M14 cells, using a miR-25-specific antagomiR, resulted in an approximately twofold induction of PTEN protein expression (Fig. 6d); conversely, an miR-25 mimic decreased PTEN expression by approximately 50% (Fig. 6e).
PTEN induction is a crucial mediator of PD0325901 growth inhibitory and anti-angiogenic effects
We next evaluated the functional relevance of the observed induction of PTEN expression with respect to the growth inhibitory and anti-angiogenic activity of PD0325901. Consistent with recent literature reports, cancer cell lines of different histological origin [BT549 (breast), U937 (myeloid leukemia), and H1650 (lung)], in which PTEN is inactivated by either deletion or mutation were functionally resistant to the growth inhibitory effects of MEK blockade by PD0325901 (IC50 >1 μM, Fig. 7a). In M14 cells, stable tranfection with a plasmid containing a dominant negative PTEN construct (DN-PTEN [18]) resulted in increased phosphorylation of AKT, p70S6K, and 4E-BP1 (Fig. 7b) and more rapid cell growth (Fig. S3A). DN-PTEN-expressing clones produced significantly higher basal and hypoxia-stimulated VEGF levels (Fig. S3B); moreover, in DN-PTEN-expressing clones MEK inhibition by PD0325901 was less efficient at offsetting both VEGF production (Fig. S3B and Fig. 7c) and in vitro cell growth (Fig. 7d), resulting in an approximately fourfold higher IC50 (Fig. 7d, inset), as compared to vector control-transfected cells. Most importantly, in vivo PD0325901 significantly inhibited AKT phosphorylation and cell proliferation, as assessed by Ki67 expression, only in xenografts derived from control vector-transfected M14 cells (M14), but not in those derived from DN-PTEN-expressing clone 2 (Fig. 7e, f).
PTEN induction is an important mediator of PD0325901 growth inhibitory and anti-angiogenic effects. a Tumor cell lines of different histological origin with either wild-type (M14 and ME1007), deleted (BT549 and H1650), or mutated (U937) PTEN were exposed to PD0325901, and cell growth was assessed by MTT assay after 72 h. PTEN levels were analyzed by western blot. b M14 clones stably transfected with either an empty plasmid vector (pEGFP-C2, vector) or a plasmid encoding a DN-PTEN construct (clone 1 and clone 2) were analyzed by western blot using the indicated antibodies. GFP-Tag identifies the transfected DN-PTEN construct as detected by an anti-GFP antibody, while PTEN identifies the endogenous PTEN protein. Results of one experiment representative of three independent experiments performed with superimposable results are shown. c Vector control- and DN-PTEN-transfected clones were exposed to the indicated concentrations of PD0325901 for 24 h under normoxic and hypoxic conditions, as indicated. VEGF release in conditioned medium was evaluated by ELISA. Results are expressed as percentage inhibition of VEGF production induced by 10 nM PD0325901 (*p < 0.007 by two-tailed Student’s t test for the comparison between vector control- and DN-PTEN-transfected clone 2) (d). Vector control- and DN-PTEN-transfected clones were exposed to PD0325901 and cell growth was assessed by MTT assay after 72 h. Results are expressed as percentage of untreated control cells and represent the average of three independent experiments; continuous lines represent calculated simulations, while solid dots and grey squares and triangles represent actual experimental data points ± SD for each cell line. Calculated IC50 are shown in the inset. e, f Effects of PD on Ki67 and pAKT expression in tumor xenografts derived from either control vector-transfected M14 cells (M14) or from DN-PTEN-epressing clone 2 (CS9). Results are expressed as percentage of Ki67-expressing cells (e) or using a semiquantitative staining score taking into account both pAKT staining intensity and percentage of positive cells (f). Student’s t test, *p < 0.05
Discussion
In this study, we show that the lipid phosphatase PTEN constitutes a crucial point of crosstalk between the MAPK and PI3K pathways. Our data indicate that MEK inhibition is per se able to cross-modulate signaling through the AKT/mTOR module and that, under certain conditions, combined MEK/mTOR blockade may exert frankly antagonistic effects in terms of inhibition of VEGF production and tumor cell growth. This observation led us to the identification of a novel crosstalk mechanisms, whereby constitutive ERK activity suppresses PTEN expression, while its inhibition restores PTEN levels and results in the concomitant inhibition of the PI3K/AKT/mTOR pathway. Originally identified in melanoma models, this crosstalk is operational in cancer and normal cells and appears to be mediated by multiple mechanisms, such as modulation of c-Jun and miR-25 expression. Finally, we suggest that MEK inhibition-dependent PTEN induction plays an important, albeit not exclusive, role in the growth inhibitory and anti-angiogenic activity of MEK inhibitors.
The MEK/ERK and PI3K/AKT/mTOR pathways extensively crosstalk to each other [4], providing the rationale for combined therapeutic strategies that are currently being tested with promise in experimental and human cancers [5, 6]. However, the functional outcome of combined pathway inhibition appears to be influenced by the genetic background of the models employed to test therapeutic synergism; for example, the cooperative tumor growth inhibition observed with simultaneous MEK and PI3K/mTOR blockade occurs most prominently in PTEN null mouse models of prostate cancer [6], suggesting that PTEN may be a crucial point of convergence of these two signaling pathways. Pharmacologic or genetic inhibition of the RAF/MEK/ERK pathway induces PTEN expression not only in BRAF mutant melanoma models but also in BRAF-wt melanomas (ME1007, ME4686) and in a proportion of short-term human melanoma cultures and patient-derived melanoma stem cells, as well as in cancer cell lines of different histological origin. Consistently, enforced expression of a constitutively active MEK/ERK fusion protein [17] in nontransformed mouse fibroblasts suppresses PTEN expression (Fig. 5b), suggesting that ERK-dependent modulation of PTEN expression may be a physiological regulatory mechanism that takes place in both normal and cancer cells, independent of the specific oncogenic mutation. These findings are consistent with previously reported evidence that transcriptional PTEN suppression constitutes a critical step in RAS-mediated transformation and is predominantly sustained by the activation of the RAF/MEK/ERK, rather than the PI3K/AKT, axis [21, 24].
Consistent with previous reports identifying c-Jun as a transcriptional regulator of PTEN expression [14, 21], our findings indicate that c-Jun is a crucial effector of ERK-dependent PTEN suppression: (1) c-Jun and PTEN are counterregulated with very close time and dose dependency upon pharmacologic or genetic modulation of ERK activity and (2) ERK-independent modulation of c-Jun by enforced expression or shRNA-mediated knockdown exerts essentially the same effects on PTEN expression as constitutive ERK activation or its pharmacologic inhibition. The molecular mechanisms by which ERK activation (or inhibition thereof) controls c-Jun, and hence PTEN, expression, in turn, appear to be consistent with a rewired ERK-JNK signaling pathway recently described in melanoma [16]. Indeed, MEK blockade by PD0325901 inhibits CREB and GSK3 phosphorylation, potentially affecting both c-Jun transcription and protein stability [16, 25]. In M14 melanoma cells, JNK phosphorylation was also strikingly inhibited by PD0325901 (Fig. 6a). While studies on the isolated kinase rule out the possibility that PD0325901-mediated inhibition of JNK phosphorylation is due to direct, off target activity of the compound [26], whether JNK deactivation in this context is the cause or consequence of c-Jun modulation remains to be addressed.
Our findings also suggest that, in addition to c-Jun modulation, MEK inhibition may regulate PTEN expression by selectively interfering with the expression of miR-25, that has recently been shown to finely regulate PTEN levels in human tumors and contribute to experimental tumorigenesis in vivo [23]. A possible relationship between these two mechanisms of regulation of PTEN expression (i.e. c-Jun activity and miR-25 expression) remains speculative at present and will require further studies.
PTEN is a major tumor suppressor, involved in cancer development and progression [27]. Although PTEN is one of the most commonly lost tumor suppressors in human cancers, homozigous gene deletion/mutation is much less frequent than partial loss of expression and it is now well established that PTEN haploinsufficiency can promote tumor development [28–30]. This seems to be the case also for human melanoma where PTEN is frequently lost during tumor progression [31, 32], can be suppressed by UV radiation [24, 31], and, most importantly, critically cooperates with oncogene aberrations such as NRAS and BRAF mutations and AKT3 overexpression [10, 33]. PTEN mutations occur in approximately 20% BRAF-mutated melanomas [34, 35], suggesting that deregulation of both pathways is necessary for the full expression of their malignant potential [11]; our data on ERK-mediated PTEN suppression extend this concept further, suggesting that in a proportion of cases, deregulation of the RAF/MEK/ERK pathway per se is able to also inactivate PTEN, without the need for a second genetic event, thereby fostering melanoma (and possibly other cancers) progression. Moreover, the data presented here on M14 melanomas in which PTEN function is partially offset by the expression of a DN-PTEN construct support an important role this tumor suppressor in the regulation of both melanoma cell growth and production of pro-angiogenic factors, such as VEGF [27]. Most importantly, from a therapeutic perspective, we identify here PTEN induction as a crucial effector of the anti-angiogenic and growth inhibitory activity of MEK inhibitors, such as PD0325901. This is consistent with recent data showing that breast cancer cell lines with deleted/mutant or shRNA-silenced PTEN are functionally resistant to MEK inhibition by PD0325901 [36] and that functionally sensitive, but not MEK inhibition-resistant, melanoma cell lines are able to upregulate PTEN in response to MEK blockade [37], as well as with our own data in acute myeloid leukemias and breast and lung cancers, showing that cell lines harboring a PTEN mutation/deletion are consistently more resistant to MEK inhibitors, including PD0325901.
Overall, these data describe a novel signaling pathway proceeding from constitutive MEK/ERK activation to c-Jun/miR-25 overexpression which, in turn, repress PTEN expression, resulting in the transactivation of the PI3K/AKT/mTOR cascade. This pathway, potentially operating in multiple tumor models, can be dysrupted by pharmacological MEK blockade which leads, in cellular contexts where PTEN protein expression is reversibly lost, to PTEN re-expression and inhibition of cell growth and VEGF production. In addition to depicting a novel crosstalk mechanism between two major signaling pathways involved in cancer development, these findings bear potentially important clinical implications in that they suggest that: (1) PTEN-deleted/mutant patients should probably be excluded from MEK inhibitor treatment and (2) combined inhibition of the MEK/ERK and PI3K/AKT/mTOR pathways could be more advantageous in PTEN-defective tumors. This is important as several novel, potent, and selective MEK inhibitors are entering the clinical arena, and no suitable markers of sensitivity/resistance to such therapeutic strategy have been identified, besides BRAF mutations [8]; thus, successful clinical development of MEK inhibitors will crucially depend upon the identification of molecularly defined mechanisms of action, such as the one involving c-Jun/miR-25 and PTEN modulation described herein.
References
Martin GS (2003) Cell signaling and cancer. Cancer Cell 4:167–174
Milella M, Ciuffreda L, Bria E (2009) Signal transduction pathways as therapeutic targets in cancer therapy. 37: 83
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Ciuffreda L, McCubrey JA, Milella M (2009) Signaling intermediates (PI3K/PTEN/AKT/mTOR and RAF/MEK/ERK pathways) as therapeutic targets for anti-cancer and anti angiogenesis treatments. Curr Signal Transduct Ther 4:130–143
Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y et al (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14:1351–1356
Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C et al (2008) Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest 118:3051–3064
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I et al (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439:358–362
Ciuffreda L, Del BD, Desideri M, Di SC, Stoppacciaro A, Ricciardi MR, Chiaretti S, Tavolaro S, Benassi B, Bellacosa A et al (2009) Growth-inhibitory and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia 11:720–731
Robertson GP, Herbst RA, Nagane M, Huang HJ, Cavenee WK (1999) The chromosome 10 monosomy common in human melanomas results from loss of two separate tumor suppressor loci. Cancer Res 59:3596–3601
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M, Bosenberg M (2009) BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41:544–552
Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di VA, Conticello C, Ruco L, Peschle C, De MR (2008) Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 15:504–514
Milella M, Trisciuoglio D, Bruno T, Ciuffreda L, Mottolese M, Cianciulli A, Cognetti F, Zangemeister-Wittke U, Del Bufalo D, Zupi G (2004) Trastuzumab down-regulates Bcl-2 expression and potentiates apoptosis induction by Bcl-2/Bcl-XL bispecific antisense oligonucleotides in HER-2 gene–amplified breast cancer cells. Clin Cancer Res 10:7747–7756
Hettinger K, Vikhanskaya F, Poh MK, Lee MK, de Belle I, Zhang JT, Reddy SA, Sabapathy K (2007) c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ 14:218–229
von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR, Troppmair J (2001) Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol Cell Biol 21:2324–2336
Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S et al (2007) Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell 11:447–460
Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH (1998) A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol 8:1141–1150
Leslie NR, Gray A, Pass I, Orchiston EA, Downes CP (2000) Analysis of the cellular functions of PTEN using catalytic domain and C-terminal mutations: differential effects of C-terminal deletion on signalling pathways downstream of phosphoinositide 3-kinase. Biochem J 346(Pt 3):827–833
Pisano C, De CM, Beretta GL, Zuco V, Pratesi G, Penco S, Vesci L, Fodera R, Ferrara FF, Guglielmi MB et al (2008) Preclinical profile of antitumor activity of a novel hydrophilic camptothecin, ST1968. Mol Cancer Ther 7:2051–2059
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Vasudevan KM, Burikhanov R, Goswami A, Rangnekar VM (2007) Suppression of PTEN expression is essential for antiapoptosis and cellular transformation by oncogenic Ras. Cancer Res 67:10343–10350
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G et al (2010) Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 3:ra29
Ming M, Han W, Maddox J, Soltani K, Shea CR, Freeman DM, He YY (2010) UVB-induced ERK/AKT-dependent PTEN suppression promotes survival of epidermal keratinocytes. Oncogene 29:492–502
Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG Jr (2005) The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8:25–33
Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315
Jiang BH, Liu LZ (2009) PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 102:19–65
Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M (2001) Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A 98:11563–11568
Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382:1–11
Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di CA, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van DT et al (2003) Pten dose dictates cancer progression in the prostate. PLoS Biol 1:E59
Ming M, He YY (2009) PTEN: new insights into its regulation and function in skin cancer. J Invest Dermatol 129:2109–2112
Tsao H, Zhang X, Benoit E, Haluska FG (1998) Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene 16:3397–3402
Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP (2004) Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res 64:7002–7010
Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, Marmion JM, Laine E, Greulich H, Tseng H, Gates C et al (2008) Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res 68:664–673
Tsao H, Goel V, Wu H, Yang G, Haluska FG (2004) Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol 122:337–341
Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, Sellers WR, Lengauer C, Stegmeier F (2009) PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res 69:4286–4293
Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, Davies MA (2010) Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 70:8736–8747
Acknowledgments
This work was supported in part by grants from the Italian Association for Cancer Research (AIRC), the Cariplo Foundation, and the Italian Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
PDF 123 kb
Rights and permissions
About this article
Cite this article
Ciuffreda, L., Di Sanza, C., Cesta Incani, U. et al. The mitogen-activated protein kinase (MAPK) cascade controls phosphatase and tensin homolog (PTEN) expression through multiple mechanisms. J Mol Med 90, 667–679 (2012). https://doi.org/10.1007/s00109-011-0844-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0844-1