Abstract
Synaptic plasticity in the spinal cord and the cortex is believed to be important for the amplification of painful information in chronic pain conditions. The investigation of molecular mechanism responsible for maintaining injury-related plastic changes, such as through the study of long-term potentiation in these structures, provides potential novel targets for designing new medicine for chronic pain. Recent studies using integrative neurobiological approaches demonstrate that protein kinase M zeta (PKMζ) maintains pain-induced persistent changes in the anterior cingulate cortex (ACC), and inhibiting PKMζ by ζ-pseudosubstrate inhibitory peptide produces analgesic effects in animal models of chronic pain. We propose that targeting PKMζ, or its up- or downstream signaling proteins, in the ACC may provide novel clinical treatment for chronic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activity-dependent synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) is a key neuronal function for the brain. To potentiate or depress synaptic responses in response to incoming inputs from the periphery allow animals, including humans, to “learn” new information, and “store” important messages in central synapses. Under physiological conditions, such central plasticity is believed to contribute to the ability to adapt to the natural environment. To investigate such mechanisms and thereby to discover drugs to enhance such ability is critical for us to treat various forms of dementia and mild cognitive impairment and to improve cognitive capacity. However, recent cumulative evidence suggests that the brain may employ similar plastic mechanisms to handle injury information. Under pathological conditions, synaptic plasticity acts as positive amplification to exaggerate disease-related conditions. Chronic pain is a typical example of such central amplification.
Chronic pain is a major health issue and is mainly caused by tissue or nerve injury. In addition to spontaneous pain, there are two common pathological conditions that develop after tissue or nerve injury: allodynia and hyperalgesia. In allodynia, there is a reduction in pain threshold, and consequently, non-noxious stimuli that normally do not cause pain now induce pain. In hyperalgesia, there is an enhanced response to noxious stimuli. Peripheral sensitization and central plasticity are likely to contribute to chronic pain. Major focuses are at peripheral and spinal plastic changes, since these two areas are the best targets for medical manipulation or treatment for chronic pain with less or no central side effects. However, increasing evidence suggest cortical plasticity also takes place right after the injury; and some plastic changes may even occur at cortical levels without the requirement of continuous spinal or peripheral activity (Fig. 1). Here, we will review recent progress made related to injury-triggered cortical plasticity and explore possible molecular mechanism for maintaining LTP. We suggest that understanding these molecular mechanisms will provide new insights for treating chronic pain in the future.
A model of sensory circuits for pain transmission and modulatory in the central nervous system. Under normal physiological conditions, noxious information is sent to the spinal cord, and some spinal cord neurons send projection terminals to supraspinal structures. Neurons in the thalamus play key roles in relaying most of these ascending sensory inputs. The cortical areas, including the ACC, IC, S1, S2, and PFC, are activated and contribute to different aspects of pain perception. Neurons in the hippocampus are also activated and can contribute to the formation of pain-related spatial memory and mood responses. Activation of the amygdala (as well as the ACC) also contributes to pain-related fear memory and pain modulation. As protective responses to noxious stimuli, descending facilitatory systems can also be activated. Facilitated spinal nociceptive transmission might trigger faster escape responses. In the case of an inescapable situation, the descending inhibitory system can be activated, and the neurotransmitters such as serotonin (5-HT) and norepinephrine (NE) will be released from descending projection fibers in the dorsal horn of the spinal cord. They then inhibit dorsal horn synaptic transmission
LTP as a model for synaptic learning
LTP is the most extensively studied physiological process by those interested in understanding the molecular and cellular basis of learning and memory in vertebrates [1]. LTP is a widespread phenomenon exhibited by most excitatory synapses so far investigated, where it encodes regionally relevant information such as spatial learning in the hippocampus and persistent pain in the anterior cingulate cortex (ACC). It is well established that most forms of LTP are induced by the transient activation of N-methyl-d-aspartate receptors (NMDARs), though there are some NMDAR-independent forms of LTP in the central nervous system (CNS). Upon NMDAR activation, there is a transient elevation of Ca2+ in the activated postsynaptic spine, which triggers a signaling cascade involving a variety of protein kinases, such as Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII), protein kinase A (PKA), and mitogen-activated protein kinase (MAPK), as well as other signaling molecules. LTP is expressed by a variety of different mechanisms that include a long-lasting enhancement in l-glutamate release, alterations in postsynaptic voltage-dependent conductance, and changes in NMDARs and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), the receptors that mediate most fast synaptic transmission in the CNS.
With respect to AMPARs, there is evidence for changes in both their single channel conductance properties and in their number that are expressed in the postsynaptic membrane [2]. An increase in single channel conductance could be due to a number of factors, including (a) a higher l-glutamate concentration in the vicinity of the receptors, (b) an alteration in phosphorylation, such as of Ser845 of the GluA1 subunit, or (c) an alteration in subunit composition, caused by the rapid exchange of Ca2+-impermeable (i.e., GluA2 containing) with Ca2+-permeable (i.e., GluA2 lacking) AMPARs. AMPAR number could be altered via a variety of mechanisms, though it is likely to involve a two-component mechanism that involves (a) insertion of AMPARs into the plasma membrane followed by (b) their lateral diffusion into the postsynaptic membrane. AMPARs are then stabilized within the synapse with an interaction with N-ethylmaleimide sensitive factor (NSF) [3].
In addition to a long-lasting enhancement of synaptic transmission, information can also be stored by the long-lasting decrease in synaptic transmission, via the process of LTD. LTD is often also triggered by the synaptic activation of NMDARs, using different patterns of activation and involving different transduction mechanisms from LTP. A second major form of LTD can be triggered via the activation of metabotropic glutamate receptors.
Protein kinase M zeta and the maintenance of LTP
So far, there have been numerous studies to understand the molecular mechanisms of initiation and acquisition of LTP, primarily studied in the hippocampus. Accordingly, now we know several of the signaling molecules that are implicated in these stages. However, less is known about how LTP is maintained and thus needs to be elucidated. Recently, Todd C. Sacktor and his colleagues have revealed that protein kinase M zeta (PKMζ) is one of the key factors in maintaining LTP [4–6]. Originally, PKMζ was considered to be a cleavage product of protein kinase C zeta (PKCζ) [4, 7]. However, it was reported that PKMζ is produced from its own mRNA transcript, distinct from PKCζ synthesis [8]. Usually, most protein kinases consist of two components; regulatory and catalytic domains or subunits. However, PKMζ contains the same catalytic domain as PKCζ, yet without the regulatory component. This unique feature confers constitutive catalytic activity upon PKMζ although it requires phosphorylation by phosphoinositide-dependent protein kinase 1 (PDK1; Fig. 2) [9, 10]. The PKMζ could be an attractive candidate for sustaining synaptic strength because of its persistent activation mode. Previous studies suggested the possibility that PKMζ is a key factor in maintaining late LTP [4]. Among PKC isoforms, only PKMζ increases during LTP maintenance phase in hippocampal slices. Although other isoforms of PKC are enhanced immediately during the induction phase of LTP initiated by single tetanic stimulation, their expression returns to the basal level within 30 min after tetanus. More direct evidence showing the role of PKMζ came from pharmacological studies with a PKMζ inhibitor—ζ pseudosubstrate inhibitory peptide (ZIP). The application of ZIP during LTP maintenance phase in tetanized slices reverses the enhanced synaptic strength [5, 6] . This phenomenon was shown in vivo system as well [11, 12].
A simple model for the role of PKMζ in hippocampal LTP. In the hippocampus, activities trigger the release of glutamate, the activations of NMDARs result in the influx of Ca2+, which then triggers several Ca2+-related signaling pathway. Especially, for the late-phase LTP (L-LTP), PKA activity is important. Activated PKA and MAPK release the translational blocker, and PKMζ mRNA is translated and further phosphorylated by PDK1. The activating PKMζ potentiate the AMPAR responses by increasing the possible number of the GluA2 containing AMPARs through the action of N-ethylmaleimide-sensitive factor (NSF)
Since PKMζ was identified as one of the factors maintaining LTP, many attempts have been made to examine whether PKMζ has a role in the maintenance of long-term memory because LTP has been considered as a synaptic mechanism of memory. Using the PKMζ inhibitor ZIP, it has been reported that PKMζ is indeed required for the maintenance of several types of memory in various brain regions. For example, in the hippocampus, PKMζ is necessary for sustaining spatial information (place avoidance task, radial arm maze, water maze) and fear memory (trace eyeblink conditioning) [11–13]. When PKMζ is inhibited in the amygdala, cued fear memory, contextual fear memory, and inhibitory avoidance memory are erased during the examined time [13–16]. In cortical regions, persistent PKMζ activity is involved in the storage of long-term memory as well. It has been shown that ZIP infusion into the insular cortex eliminates the memory of conditioned taste aversion [17, 18]. Recently, it has been further demonstrated that overexpression of PKMζ in the insular cortex enhanced long-term memory, whereas a dominant negative PKMζ disrupted memory [19]. Moreover, emotional fear memory was impaired by ZIP infusion into the secondary sensory cortices [20]. Besides rodent studies, PKMζ is found in the head of Drosophila and has a similar role related to memory maintenance in rodents [21].
LTP in pain-related central synapses
Unlike hippocampal LTP, the studies of LTP in pain-related synapses were slow in the beginning. In the dorsal horn of the spinal cord, it is not easy to obtain field recordings of synaptic responses. Due to the mixed neuronal populations, tetanic stimulation of the dorsal root afferent fibers often leads to LTP or LTD in different cells recorded. The use of whole-cell patch recording technique, especially recording from retrograde-labeled projecting cells, significantly improves our understanding of spinal cord LTP [22, 23].
Several cortical areas including the ACC and insular cortex have been reported to contribute to chronic pain. Genetic, pharmacological, and electrophysiological approaches have been used to investigate the basic mechanisms for LTP at ACC synapses [24, 25]. Different stimulation protocols can be used for inducing LTP in ACC pyramidal cells. A conventional pairing protocol (synaptic activity paired with postsynaptic depolarization), a spike-excitatory postsynaptic potential pairing protocol, and a theta burst stimulation (TBS) protocol all induce LTP in ACC pyramidal neurons. The activation of NMDARs is critical for the induction of ACC LTP [24]. Blocking LTP in the ACC by microinjection of a NMDA receptor antagonist AP-5 suppresses injury-induced allodynia [26]. Ca2+-stimulated, neuron-specific adenylyl cyclase subtype 1 (AC1) is highly expressed in the ACC neurons [26], and LTP requires the activation of AC1 activity as demonstrated by the use of gene knockout mice lacking AC1 [27] or a selective inhibitor of AC1 [28]. For the expression of LTP, AMPARs containing the GluA1 subunit are involved. For example, ACC LTP is absent GluA1 knockout mice [29].
LTP has also been reported in other pain-related cortical areas. These include prefrontal cortex, somatosensory cortex area, and insular cortex. However, the exact molecular mechanisms for the induction and expression of LTP are mostly unknown and remain to be investigated in future studies. Recent studies have indicated that amygdala synapses undergo plastic changes after injury [30]. Different forms of amygdala LTP have been reported, mainly in the areas related to the study of mechanisms of fear memory. It is possible that some of the synaptic potentiation may be shared by chronic pain and emotional fear. Future studies are clearly needed to determine any distinct changes related to chronic pain.
ACC and chronic pain
It has been known that the ACC is important for mediating the emotional and the attentive responses to internal and external stimulation [31–33]. Brain imaging studies provide direct evidence that several limbic areas can be activated by peripheral pain stimulation. Among them, the ACC is found to be the most reliable area, which can respond to different noxious or painful stimuli [34, 35]. In vivo and in vitro electrophysiological experiments have demonstrated that the ACC neurons respond to peripheral somatosensory or visceral nociceptive stimulation in various types of animals, such as mouse, rat, rabbit, and monkey [28, 36–42]. The ACC may exert a top–down modulation on spinal nociceptive transmission through the descending regulatory systems, since electrical and chemical stimulation of the ACC facilitated behavioral responses to noxious stimulation [34, 43, 44]. While lesions of the ACC significantly reduced the animal’s sensitivity to noxious stimuli [45], inhibiting glutamatergic activity by injection of 6-cyano-7-nitroquinoxaline-2,3-dione into the ACC reduced the mechanical allodynia and pain-related vocalization [46, 47]. Consistent with animal studies, clinical studies found that frontal lobotomies or cingulotomies abolished the unpleasantness of pain in patients [48, 49]. Furthermore, unlike the somatosensory cortex, it has been proposed that the ACC may not code information for the location, intensity and duration of peripheral stimuli, but rather contributes to the affective content or the unpleasantness of pain [33, 34, 41]. It has been proved that, in freely moving animals, stimulation of the ACC also produced aversive behaviors or fear responses, which are typical emotional reactions related to pain [44, 50] .
ACC LTP after injury: pre- and postsynaptic mechanisms
Different approaches have been employed to investigate the changes of cingulate synaptic transmission under chronic pain condition. It has been found that synaptic transmission in the ACC was enhanced by periphery inflammation [51], nerve injury [37, 46], and digit amputation [52]. This enhancement shares similar neuronal mechanism with LTP because it occluded the plasticity of synaptic transmission in the ACC. For example, the amputation of a third hindpaw digit in adult rats caused loss of LTD, which could last for at least 2 weeks [52]. Recently, we examined the effects of nerve injury on LTP induction in the ACC and found that the L-LTP induced by TBS from nerve injury mice was significantly smaller than that from sham or naïve mice [37]. In addition, inhibiting the enhanced glutamatergic synaptic transmission decreased peripheral allodynia [46].
The enhancements of synaptic transmission induced by chronic pain in the ACC were mediated by both pre- and postsynaptic mechanisms [34]. It was found that glutamate release was increased by chronic pain. For example, it has been reported that paired-pulse facilitation was decreased and that the frequency of miniature EPSCs was increased by periphery inflammation [51], and similar results were observed under neuropathic pain condition [46]. Further experiments found that AC1 was involved in the modulation of the presynaptic release of glutamate [53, 54].
AMPARs, kainate receptors, and NMDARs are the major targets of glutamate in the ACC [55]. Integrative evidences showed that the postsynaptic components are also involved in the enhancement of synaptic transmission. The expression of AMPARs in the postsynaptic sites was increased under chronic pain condition [46]. This was further confirmed for the ACC neurons activated by allodynia by using the FosGFP transgenic mice, in which the expression of green fluorescent protein is controlled by the promoter of the c-fos gene [37]. Calcium signaling pathway in the postsynaptic cells may be involved in the initiation of LTP-like change induced by chronic pain. It was found that Ca2+ elevation in the postsynaptic sites was critical for the LTP induction in the ACC. Ca2+/CaM leads to the activation of calcium-stimulated signaling pathways [56, 57]. In turn, Ca2+/CaM can stimulate the activities of AC1, which can convert adenosine-5′-triphosphate to a second messenger cyclic adenosine monophosphate (cAMP). AC1 is critical for the induction of LTP in the ACC. Using a new selective AC1 inhibitor, we found that inhibiting of cingulate AC1 significantly reduced mechanical allodynia induced by nerve injury [27, 28]. The increased cAMP further activates PKA-dependent signaling pathway, including MAPK [58] and cAMP response element-binding protein (CREB). Ca2+/CaM can also activate different forms of CaM kinases, among them, CaMKIV is distinguished in its capacity to activate CREB-dependent transcription (Fig. 3) [59].
A model for LTP in the ACC. Activation of postsynaptic NMDARs leads to an increase in postsynaptic Ca2+ in dendritic spines. Ca2+ binds to CaM and leads to the activation of calcium-stimulated ACs, primarily AC1. Other Ca2+-/CaM-dependent protein kinases (e.g., PKC, CaMKII, and CaMKIV) are also activated. Activation of CaMKIV, a kinase predominantly expressed in the nuclei, will trigger CREB signaling pathways. In addition, the activation of AC1 leads to the activation of PKA, and subsequently CREB activation as well. Subsequently, AMPARs likely undergo long-term plastic upregulation. The upregulated PKMζ potentiates the response of AMPAR by modulating GluA1-containing AMPARs in the ACC
Molecular regulation of PKMζ in chronic pain
Painful stimulation on the periphery induces LTP-like change, especially in the ACC [52]. This implies that sustained LTP in the ACC maybe at least partially responsible for chronic pain induced by peripheral nerve injury. How is LTP maintained in the ACC? We recently showed that PKMζ is necessary for maintaining LTP in the ACC [37], like in the hippocampus. Calcium-dependent cAMP-PKA signaling pathway acts upstream of PKMζ. In AC1 knockout mice, nerve injury neither induced hyperalgesia nor increased PKMζ and phosphorylated PKMζ (p-PKMζ) levels in the ACC. Moreover, elevated cAMP level after forskolin treatment enhances both PKMζ and p-PKMζ in a time-dependent manner in the ACC slices. It is noteworthy that protein level of PKMζ increases even 5 min after forskolin treatment. This rapid increase of PKMζ by elevated cAMP is most likely due to the increase in translation rate, not due to transcriptional regulation [37].
In addition to the cAMP-PKA pathway, other molecules were suggested as upstream of PKMζ [4, 9]. Blocking NMDARs hampers PKMζ expression induced by tetanic stimulation [4]. NMDAR signaling especially through an involvement of the GluN2B subunit in the ACC is required for pain processing [60–62]. This implies that the peripheral nerve injury can increase neuronal Ca2+ level through the activation of NMDARs in the ACC and then, increased Ca2+ level can induce the PKMζ activation. Indeed, elevated Ca2+ can activate various kinases, including CaMKII, extracellular-signal-regulated kinases, PKA, PKC, PI3-kinase, and mammalian target of rapamycin. These activated kinases can increase the expression of PKMζ [9]. Although PKMζ expression is controlled by various kinases, newly synthesized PKMζ seems to be phosphorylated by PDK1. After strong tetanization of hippocampal slices, PKMζ and p-PKMζ are simultaneously increased at a similar rate. The ratio of PKMζ and p-PKMζ does not change even after tetanization [9]. This phenomenon seems to occur in neuropathic pain as well. PKMζ and p-PKMζ in the ACC increased to similar levels 3 days after nerve injury. However, the expression of PKMζ returns to basal level within 7 days after nerve injury, whereas p-PKMζ still remains at elevated levels even up to 14 days after nerve injury. Thus, at the initial stage of chronic pain, PKMζ might be increased via protein translation and autonomously activated by PDK1. At later stage, expression of PKMζ might be suppressed by some homeostatic mechanism. PDK1 still can activate PKMζ via a signaling pathway that is unknown so far and thus requires further studies.
AMPA receptor and PKMζ
Given the importance of PKMζ to the expression of synaptic plasticity, long-term memory and chronic pain, knowing the downstream effectors of PKMζ will help to understand the neuronal mechanism of chronic pain. In the ACC, integrative evidence showed that GluA1 is the downstream target of PKMζ. First, deletion of the GluA1 subunit abolished LTP in the ACC, while enhanced LTP was observed in the ACC of GluA2 null mice [29]. Interfering with the interaction between the C terminus of GluA1 and post-synaptic density (PDZ) domain proteins blocked the induction of cingulate LTP [63]. Furthermore, bath application of PhTx-433, an antagonist of Ca2+-permeable (i.e., GluA2-lacking) AMPARs, 5 min after LTP induction reduced synaptic potentiation [63]. These data suggest that Ca2+-permeable GluA2-lacking receptors contribute to the expression of LTP. Blocking the activities of PKMζ by ZIP erased late-phase LTP induced by theta burst stimulation (Fig. 4) [37]. Therefore, PKMζ may interact with GluA1 to maintain LTP in the ACC.
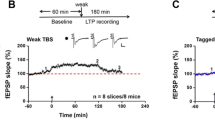
ZIP blocked L-LTP in the ACC. Examples showed that TBS can induce L-LTP for at least 5 h in ACC slices of adult mice (blue squares). Bath application of ZIP (5 μM) erased the maintenance of L-LTP in the ACC (orange squares). Inset Individual traces of field EPSCs are shown at three different time points (before training: a L-LTP before ZIP application, b after ZIP application, or c control; modified from Li et al. [37])
Periphery nerve injury resulted in an increase in GluA1 and in its phosphorylation on Ser845, but not GluA2/3, in the ACC neurons [46]. We found that bath application of ZIP decreased the amplitude of evoked EPSCs, and by applying non-stationary fluctuation analysis, we found that the effect of ZIP on the eEPSCs was due to a decrease in the number of active channels, rather than a decrease in the unitary conductance of AMPARs. We further analyzed the subunits of AMPARs and found that blocking the activities of PKMζ in the ACC decreased the protein level of GluA1 in the synapses [37]. Therefore, our results suggest that under neuropathic pain condition, PKMζ interacts with the GluA1 subunit of AMPARs in the ACC.
The downstream effectors of PKMζ were also investigated in other brain areas such as hippocampus [64] and amygdala [15]. In these brain areas, PKMζ maintains the L-LTP and long-term memory through persistently modifying NSF/GluA2-dependent AMPAR trafficking at the synapse [15, 64]. Considering the differences between the hippocampus and the ACC [25], it is possible that the downstream of PKMζ may be different between the hippocampus and ACC or they may share at least some of common mechanisms. Future studies need to be done to investigate the contributions of PKMζ to the synaptic potentiation in other pain-related brain areas under chronic pain condition.
Physiological and pathological implications
From the physiological points of view, ACC neurons have multiple dimensions in their functions. They not only respond to sensory stimuli, such as noxious or painful stimuli, but also respond to emotional stimuli. At the single cell level, each cell has a diffuse receptive field that covers almost the whole body area [33, 36]. It is responsive to inputs from somatosensory areas and internal visceral organs. Enhancing excitatory transmission in the ACC after injury may contribute to enhanced attentional state and facilitate individual responses to future potential dangerous stimulation. Recent studies indeed indicate that ACC sends top–down modulatory influences to the spinal cord and facilitates spinal nociceptive responses [50].
ACC influences may not be limited at sensory transmission and modulation. Altered ACC excitability may contribute to emotional anxiety and fear. Recent studies using genetic and pharmacological manipulations demonstrate that ACC neurons contribute to anxiety- and fear-like behaviors [65]. It remains to be determined if ACC produces these effects through other cortical or subcortical structures. It has been known that ACC forms a high density of inter-connections with other brain areas [33].
In summary, cumulative evidence using integrative experimental approaches consistently demonstrate that peripheral injury triggers LTP of excitatory synaptic transmission within the ACC. Enhanced excitatory synaptic transmission may facilitate responses of ACC neurons to subsequent sensory stimulation. In case of chronic pain, ACC LTP may be responsible for increased responses of ACC neurons to peripheral sensory stimuli, including non-noxious stimuli that trigger behavioral allodynia. Potentiated synaptic responses may also contribute to abnormal spontaneous neuronal activity within the ACC, a possible synaptic mechanism for spontaneous pain during chronic pain. Therefore, understanding molecular mechanisms for the induction and expression of ACC LTP may help us to identify new protein targets for the treatment of chronic pain. The discovery of the key role of PKMζ in the maintenance of injury-related cortical synaptic potentiation may help us to design novel medicine such as a selective chemical inhibitor of PKMζ for the treatment of chronic pain in patients.
References
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Benke TA, Luthi A, Isaac JT, Collingridge GL (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393:793–797
Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21:87–97
Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E (1993) Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA 90:8342–8346
Serrano P, Yao Y, Sacktor TC (2005) Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci 25:1979–1984
Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC (2002) Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 5:295–296
Kishimoto A, Kajikawa N, Shiota M, Nishizuka Y (1983) Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem 258:1156–1164
Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC (2003) Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem 278:40305–40316
Kelly MT, Crary JF, Sacktor TC (2007) Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation. J Neurosci 27:3439–3444
Sacktor TC (2011) How does PKMzeta maintain long-term memory? Nat Rev Neurosci 12:9–15
Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313:1141–1144
Madronal N, Gruart A, Sacktor TC, Delgado-Garcia JM (2010) PKMzeta inhibition reverses learning-induced increases in hippocampal synaptic strength and memory during trace eyeblink conditioning. PLoS One 5:e10400
Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA (2008) PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol 6:2698–2706
Parsons RG, Davis M (2011) Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci 14:295–296
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K (2010) PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13:630–634
Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ (2009) Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci 123:844–850
Shema R, Sacktor TC, Dudai Y (2007) Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317:951–953
Shema R, Hazvi S, Sacktor TC, Dudai Y (2009) Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem 16:122–128
Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y (2011) Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science 331:1207–1210
Sacco T, Sacchetti B (2010) Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329:649–656
Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC (2002) Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci 5:316–324
Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J (2006) Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312:1659–1662
Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J (2003) Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299:1237–1240
Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M (2005) Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47:859–872
Zhuo M (2007) A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 23:259–271
Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M (2002) Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36:713–726
Liauw J, Wu LJ, Zhuo M (2005) Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol 94:878–882
Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M (2011) Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 3:65ra63
Toyoda H, Zhao MG, Ulzhofer B, Wu LJ, Xu H, Seeburg PH, Sprengel R, Kuner R, Zhuo M (2009) Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Molecular Pain 5:46
Neugebauer V, Li W, Bird GC, Han JS (2004) The amygdala and persistent pain. Neuroscientist 10:221–234
Derbyshire SW, Vogt BA, Jones AK (1998) Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118:52–60
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971
Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544
Zhuo M (2008) Cortical excitation and chronic pain. Trends Neurosci 31:199–207
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484
Sikes RW, Vogt BA (1992) Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol 68:1720–1732
Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M (2010) Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330:1400–1404
Koga K, Li X, Chen T, Steenland HW, Descalzi G, Zhuo M (2010) In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol Pain 6:62
Koyama T, Tanaka YZ, Mikami A (1998) Nociceptive neurons in the macaque anterior cingulate activate during anticipation of pain. Neuroreport 9:2663–2667
Sikes RW, Vogt LJ, Vogt BA (2008) Distribution and properties of visceral nociceptive neurons in rabbit cingulate cortex. Pain 135:160–174
Kuo CC, Yen CT (2005) Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J Neurophysiol 94:1825–1836
Bie B, Brown DL, Naguib M (2011) Increased synaptic GluR1 subunits in the anterior cingulate cortex of rats with peripheral inflammation. Eur J Pharmacol 653:26–31
Calejesan AA, Kim SJ, Zhuo M (2000) Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain 4:83–96
Johansen JP, Fields HL (2004) Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci 7:398–403
Johansen JP, Fields HL, Manning BH (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 98:8077–8082
Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28:7445–7453
Harte SE, Spuz CA, Borszcz GS (2011) Functional interaction between medial thalamus and rostral anterior cingulate cortex in the suppression of pain affect. Neuroscience 172:460–473
Yarnitsky D, Barron SA, Bental E (1988) Disappearance of phantom pain after focal brain infarction. Pain 32:285–287
Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–484
Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M (2005) Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain 1:6
Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M (2006) Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 26:8923–8930
Wei F, Zhuo M (2001) Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 532:823–833
Toyoda H, Zhao MG, Zhuo M (2009) Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain 5:4
Wu LJ, Steenland HW, Kim SS, Isiegas C, Abel T, Kaang BK, Zhuo M (2008) Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol Pain 4:40
Wu LJ, Li X, Chen T, Ren M, Zhuo M (2009) Characterization of intracortical synaptic connections in the mouse anterior cingulate cortex using dual patch clamp recording. Mol Brain 2:32
Wei F, Xia XM, Tang J, Ao H, Ko S, Liauw J, Qiu CS, Zhuo M (2003) Calmodulin regulates synaptic plasticity in the anterior cingulate cortex and behavioral responses: a microelectroporation study in adult rodents. J Neurosci 23:8402–8409
Toyoda H, Zhao MG, Mercaldo V, Chen T, Descalzi G, Kida S, Zhuo M (2010) Calcium/calmodulin-dependent kinase IV contributes to translation-dependent early synaptic potentiation in the anterior cingulate cortex of adult mice. Mol Brain 3:27
Toyoda H, Zhao MG, Xu H, Wu LJ, Ren M, Zhuo M (2007) Requirement of extracellular signal-regulated kinase/mitogen-activated protein kinase for long-term potentiation in adult mouse anterior cingulate cortex. Molecular Pain 3:36
Soderling TR (1999) The Ca–calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24:232–236
Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M (2001) Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4:164–169
Lei LG, Sun S, Gao YJ, Zhao ZQ, Zhang YQ (2004) NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp Neurol 189:413–421
Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M (2005) Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 25:11107–11116
Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M (2007) Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol 67:498–509
Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 28:7820–7827
Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, Wu LJ, Zhuo M (2011) Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain 4:6
Acknowledgments
This work was supported by grants from the EJLB-CIHR Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair and CIHR operating grants (CIHR66975 and CIHR84256) (M. Z.). M.Z., B.-K.K., and G.L.C. are also supported by the World-Class University (WCU) program of the Ministry of Education, Science and Technology in Korea through KOSEF (R32-10142). X.-Y. Li and T. Chen are supported by postdoctoral fellowships from Fragile X Research Foundation of Canada. B.-K.K. is supported by the National Creative Research Initiative Program of the Korean Ministry of Science and Technology, Korea. H.-G. K. is supported by a BK21 fellowship, Korea. G.L.C. receives support from the MRC (UK) and is a WCU International Scholar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XY., Ko, HG., Chen, T. et al. Erasing injury-related cortical synaptic potentiation as a new treatment for chronic pain. J Mol Med 89, 847–855 (2011). https://doi.org/10.1007/s00109-011-0768-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0768-9