Abstract
In contrast to the transforming members of the Wnt family, shown to be upregulated in many cancers, the role of Wnt 5a is still controversial. While it has been attributed a tumour suppressor function in some malignancies, there is increasing evidence of promigratory and proinvasive effects in others, mediated predominantly through the planar cell polarity pathway and activation of protein kinase C. Obviously, the outcome of an individual Wnt 5a signal is dependent on a multitude of variables, ranging from availability of receptors, downstream effectors, and inhibitors to external influences coming from the tumour microenvironment and the extracellular matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wnt ligands belong to a family of at least 19 secreted glycoproteins with multiple functions in cell proliferation and migration, as well as tissue organisation. They are best known for their role in embryonic development and in tissue homeostasis in the adult organism (for recent reviews see [1] and [2]). However, the same capabilities, which are beneficial during ontogeny, render them powerful contributors to cancer initiation and progression in case of deregulation.
Among the multitude of Wnts, we will focus in this study on Wnt 5a, which in the past has been outshone in the field of cancer research by the oncogenic members of the family, such as Wnt 1, 3 and 7. In contrast to its intensely investigated relatives, Wnt 5a lacks transforming activity [3, 4]. It does not typically signal via the canonical β-catenin pathway, which is known to be involved in (cancer) stem cell renewal [5] and whose constitutive activation is the critical event in the majority of colon cancers (reviewed in [6]). Despite the lack of comparatively well-defined clinical models, recent data provide strong evidence that Wnt 5a also plays an essential role in cancer, contributing not so much to its initiation but more to malignant progression. However, the exact nature of this role, either as a tumour suppressor or promotor, is still controversial.
Signalling pathways of Wnt 5a
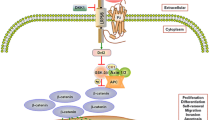
Wnt 5a has been classified as a so-called non-canonical ligand, which uses alternative signalling pathways in contrast to the canonical Wnt 1-type ligands. Signal transmission through either of these pathways has been considered mutually exclusive. However, recent data suggest a much more complicated transduction pattern (Fig. 1).
Signaling pathways initiated by Wnt 5a (β-Cat β-catenin, CamKII calmodulin-dependent kinase II, Dvl disheveled, Fz frizzled, JNK Jun-N-terminal kinase, LRP 5/6 low-density lipoprotein receptor-related protein, NFAT nuclear factor of activated T cells, PKC protein kinase C, ROCK RhoA/Rho kinase, TCF/LEF T cell factor/lymphocyte enhancer factor)
The canonical cascade is activated by simultaneous binding of Wnt 1-type ligands to receptors of the Frizzled (Fz)-family and the co-receptor low density lipoprotein-related protein (LRP) 5/6 (reviewed in [1, 2]). This recruits the multidomain protein dishevelled (Dvl; reviewed in [7]) to the cell membrane and leads to the inactivation of a destruction complex, which keeps the concentration of transcriptionally active β-catenin low in the absence of Wnt stimulation. Released β-catenin then translocates into the nucleus, binds to transcription factors of the TCF/LEF-family (T cell factor/lymphoid enhancer factor) and activates the transcription of target genes [8], such as c-myc [9], matrix metalloprotease-7 (mmp-7) [10] and fibronectin [11].
Non-canonical signalling by Wnt 5a activates the planar cell polarity (PCP) and the Wnt/Ca2+ pathway. Signalling through the latter occurs predominantly via Fz 2 and is mediated by increased intracellular Ca2+-realease [12, 13]. This stimulates protein kinase C (PKC), nuclear factor associated with T cells (NFAT) or calmodulin-dependent kinase II (CamKII). Channelling of Wnt 5a-signals into the PCP pathway is mediated by the PDZ and DEP regions of Dvl and results in activation of the small Rho-GTPases RhoA and Rac [7]. They, in turn, induce the Rho-associated kinase (ROCK), leading to reorganisation of the cytoskeleton, as well as activation of the Jun-N-terminal kinase (JNK). The latter phosphorylates c-jun and transcriptionally activates AP-1. Wnt 5a-triggered PCP signalling mediates epithelial cell polarity in Drosophila development and convergent extension movements during vertebrate gastrulation [14, 15]. Recently, Wnt 5a has also been shown to be critical for PCP signalling in mammalians, where it is involved in embryonic development of the cochlea in mice [16].
There are multiple ways how the non-canonical Wnt 5a-cascades can interact with the canonical pathway. Signalling through CamKII or other GSK-3-independent mechanisms exerts an antagonistic effect [13, 17, 18]. Alternatively, Wnt 5a can bind to the receptor tyrosine kinase Ror2 [19, 20], which results in Rho GTPase-independent activation of JNK and also inhibits canonical signalling [21]. On the other hand, Wnt 5a has been shown to stabilise β-catenin via PKC [22]. Phosphorylation of c-jun by JNK acts synergistically with TCF/LEF on the promoter of the canonical target gene c-jun [23]. In the presence of appropriate receptor constellations, Wnt 5a is even able to directly activate canonical signalling, as has been shown by simultaneous overexpression of Wnt 5a and Fz 5 in Xenopus [24] and Fz 4 and LRP 5 in human embryonic kidney cells [21].
Obviously, there are multiple options for transduction of Wnt 5a signals, which are closely cross-linked. General prediction of its signalling output is difficult, as it is not only regulated by the characteristics of the protein itself but is also controlled by availability of receptors and other mediators of the signalling cascade and by the presence of natural inhibitors, such as the secreted Fz-related proteins (sFRP) and the members of the Dickkopf (DKK) family (reviewed in [25, 26]).
Physiologic effects with implications for cancer progression
Usually, malignancies are a problem of the adult organism. Thus, it seems likely that of the multitude of Wnt 5a functions predominantly those are involved in tumour promotion, which play a role also beyond embryonic development. Wnt 5a effects on migration, invasion and angiogenesis in adult tissues are of special interest, as they are, on the one hand, necessary for tissue homeostasis and repair, on the other, known as critical steps of cancer progression. E.g. Wnt 5a can activate the PCP pathway and induce actin reorganisation. Interestingly, upregulation of mediators of PCP signalling, the Rho GTPases, is found in many metastatic cancers [27, 28], as acquisition of a motile cell phenotype via cytoskeletal rearrangement is a prerequisite for cancer dissemination.
In the embryo, Wnt 5a is localised to the distal compartments of several developmental systems that extend from the primary body axis, such as limbs, genitals, face and outer ear. Mice lacking Wnt 5a show defects in these structures, including loss of tail and digits as well as shortening of the body axis, mandible, head and tongue [29]. Wnt 5a regulates lung branching, as it is normally expressed in the distal lung bud, and its complete loss leads to severe, perinatally lethal defects in lung development [30]. The ability of Wnt 5a to induce spatially defined outgrowth of tissues can also be observed in the adult organism. In the terminal end buds of the breast, it regulates postnatal branching morphogenesis in the maturing mammary gland, a process which closely mirrors embryonic lung development [31]. Moreover, Wnt 5 has been described in so-called side population cells of the mammary gland, a subpopulation with cancer stem cell characteristics, which has been implicated in tumorigenesis [32]. Interestingly, the majority of early disseminated breast cancer cells in the bone marrow display a stem cell-like phenotype, in contrast to their non-metastatic counterparts in the primary tumour [33].
Wnt 5a is expressed in the mesenchymal compartment of the uterus, where it is required for the development of epithelial glands that are essential for mature function [34]. It is also involved in the mesenchymal–epithelial interactions during trophoblast invasion [35] by increasing trophectoderm cell migration in a ROCK-dependent manner [36]. Invasion, be it benign or malignant, requires remodelling of adjacent tissues through induction of MMPs. MMP-7, usually considered a target of canonical signalling, has recently been shown to be upregulated by Wnt 5a [37] and by other non-canonical signals [38]. In addition to its general proteolytic function, it can serve as a shedding protease for TNF-α, a cytokine well-known for its tumour-promoting activity [39]. Wnt 5a induces production of MMP-1 in mouse mammary epithelial [40] and in cultured endothelial cells [41]. In the latter, it enhances the formation of capillary-like networks, another critical step in tumour progression.
Wnt signalling is necessary for normal skin development and hair follicle morphogenesis in the embryo (reviewed in [1] and [5]). However, the adult skin is capable of responding to the same morphogenetic signals. Wound repair after full-thickness skin loss is associated with enhanced Wnt signalling: β-catenin-independent within the wound and β-catenin-dependent in adjacent regions. Despite the precisely orchestrated Wnt activation and the presence of multipotent epidermal stem cells in the immediate neighbourhood, this usually leads to functionally inferior scar tissue with only one single epithelial layer. True regeneration with epithelial appendage formation can be achieved by forced expression of Wnt 5a in mesenchymal cells of the deeper dermis [42]. The repopulating interfollicular epithelial cells migrate along the morphogenetic Wnt 5a-gradient into the wound tissues, forming large keratin-containing epithelial cysts, small sebaceous glands and rudimentary hair follicles. No tumours could be observed. Nevertheless, it is tempting to speculate that this may represent a benign equivalent of malignant invasion, which may occur, when the gradients of morphogenetic signals are not provided in the correct environmental context.
Expression in cancer tissues and clinical impact
Considering the complexity of the Wnt signalling cascades and the multitude of regulating factors, it is not surprising that the data on expression of Wnt 5a in cancer tissues are contradictory. Aberrant Wnt 5a mRNA and/or protein expression has been demonstrated in a variety of cancers of epithelial and mesenchymal origin.
Consistent with the concept of Wnt 5a as a tumour suppressor, it has been found downregulated in comparison to the respective normal tissue in endometrial cancer and high-risk neuroblastomas [43, 44]. In contrast to human peripheral blood B and myeloid cells, where Wnt 5a transcripts were readily detectable, they were absent or greatly reduced in the respective acute leukemias [45]. Loss of expression was associated with dedifferentiation in thyroid carcinomas [46], shortened survival in stage Dukes B colon cancer [47] and with advanced stage and metastatic disease in breast cancer [48]. Although Wnt 5a was an independent variable upon multivariate analysis, the positive effect of Wnt 5a expression on breast cancer survival was detectable predominantly in women with concomitant overexpression of the non-receptor tyrosine kinase Syk [49] suggesting cross-talk with other signalling pathways.
On the other hand, Wnt 5a expression has been described to be associated with aggressive tumour biology and poor clinical outcome. It was found overexpressed in comparison to the respective normal tissues in cancers of the lung, breast, prostate, and nasopharynx [50–53]. Wnt 5a was upregulated during early pancreatic cancerogenesis, its levels continuously increasing from intraepithelial neoplasias to overtly invasive adenocarcinomas [54]. In human squamous cancer cells [55], Wnt 5a expression was associated with loss of E-cadherin and epithelial–mesenchymal transition (EMT), which is a key step in metastasis.
Wnt 5a-positivity of non-small cell lung cancers was a significant predictor of shortened survival upon multivariate analysis [56]. In gastric cancers, Wnt 5a-positivity was correlated with advanced stages and poor survival [57]. Gene expression studies identified Wnt 5a as one of the most robust markers of aggressive behaviour of cutaneous melanomas [58], which was supported by the detection of a direct correlation between immunohistologic Wnt 5a-positivity and high tumour grade [59]. The same correlation between Wnt 5a expression and advanced tumour stage with higher rate of lymph node involvement was found in colon cancer [60]. Interestingly, Wnt 5a mRNA was not present in the tumour cells themselves, but in macrophages within the tumour stroma, pointing to a role of the benign tumour surrounding as a source and regulator of the Wnt network.
Functional effects in cancer
The ambiguity of the mentioned findings is reflected by the controversial results of the functional studies. A tumour suppressor effect was described in Wnt 5a +/− mice. Haematopoetic progenitor cells showed increased proliferation upon reduction of Wnt 5a expression due to downregulation of CamKII activity. This presumably facilitates acquisition of additional genetic abnormalities and may explain the observed increased incidence of haematologic malignancies in these animals [45]. Transformation of C57MG mammary epithelial cells via Wnt 1 overexpression could be partially reverted by the presence of ectopic Wnt 5a [61]. In the human mammary cell line HB2, Wnt 5a abolished hepatocyte growth factor-stimulated migration and enhanced cell-to-collagen binding via the discoidin domain receptor 1 [62]. Similarly, the migratory capacity of the highly invasive colon cancer cell line SW 480 was inhibited by addition of Wnt 5a [47]. A synthetic hexapeptide, based on the sequence of Wnt 5a, mimicked this effect in a Wnt/Ca2+-dependent way [63]. Transfection of Wnt 5a in thyroid carcinoma cell lines lead to increased intracellular Ca2+-release and activation of CamKII [46]. This was followed by downregulation of migration, invasiveness and clonogenicity as well as by membrane translocation of β-catenin.
The latter finding suggests that many of these results may be due to negative interference of Wnt 5a with the canonical pathway, particularly, when it signals via CamKII. Most of the above investigated cell lines owe their migratory capacity to an either constitutively or artificially activated β-catenin cascade. It stands to reason that cells with unaltered β-catenin signalling will not necessarily respond in the same way.
In fact, there is a preponderance of observations of Wnt 5a-induced tumour-promoting effects in other systems. Scratch assays with HUVEC cells showed reduced migration into wounded areas upon reduction of Wnt 5a expression via shRNA [41]. Wnt 5a enhanced cell migration in mouse embryonic fibroblasts [64] via Ror2-induced filopodia formation and actin reorganisation. Knock-down of Wnt 5a inhibited migration of HeLaS3 cells, while its addition stimulated adhesion-dependent migration and invasion of NIH3T3 and L cells [57]. This was associated with an increased number of lamellipodia and with activation of focal adhesion kinase and accelerated turnover of paxillin in these structures.
Wnt 5a was identified as a downstream target of the homeobox transcription factor CUTL1 in pancreas carcinoma cell lines, where it mediated CUTL1-induced enhanced migration and invasion [54]. In melanomas, Wnt 5a stimulated motility of the cancer cells via activation of PKC [59]. PKC inhibition in cells with high Wnt 5a expression resulted in marked prolongation of wound closure in scratch assays [65]. Wnt 5a upregulated CD 44, a tumour cell homing and metastasis-associated gene. Additionally, it triggered expression of vimentin, an intermediate filament protein, and activation of the transcriptional repressor snail, which, in turn, downregulated E-cadherin and stimulated production of MMP-2. Obviously, Wnt 5a can potentiate melanoma metastasis by inducing EMT. The fact that EMT was easily reversible upon Wnt 5a downregulation could suggest it as a potential target for therapeutic strategies, at least in melanomas.
Apart from tissue-specific variations in receptor expression and signalling pathways, it seems very likely that differences in the applied experimental systems additionally contribute to the cited contradictions. With a morphogen such as Wnt 5a, the slope of a given concentration gradient and the direction, from which it hits the cell, is of essential significance. In consequence, tools like ectopic overexpression or assays in an unphysiological surrounding, such as plastic surfaces, will most probably yield results, which are not transferable to other contexts, let alone the in vivo situation.
Wnt 5a and the tumour microenvironment
The role of the benign microenvironment for tumour progression has only recently come into focus. Migration and invasion are not only dependent on the biological characteristics of the tumour cells themselves, but are subject to influences from the surrounding tissue compartment, which contains cells of the innate and adaptive immune system, fibroblasts, endothelia and extracellular matrix (ECM).
ECM is mainly composed of collagens, fibronectin, laminins, elastin and glycosaminoglycans. For a long time considered a mere structural scaffold for the embedded epithelial cells, it is now known to be a dynamic signalling network, which can either give rise to or modulate the activity of signalling molecules (reviewed in [66, 67]). Due to their chemical structure, the lipid-modified Wnt ligands (reviewed in [68]) are predestined for modification by the ECM. They bind to heparan sulphate proteoglycans, which influences their transport from cell to cell and alters their signalling activity [69]. Much of the secreted Wnt 5a—if correctly palmitoylated—is bound to components of the ECM [70]. Chemical linkage very likely interferes with the establishment of morphogenetic gradients, needed especially for directional cell movement, cell polarity and the organisation of tissues.
Apart from direct modification of Wnt effects, ECM and the benign cells of the stromal compartment provide a variety of molecules, which are known to induce accumulation of β-catenin and EMT (reviewed in [71]). Nuclear localisation of β-catenin is found especially at the invasive front of colon cancers [72]. This supports the assumption that regulatory effects from the microenvironment additionally contribute to the constitutive activation of canonical signalling due to loss of APC function or β-catenin mutations in the tumour cells. For non-canonical Wnt signalling, the situation is less clear. However, the fact that Wnt 5a in melanomas was not expressed homogenously, but predominantly at the leading edge of invasion [59], is highly suggestive of interactions between tumour and stroma.
Wnts are not only produced by the malignant but also by the benign cellular components of the tumour [73]. In this context, the role of the tumour-associated macrophages (TAM) is of special interest. TAM contribute to malignant progression by enhancing invasiveness via upregulation of MMPs and production of TNF-α as well as other tumour-promoting cytokines (reviewed in [74, 75]). It is still largely unclear what switches them from their usual proinflammatory, cytotoxic to a tumour-permissive, proinvasive phenotype. A possible explanation comes from recent data, demonstrating that co-culture of macrophages with otherwise weakly invasive breast cancer cells induces upregulation of Wnt 5a in the former and enhances invasiveness in the latter [37, 76]. Although a functional canonical pathway in the tumour cells was a necessary prerequisite, non-canonical signalling via the JNK-pathway was critical for this effect. Consistent with the description of Wnt 5a-positive TAM in colon cancers [60], Wnt 5a could also be detected in TAM in primary breast cancers and, in particular, at the invasive front of lymph node metastases, underlining the biological significance of these findings.
Apart from its pro-migratory function, this suggests a role for Wnt 5a as a potential immune modulatory agent. The concept is further supported by the observation that Wnt 5a is upregulated in macrophages in granulomatous lesions due to infection with Mycobacterium tuberculosis [77], a condition sharing many common features with the “inflammatory reaction” in cancers.
Conclusion
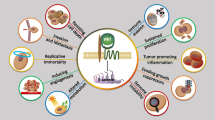
Taken together, there is strong evidence that Wnt 5a is involved in cancer progression. Although in part still controversial, many findings point to a pro-migratory and pro-invasive role of Wnt 5a. However, its function either as a tumour suppressor or promotor is obviously dependent on the individual intra- and intercellular context. Not only the presence of receptors, downstream effectors and inhibitors but also interactions between different cell types and modulation of Wnt 5a gradients by matrix components influence the complex signalling network and determine the actual signalling output (Fig. 2). In general, Wnt 5a signalling seems an attractive target for specific anticancer strategies. However, to achieve this goal, the mentioned variables have to be clarified in more detail, and models to predict the individual signalling outcome have to be developed.
Hypothetical model of intratumoral Wnt 5a signalling and its potential effects A tumor promoter function of Wnt 5a has been described predominantly as a result of signaling through PKC and the PCP pathway, while activation of CamKII was associated with tumor suppressor effects. The outcome of an individual Wnt 5a signal obviously depends on which signaling cascades are activated/inactivated in a special context. Apart from the availability of receptors and downstream effectors on/in the tumor cells themselves (T tumour cells), this is influenced by the surrounding stromal compartment, containing benign cells (F fibroblasts, L lymphocytes, M macrophages) and extracellular matrix (ECM). Wnt 5a, produced by the tumor cells, can act in an either autocrine or paracrine fashion on adjacent malignant and benign cells. Wnt 5a can also be produced by stromal cells, thus providing different directional gradients, which can be further modulated by chemical linkage to components of the ECM. Secreted physiological inhibitors, presence of other ant/agonistic Wnt ligands and promigratory molecules from the stromal compartment, e.g. laminin-5 and cytokines, additionally interfere with signalling and functional effects
References
Clevers H (2006) Wnt/β-catenin signaling in development and disease. Cell 127:469–480
Nusse R (2005) Wnt signaling in disease and development. Cell Res 15:28–32
Wong GT, Gavin BJ, McMahon AP (1994) Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol 14:6278–6286
Olson DJ, Papkoff J (1994) Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ 5:197–206
Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434:843–850
Fodde R, Brabletz T (2007) Wnt/beta-catenin signaling in cancer stemness and malignant behaviour. Curr Opin Cell Biol 19:150–158
Wallingford JB, Habas R (2005) The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132:4421–4436
Wnt homepage (2007) http://www.stanford.edu/~rnusse/wntwindow.html
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T (1999) β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155:1033–1038
Gradl D, Kühl M, Wedlich D (1999) The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol 19:5576–5587
Slusarski DC, Corces VG, Moon RT (1997) Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413
Pandur P, Maurus D, Kühl M (2002) Increasingly complex: new players enter the Wnt signaling network. Bioessays 24:881–884
Seifert JR, Mlodzik M (2007) Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8:126–138
Jones C, Chen P (2007) Planar cell polarity signaling in vertebrates. Bioessays 29:120–132
Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P (2007) Wnt 5a functions in planar cell polarity regulation in mice. Dev Biol 306:121–133
Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y (2003) Wnt 5a inhibits the canonical Wnt pathway by promoting GSK-3-independent . b. -catenin degradation. J Cell Biol 162:899–908
Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC (2003) Wnt 5a/pipetail fundctions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin signaling. J Cell Biol 162:889–898
Oishi I, Suzuki H, Onishi N, Takada R, Kanai S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Schambony A, Wedlich D (2007) Wnt-5a/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 5:779–792
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits . b. -catenin-TCF signaling depending on receptor context. PLoS Biol 4:570–582
Chen RH, Ding VW, McCormick F (2000) Wnt signaling to . b. -catenin involves two interactive components, glycogen synthase kinase-3b inhibition and activation of protein kinase C. J Biol Chem 275:17894–17899
Nateri AS, Spencer-Dene B, Behrens A (2005) Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437:281–285
He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the frizzled protein family mediating axis induction by Wnt 5a. Science 275:1652–1654
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634
Niehrs C (2006) Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481
Burbelo P, Wellstein A, Pestell RG (2004) Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat 84:43–48
Liu N, Zhang G, Bi F, Pan Y, Xue Y, Shi Y, Yao L, Zhao L, Zheng Y, Fan D (2007) RhoC is essential for the metastasis of gastric cancer. J Mol Med 85:1149–1156
Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223
Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) Wnt5a participates in distal lung morphogenesis. Dev Biol 248:68–81
Kouros-Mehr H, Werb Z (2006) Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn 235:3404–3412
Behbod F, Xian W, Shaw CA, Hilsenbeck SG, Tsimelzon A, Rosen JM (2006) Transcriptional profiling of mammary gland side population cells. Stem Cells 24:1065–1074
Balic M, Lin Y, Young L, Haws D, Guiliano A, McNamara G, Datar RH, Cote RJ (2006) Most early disseminated cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12:5615–5621
Mericskay M, Kitajewski J, Sassoon D (2004) Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131:2061–2072
Sonderegger S, Husslein H, Leisser C, Knöfler M (2007) Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 28(Suppl A):97–102
Hayashi K, Burghardt RC, Bazer FW, Spencer TE (2007) WNTs in the ovine uterus: potential regulation of periimplantation ovine conceptus development. Endocrinology 48:3496–3506
Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C (2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci 103:5454–549
Le Floch N, Rivat C, De Wever O, Bruyneel E, Mareel M, Dale T, Gespach C (2005) The proinvasive activity of Wnt-2 is mediated through a noncanonical Wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J 19:144–146
Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 25:409–416
Prieve MG, Moon RT (2003) Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev Biol 3:2
Maskauchuan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J (2006) Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 17:5163–5172
Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon RT, Isik F (2006) Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 7:4
Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL (1997) Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer 75:1131–1136
Blanc E, Goldschneider D, Douc-Rasy S, Benard J, Raguenez G (2005) Wnt-5a gene expression in malignant human neuroblasts. Cancer Lett 228:117–123
Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN (2003) Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoetic tissue. Cancer Cell 4:349–360
Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G (2005) Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24:2144–54
Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T (2005) Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 65:9142–9146
Jönsson M, Dejmek J, Bendahl PO, Andersson T (2002) Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 62:409–416
Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, Landberg G, Andersson T (2005) Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res 11:520–528
Iozzo RV, Eichstetter I, Danielson KG (1995) Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res 55:3495–3499
Lejeune S, Huguet EL, Hamby A, Poulsom R, Harris AL (1995) Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res 1:215–222
Fernandez-Cobo M, Zammarchi F, Mandeli J, Holland JF, Pogo BG (2007) Expression of Wnt5A and Wnt10B in non-immortalized breast cancer cells. Oncol Rep 17:903–907
Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, Luo XM, Wu MH, Yang YX, Huang C, Cao L, Tang K, Qian J, Shen SR, Li GY (2007) Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol 38:120–133
Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, Downward J, Gress T, Michl P (2007) WNT5A-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 6:1178–1187
Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S, Nagayama M (2003) Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci 94:593–597
Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, Ueno M (2005) Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor - an expression in non-small-cell lung cancer. J Clin Oncol 23:8765–8773
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A (2006) Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66:10439–10448
Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536–540
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1:279–288
Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL (1999) Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer 81:496–502
Olson DJ, Gibo DM (1998) Antisense wnt-5a mimics wnt1-mediated C57MG mammary epithelial cell transformation. Exp Cell Res 241:134–141
Jönsson M, Andersson T (2001) Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001 114:2043–2053
Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T (2006) A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem 281:2740–49
Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y (2006) Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175:555–562
Dissanayake SK, Wade M, Johnson CE, O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT (2007) The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 282:17259–17271
Schenk S, Quaranta V (2003) Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 13:366–375
Mareel M, Leroy A (2003) Clinical, cellular and molecular aspects of cancer invasion. Physiol Rev 83:337–376
Mikels AJ, Nusse R (2006) Wnts as ligands: processing, secretion and reception. Oncogene 25:7461–7468
Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP (2003) QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol 162:341–351
Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A (2007) Post-translational palmitoylation and glycosylation of Wnt-5a are necassary for its signaling. Biochemical J 402:515–523
Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T (2005) Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and ß-catenin. Cells Tissues Organs 179:56–65
Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T (1998) Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasive front. Pathol Res Pract 194:701–704
Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H (2005) Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129:626–38
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612
Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C (2004) Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 8:1543–1549
Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N (2006) The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108:965–973
Acknowledgement
We thank Annette Borchers and Florian Klemm for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pukrop, T., Binder, C. The complex pathways of Wnt 5a in cancer progression. J Mol Med 86, 259–266 (2008). https://doi.org/10.1007/s00109-007-0266-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-007-0266-2