Abstract
Sequelae of sepsis include anemia which presumably results from accelerated clearance of erythrocytes from circulating blood. The underlying mechanisms, however, remained hitherto elusive. Most recent studies disclosed that increased cytosolic Ca2+ activity and ceramide both trigger suicidal erythrocyte death (i.e., eryptosis), which is characterized by lipid scrambling of the cell membrane leading to phosphatidylserine exposure at the erythrocyte surface. Phosphatidylserine exposing erythrocytes may adhere to vascular walls or may be engulfed by macrophages equipped with phosphatidylserine receptors. To explore whether sepsis leads to eryptosis, erythrocytes from healthy volunteers were exposed to plasma of patients suffering from sepsis, or to supernatants from sepsis producing pathogens. Then, phosphatidylserine exposure (annexin V binding), cell volume (forward scatter), cytosolic Ca2+ activity (Fluo3 fluorescence), and ceramide formation (anti-ceramide antibody) were determined by flow cytometry. Challenge of erythrocytes with plasma from the patients but not with plasma from healthy individuals triggered annexin V binding. The effect of patient plasma on erythrocyte annexin V binding was paralleled by formation of ceramide and a significant increase of cytosolic Ca2+ activity. Exposure of erythrocytes to supernatant of pathogens similarly induced eryptosis, an effect correlating with sphingomyelinase activity. The present observations disclose a novel pathophysiological mechanism leading to anemia and derangement of microcirculation during sepsis. Exposure to plasma from septic patients triggers phosphatidylserine exposure leading to adherence to the vascular wall and clearance from circulating blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening condition during overwhelming infection with a variety of pathogens [1, 2]. Characteristic sequelae of sepsis include rapidly developing anemia which cannot be accounted for by decreased formation of erythrocytes but must involve accelerated clearance of erythrocytes from circulating blood [1].
The clearance of circulating erythrocytes is at least in part the result of hemolysis [3, 4]. Recent in vitro experiments disclosed a novel mechanism affecting erythrocyte survival. Erythrocytes exposed to oxidative stress, hyperosmotic shock, or energy depletion activate a Ca2+-permeable cation channel [5] with subsequent entry of Ca2+. Ca2+ then activates Ca2+-sensitive K+ channels leading to cell shrinkage [6]. Moreover, the entry of Ca2+ triggers Ca2+ sensitive scrambling of the cell membrane [7] with transbilayer movement of plasma membrane phospholipids and exposure of phosphatidylserine at the erythrocyte surface [5]. The erythrocytes are sensitized towards Ca2+ by the sphingolipid metabolite ceramide, which is also released after erythrocyte injury [8].
Phosphatidylserine exposing erythrocytes may adhere to endothelial cells of the vascular wall and thus impede microcirculation [9]. On the other hand, macrophages are equipped with receptors specific for phosphatidylserine [10], and erythrocytes exposing phosphatidylserine at their surface are recognized, engulfed, and degraded [11]. Thus, erythrocytes exposing phosphatidylserine at their surface are prone to be eliminated from circulating blood. Erythrocytes destroyed by suicidal death (i.e., eryptosis) [12] may undergo similar but not necessarily identical changes as those undergoing erythrocyte senescence [12–16] or neocytolysis [15].
Eryptosis may be triggered by complement activation in the course of hemolytic–uremic syndrome [17] or by bacterial toxins, such as hemolysin Kanagawa [18]. Several sepsis-inducing bacterial pathogens are known to produce sphingomyelinases, which in turn could stimulate ceramide formation [8]. Along those lines, the β-toxin of Staphylococcus aureus, one of the most frequent causative agents of sepsis, is a secretory sphingomyelinase [19].
The present study has been performed to explore whether erythrocyte phosphatidylserine exposure participates in the pathophysiology of sepsis.
Materials and methods
Patients and plasma
Heparinized plasma was isolated from healthy volunteers or from patients suffering from severe sepsis who have been treated in the intensive care unit of the University Hospital of Tübingen. Table 1 lists the clinical data of the patients included in this study. The heparinized plasma was added to erythrocytes with identical blood group from healthy volunteers in vitro. The relatives of the patients and the volunteers providing erythrocytes gave informed consent. The study has been approved by the ethics committee of the University of Tübingen.
Solutions and chemicals
Experiments were performed at 37°C either in a CO2 free (Ringer) or in a 5% CO2 atmosphere (plasma) with blood group matched isolated erythrocytes drawn from healthy volunteers. Ringer solution contained (in millimolar) 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), 5 glucose, 1 CaCl2; pH 7.4. Ionomycin was used at a concentration of 1 μM; d-erythro-N-hexanoylsphingosine and ionomycin were purchased from Sigma (Taufkirchen, Germany), the Ca2+ dye Fluo-3/AM from Calbiochem (Bad Soden, Germany).
Isolation of pathogen supernatant
Bacteria were grown in TSB (Difco Laboratories; Sparks, MI, USA) medium; pH 7.2, with 180 rpm shaking at 37°C. Media were supplemented with glucose to a final concentration of 0.5% (w/v). All ingredients were mixed before autoclaving, and the medium pH did not change after autoclaving. Late-exponential-phase cultures were used to inoculate 50 ml of the same medium. The initial optical density (OD578) was adjusted to 0.1. After a growth period of 14–16 h, cells were harvested by centrifugation at 10,000 rpm for 30 min at 4°C. The supernatants were sterilized by passing through a 0.22 μm filter (Millipore; Schwalbach, Germany), preserved at 4°C and then used for further studies.
Measurements of intracellular Ca2+
For intracellular Ca2+ measurements, erythrocytes were loaded with Fluo-3/AM (Calbiochem; Bad Soden, Germany) by addition of 2 μl of a Fluo-3/AM stock solution (2.0 mM in DMSO) to 1 ml erythrocyte suspension (0.3% hematocrit in Ringer). Cells were incubated at 37°C for 15 min under protection from light. Subsequently, an additional 2 μl aliquot of Fluo-3/AM was added, and cells incubated for further 25 min. Fluo-3-AM-loaded erythrocytes were centrifuged at 1,000×g for 5 min at 22°C and washed two times with Ringer solution containing 1% fetal calf serum (Sigma) and one time with Ringer. Fluo-3/AM-loaded erythrocytes were resuspended in 0.5 ml plasma from patients with sepsis or from healthy volunteers (0.3% hematocrit) and incubated for different time periods at 37°C. Then, Ca2+-dependent fluorescence intensity was measured by fluorescence-activated cell sorter (FACS) analysis on a FACS-Calibur from Becton Dickinson (Heidelberg, Germany) in the fluorescence channel FL-1 with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. As a positive control, labeled erythrocytes were incubated for 5 min in Ringer solution containing 1 μM of ionomycin.
Determination of ceramide formation
For determination of ceramide, cells were stained for 1 h at 4°C with anti-ceramide antibody or isotype matched pure mouse antibody in phosphate buffered saline (PBS) containing 1% fetal calf serum (FCS) at a dilution of 1:5 as described recently [8]. After three washes with PBS/1% FCS, cells were stained with polyclonal fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Ig specific antibody (Pharmingen, Hamburg, Germany) in PBS/1% FCS at a dilution of 1:50 for 30 min. Unbound secondary antibody was removed by washing the cells two times with PBS/1% FCS and samples were analyzed by flow cytometric analysis on a FACS-Calibur. FITC-fluorescence intensity was measured in FL-1. As a positive control, erythrocytes were treated for 5 min with 1 U/ml Streptomyces sp. sphingomyelinase from Sigma.
Sphingomyelinase assay
Sphingomyelinase (SMase) content in bacterial supernatants was measured using the Sphingomyelinase [3H] SPA (scintillation proximity assay) enzyme assay (code TRKQ7140) from Amersham Bioscience (Freiburg, Germany) according to the manufacturer’s protocol. Briefly, 10 μl bacterial supernatant, 10 μl of 10-fold enzyme buffer containing 1 M Tris–HCl (pH 7.4), 50 mM MgCl2 and 0.05% NaN3, 70 μl distilled water, and 10 μl tracer solution (biotinylated [3H]-labeled sphingomyelin) were mixed. After addition of the radioactive tracer, the samples were incubated at 37°C with moderate shaking for 30–60 min. Then, the reaction was stopped by adding 20 μl of stop reagent containing 2 M Glycin–HCl (pH 3.6), 0.05 M ethylenediamine tetraacetic acid (EDTA), 0.2% Triton X-100, and 0.05% (w/v) NaN3 and streptavidin-coated Ytrium silicate SPA beads. Finally, the samples were counted in a ß-scintillation counter (Wallac; Freiburg, Germany). The activity of SMase in the samples was calculated from a SMase calibration curve (0.3–80 mU/tube) which was run in parallel (Fig. 5c).
Annexin V binding in FACS analysis
FACS analysis was performed essentially as described [5]. After incubation of erythrocytes (0.3% hematocrit) with plasma from sepsis patients or with plasma from healthy individuals, the cells were washed in annexin V-binding buffer containing (in milimolar) 125 NaCl, 10 HEPES; pH 7.4 and 5 CaCl2. Erythrocytes were stained with Annexin V-Fluos (Roche, Mannheim, Germany) at a 1:50 dilution. After 10 min, samples were diluted 1:5 and measured by flow cytometric analysis on a FACS-Calibur. Cells were analyzed by forward scatter and Annexin V-fluorescence intensity was measured in FL-1.
Statistics
Data are expressed as arithmetic means±SEM and statistical analysis was performed by paired student’s t test or analysis of variance (ANOVA), as appropriate.
Results
Eleven patients with sepsis were included in the study (seven male, four female, 55.6 ± 4.1 years, see Table 1). As shown in Table 2, the patients suffered from severe anemia, which may result from decreased formation of new erythrocytes or from accelerated clearance of damaged cells from circulating blood.
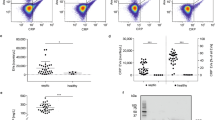
As shown in Fig. 1, the exposure of erythrocytes from healthy volunteers to heparinized plasma of septic patients for 24 h led to marked stimulation of phosphatidylserine exposure at the erythrocyte surface (Fig. 1a,b; unpaired t test P < 0.05). On the other hand, exposure of erythrocytes from either healthy individuals or septic patients to Ringer solution did not significantly increase the percentage of annexin V binding erythrocytes (Fig. 1c).
Stimulation of phosphatidylserine exposure at the erythrocyte surface by plasma of septic patients a Histograms of annexin V binding in a representative experiment of erythrocytes from a healthy volunteer incubated for 24 h in plasma from a septic patient (right panel) or from a healthy volunteer (left panel). b Arithmetic means±SEM (n = 11) of annexin V binding at erythrocytes incubated for 24 h in plasma from septic patients (right, black column) or from healthy volunteers (left white column). The asterisk indicates significant difference between the plasma from septic patients and plasma from healthy volunteers (unpaired t test P < 0.05). c Arithmetic means±SEM (n = 4) of annexin V binding of erythrocytes from septic patients (right, black column) or from healthy volunteers (left white column) both incubated for 24 h in Ringer
As shown in Fig. 2, exposure of erythrocytes from healthy volunteers to heparinized plasma of septic patients for 24 h further led to a marked decrease of the forward scatter (Fig. 2a,b; unpaired t test P < 0.05). Similar to what was demonstrated for annexin V binding, exposure of erythrocytes from either healthy individuals or septic patients to Ringer solution did not significantly modify the forward scatter (Fig. 2c,d).
Decrease of erythrocyte forward scatter after exposure to plasma from septic patients. a Histograms of forward scatter in a representative experiment of erythrocytes from a healthy volunteer incubated for 24 h in plasma from a septic patient (right panel) or from a healthy volunteer (left panel). Numbers depict the respective geo mean values. b Arithmetic means±SEM (n = 10) of forward scatter of erythrocytes incubated for 5 h in plasma from septic patients (right, black column) or from healthy volunteers (left white column). The asterisk indicates significant difference between the plasma from septic patients and plasma from healthy volunteers (unpaired t test P<0.05). c Histograms of forward scatter in a representative experiment of erythrocytes from a healthy volunteer (left panel) or a septic patient (right panel) each incubated for 24 h in Ringer. d Arithmetic means±SEM (n = 6) of forward scatter of erythrocytes from septic patients (right, black column) or from healthy volunteers (left white column) both incubated for 24 h in Ringer
In search for a possible stimulator of phosphatidylserine exposure, we tested whether plasma of septic patients enhances the intraerythrocytic Ca2+ activity. Fluo3-fluorescence revealed that exposure to the plasma of septic patients led to a slight but significant increase of the mean fluorescence of the erythrocytes pointing to increased cytosolic Ca2+ activity (Fig. 3a,b; ANOVA using Dunnett’s test as post hoc test P < 0.05). As a positive control, the Ca2+ ionophore ionomycin (1 μM) led to a marked increase of the cytosolic Ca2+ concentration in virtually all erythrocytes (Fig. 3b).
Increase of cytosolic Ca2+ activity in erythrocytes exposed to plasma from septic patients. a Representative original histograms of Ca2+-dependent Fluo3 fluorescence from erythrocytes incubated for 360 min in plasma from a septic patient (right panel) or from a healthy volunteer (left panel). b Arithmetic means±SEM (n = 10) of Fluo3 fluorescence in erythrocytes incubated for 360 min in plasma from septic patients (black column) or from healthy volunteers (white column), and of erythrocytes exposed to the Ca2+ ionophore ionomycin (1 μM, gray column) in Ringer. The asterisk indicates significant difference between the value to erythrocytes exposed to plasma of healthy individuals (ANOVA using Dunnett’s test as post hoc test. P < 0.05)
We further tested whether plasma of septic patients stimulates ceramide formation. According to binding of a FITC-labeled anti-ceramide antibody, challenge of erythrocytes with plasma from septic patients significantly stimulated the formation of ceramide (Fig. 4a, left histogram, and Fig. 4b; ANOVA using Dunnett’s test as post hoc test P < 0.05). As a positive control, purified sphingomyelinase (1 U/ml) was added to the erythrocyte suspension. As expected, treatment of the cells with sphingomyelinase for 5 min led to an increase of the mean cellular fluorescence, i.e., to an increase of the cellular ceramide levels (Fig. 4a, right histogram, and Fig. 4b).
Stimulation of ceramide formation in erythrocytes exposed to plasma from septic patients. a Left Histogram overlay of ceramide presenting erythrocytes in a representative experiment of erythrocytes incubated for 24 h in plasma of a septic patient (dotted line, left panel) or in plasma from a healthy volunteer (solid line). Right Histogram overlay of ceramide presenting erythrocytes in a representative experiment of erythrocytes incubated for 24 h in Ringer without (solid line) and with 0.1 mU bacterial sphingomyelinase (dotted line right panel). b Arithmetic means±SEM (n = 4) of ceramide-dependent fluorescence in erythrocytes incubated for 24 h in plasma from septic patients (black, middle column), plasma from healthy volunteers (white, left column), or with 1 U/ml bacterial sphingomyelinase (gray, right column). The asterisk indicates significant difference of the plasma from healthy volunteers (ANOVA using Dunnett’s test as post hoc test. P < 0.05)
Further experiments were performed to explore whether sphingomyelinase activity released from pathogens triggers phosphatidylserine exposure of erythrocytes. To this end, erythrocytes from healthy volunteers were exposed to culture medium (as a negative control) or to supernatant from sphingomyelinase-producing S. aureus. In additional control experiments, erythrocytes were exposed to supernatants from an isogenic mutant strain whose sphingomyelinase gene (hlb) is disrupted because of the integration of a prophage in the hlb gene [20]. As illustrated in subpanels a and b of Fig. 5, incubation of erythrocytes for 60 min with the supernatant of sphingomyelinase-producing S. aureus 8325-4 (for sphingomyelinase determination see Fig. 5c,d) resulted in a marked increase of annexin V-positive erythrocytes (ANOVA using Dunnett’s test as post hoc test; P < 0.05), while incubation in bacterial growth medium or in supernatant from mutated S. aureus 8325-4 lacking functional sphingomyelinase was without any appreciable effect. The analysis of different sepsis-inducing pathogens disclosed a positive correlation between the released sphingomyelinase activity and the potency of the supernatant to trigger eryptosis (Fig. 5e; R 2 = 0.891).
Stimulation of phosphatidylserine exposure at the erythrocyte surface by supernatant from sphingomyelinase producing staphylococcus aureus. a Histograms of annexin V binding in a representative experiment of erythrocytes incubated for 60 min in bacterial growth medium without pathogen (left panel) or in supernatant (middle and right panels) from wild type S. aureus 8325-4 (middle panel) or from mutated S. aureus 8325-4 lacking functional sphingomyelinase (right panel). b Arithmetic means±SEM (n = 3) of the percentage annexin V binding erythrocytes after incubation for 60 min in pathogen free medium (M) or in supernatant from wild-type sphingomyelinase expressing S. aureus 8325-4 (Wt) or from mutated S. aureus 8325-4 lacking functional sphingomyelinase (Mt). The asterisk indicates significant difference to the annexin V binding of cells incubated in pathogen free medium (ANOVA using Dunnett’s test as post hoc test. P < 0.05). c Standard curve of the radioactive measurement of SMase activity. d Arithmetic means±SEM (n = 3) of sphingomyelinase activity in the supernatant from wild-type sphingomyelinase expressing S. aureus 8325-4 (Wt) or from mutated S. aureus 8325-4 (Mt). The asterisk indicates significant difference to the sphingomyelinase activity of mutated S. aureus (unpaired t test; P ≤ 0.05). e Correlation between sphingomyelinase activity in bacterial supernatants of 20 different bacterial strains and the percentage of annexin V binding erythrocytes after treatment with those supernatants (R 2 = 0.891)
In another set of experiments, we investigated whether eryptosis occurred before hemolysis. In contrast to annexin V binding, which already reached more than 80% of the erythrocyte population after 60 min, no hemolysis was observed after exposure of the cells to sphingomyelinase-containing, bacterial supernatant during the first 60 min of incubation (Fig. 6). Within 24 h, however, almost complete hemolysis was observed.
Dissociation of annexin V binding and hemolysis after exposure to bacterial supernatant. Time course of phosphatidylserine exposure and hemolysis after incubation of erythrocytes with supernatant from S. aureus 8325-4 (Wt). Arithmetic means±SEM (n = 3) of the percentage of annexin V binding erythrocytes (●) and hemolysis (△), respectively
Discussion
The present experiments demonstrate that plasma from septic patients triggers phosphatidylserine exposure of erythrocytes in vitro leading to suicidal erythrocyte death (i.e., eryptosis).
The phosphatidylserine exposure is partially due to an increase of the cytosolic Ca2+ activity. Besides its effect on phosphatidylserine exposure, the increase of cytosolic Ca2+ could activate Ca2+-sensitive K+ channels [21], which together with Cl−channels allow the exit of KCl and thus lead to cell shrinkage [6]. Further effects of enhanced cytosolic Ca2+ concentrations include the activation of the protease calpain leading to degradation of membrane proteins and cell membrane blebbing [22, 23].
The effect of plasma from septic patients on cytosolic Ca2+ activity is, however, modest and probably does not fully account for the strong stimulation of phosphatidylserine exposure. Rather, the stimulation of ceramide formation by sphingomyelinase-mediated sphingomyelin breakdown may be more important. Activation of sphingomyelinase has previously been observed in septic patients [24] and after exposure of cells to S. aureus [25] or Escherichia coli [26]. Thus, sphingomyelinase-induced accumulation of intraerythrocytic ceramide most likely contributes to the stimulation of phosphatidylserine exposure [8]. The present study does not allow any conclusions as to the origin of the sphingomyelinase. It is noteworthy, however, that the supernatant of pathogens contains sufficient sphingomyelinase activity to trigger marked phosphatidylserine exposure in vitro. The perfect linear correlation between phosphatidylserine exposure and sphingomyelinase activity and the disappearance of phosphatidylserine exposure after loss of function mutation of the bacterial sphingomyelinase clearly demonstrates that the activity in the supernatant is largely due to sphingomyelinase. This observation does not rule out, however, that the pathogens express additional factors triggering phosphatidylserine exposure, which are not released into the supernatant. The observations, further, do not address the possibility that a sphingomyelinase from other origin, such as leukocytes, is released into the plasma of septic patients and contributes to the stimulation of phosphatidylserine exposure. Whatever the origin, sphingomyelinases are critically involved in the pathophysiology of sepsis, a conclusion consistent with most recent findings [24] demonstrating that inhibitors of the acid sphingomyelinase protect animals from liver cell apoptosis and death of the animal upon induction of sepsis by intraperitoneal injection of endotoxin.
The exposure of phosphatidylserine at the cell surface favors the binding to respective phosphatidylserine receptors expressed by macrophages [10]. Binding to those receptors triggers engulfment and subsequent degradation of the affected erythrocytes [11]. Thus, erythrocytes exposing phosphatidylserine at their surface will be cleared from circulating blood. Moreover, the erythrocytes may bind to receptors in the vascular wall and thus impede microcirculation [9]. Along those lines, we observed enhanced trapping of annexin V-binding erythrocytes in renal medulla after ischemia of the mouse kidney [27].
Pathogen-induced hemolysis may similarly contribute to accelerated erythrocyte clearance and anemia in septic patients, especially after infection with clostridium perfringens [3, 4]. Hemoglobin released from hemolytic erythrocytes is bound to the glycoprotein haptoglobin, which prevents glomerular filtration and intratubular precipitation of circulating free hemoglobin, but may be overridden during excessive hemolysis [28, 29]. As shown in this study, S. aureus-induced eryptosis preceeds hemolysis, and rapid clearance of phosphatidylserine-exposing erythrocytes [12, 30] serves to remove defective erythrocytes before hemolysis. In vitro eryptotic erythrocytes are not engulfed by macrophages and thus eventually undergo delayed hemolysis. It should be kept in mind, though, that the concentrations of sphingomyelinase in bacterial supernatants are most likely higher than those in septic patients. Moreover, protective mechanisms such as erythropoietin [31] may counteract erythrocyte death in vivo. Thus, the time course of eryptosis and subsequent hemolysis in vitro presumably does not reflect the time course of erythrocyte death in septic patients.
The present observations may not only be relevant for anemia but as well for the stimulation of thrombocytes and the pathophysiology of organ damage. Ceramide [32–34] and Ca2+ [35] have similarly been implicated in the triggering of apoptosis of nucleated cells and the pathogenic plasma components may similarly trigger apoptosis of endothelial, renal, and hepatic cells thus contributing to the pleiotropic clinical features of sepsis. For instance, lymphocyte apoptosis participates in the pathophysiology of sepsis in mice [36]. Our studies therefore shed new light on the role of bacterial sphingomyelinase toxins whose role in virulence had remained elusive. Enzymes such as the S. aureus β–toxin might well contribute to the severity of sepsis by promoting erythrocyte death and anemia.
In conclusion, the present observations provide evidence for the stimulation of erythrocyte phosphatidylserine exposure by the plasma of septic patients. It demonstrates that the effect of the plasma is at least partially due to stimulation of Ca2+ entry and ceramide formation. The study thus reveals a novel mechanism which presumably contributes to the pleiotropic pathophysiology of this severe, life-threatening disease.
References
Aird WC (2003) The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc 78:869–881
Sessler CN, Perry JC, Varney KL (2004) Management of severe sepsis and septic shock. Curr Opin Crit Care 10:354–363
McArthur HL, Dalal BI, Kollmannsberger C (2006) Intravascular hemolysis as a complication of clostridium perfringens sepsis. J Clin Oncol 24:2387–2388
Vaiopoulos G, Calpadaki C, Sinifakoulis H, Konstantopoulos K, Avlami A, Stefanou J, Pangalis GA (2004) Massive intravascular hemolysis: a fatal complication of clostridium perfringens septicemia in a patient with acute myeloid leukemia. Leuk Lymphoma 45:2157–2159
Lang KS, Duranton C, Poehlmann H, Myssina S, Bauer C, Lang F, Wieder T, Huber SM (2003) Cation channels trigger apoptotic death of erythorcytes. Cell Death Differ 10:249–256
Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM (2003) Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol 285:C1553–C1560
Woon LA, Holland JW, Kable EP, Roufogalis BD (1999) Ca2+ sensitivity of phospholipid scrambling in human red cell ghosts. Cell Calcium 25:313–320
Lang KS, Myssina S, Brand V, Sandu C, Lang PA, Berchtold S, Huber SM, Lang F, Wieder T (2004) Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ 11:231–243
Closse C, Dachary-Prigent J, Boisseau MR (1999) Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br J Haematol 107:300–302
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85–90
Boas FE, Forman L, Beutler E (1998) Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA 95:3077–3081
Lang KS, Lang PA, Bauer C, Duranton C, Wieder T, Huber SM, Lang F (2005) Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem 15:195–202
Barvitenko NN, Adragna NC, Weber RE (2005) Erythrocyte signal transduction pathways, their oxygenation dependence and functional significance. Cell Physiol Biochem 15:1–18
Bosman GJ, Willekens FL, Werre JM (2005) Erythrocyte aging: a more than superficial resemblance to apoptosis? Cell Physiol Biochem 16:1–8
Rice L, Alfrey CP (2005) The negative regulation of red cell mass by neocytolysis: physiologic and pathophysiologic manifestations. Cell Physiol Biochem 15:245–250
Schwarzer E, Kühn H, Valente E, Arese P (2005) Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell Physiol Biochem 16:133–146
Lang PA, Beringer O, Nicolay JP, Amon O, Kempe DS, Hermle T, Attanasio P, Akel A, Schafer R, Friedrich B, Risler T, Baur M, Olbricht CJ, Zimmerhackl LB, Zipfel PF, Wieder T, Lang F (2006) Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J Mol Med 84:378–388
Lang PA, Kaiser S, Myssina S, Birka C, Weinstock C, Northoff H, Wieder T, Lang F, Huber SM (2004) Effect of Vibrio parahaemolyticus haemolysin on human erythrocytes. Cell Microbiol 6:391–400
Bohach GA, Dinges MM, Mitchell DT, Ohlendorf DH, Schlievert PM (1997) Exotoxins. In: Crossley KB, Archer GL (eds) The staphylococci in human disease. Churchill Livingstone, New York, pp 83–111
Goerke C, Koller J, Wolz C (2006) Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177
Brugnara C, de Franceschi L, Alper SL (1993) Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest 92:520–526
Anderson DR, Davis JL, Carraway KL (1977) Calcium-promoted changes of the human erythrocyte membrane. Involvement of spectrin, transglutaminase, and a membrane-bound protease. J Biol Chem 252:6617–6623
Lang PA, Kempe DS, Myssina S, Tanneur V, Birka C, Laufer S, Lang F, Wieder T, Huber SM (2005) PGE(2) in the regulation of programmed erythrocyte death. Cell Death Differ 12:415–428
Claus RA, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinscherf R, Deigner HP (2005) Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J 19:1719–1721
Esen M, Schreiner B, Jendrossek V, Lang F, Fassbender K, Grassme H, Gulbins E (2001) Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis 6:431–439
Falcone S, Perrotta C, De Palma C, Pisconti A, Sciorati C, Capobianco A, Rovere-Querini P, Manfredi AA, Clementi E (2004) Activation of acid sphingomyelinase and its inhibition by the nitric oxide/cyclic guanosine 3′,5′-monophosphate pathway: key events in Escherichia coli-elicited apoptosis of dendritic cells. J Immunol 173:4452–4463
Lang KS, Myssina S, Lang PA, Tanneur V, Kempe DS, Mack AF, Huber SM, Wieder T, Lang F, Duranton C (2004) Inhibition of erythrocyte phosphatidylserine exposure by urea and Cl−. Am J Physiol Renal Physiol 286:F1046–F1053
Fowkes FJ, Imrie H, Migot-Nabias F, Michon P, Justice A, Deloron P, Luty AJ, Day KP (2006) Association of haptoglobin levels with age, parasite density, and haptoglobin genotype in a malaria-endemic area of Gabon. Am J Trop Med Hyg 74:26–30
Rogerson S (2006) What is the relationship between haptoglobin, malaria, and anaemia? PLoS Med 3:e200
Kempe DS, Lang PA, Duranton C, Akel A, Lang KS, Huber SM, Wieder T, Lang F (2006) Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J 20:368–370
Myssina S, Huber SM, Birka C, Lang PA, Lang KS, Wieder T, Lang F (2003) Inhibition of erythrocyte cation channels by erythropoietin. J Am Soc Nephrol 14:2750–2757
Kolesnick R, Golde DW (1994) The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell 77:325–328
Kolesnick RN, Kronke M (1998) Regulation of ceramide production and apoptosis. Annu Rev Physiol 60:643–665
Obeid LM, Linardic CM, Karolak LA, Hannun YA (1993) Programmed cell death induced by ceramide. Science 259:1769–1771
Perretti M, Solito E (2004) Annexin 1 and neutrophil apoptosis. Biochem Soc Trans 32:507–510
Oberholzer C, Tschoeke SK, Moldawer LL, Oberholzer A (2006) Local thymic caspase-9 inhibition improves survival during polymicrobial sepsis in mice. J Mol Med 84:389–395
Acknowledgements
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic. This study was supported by the Deutsche Forschungsgemeinschaft, Nr. La 315/6-1 and La 315/13-1, by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Center for Interdisciplinary Clinical Research: 01 KS 9602), the Promotionskolleg Molecular Medicine # 1547, and the Dr. Karl Kuhn-Stiftung. Daniela S. Kempe and Ahmad Akel equally contributed to the study and thus share first authorship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kempe, D.S., Akel, A., Lang, P.A. et al. Suicidal erythrocyte death in sepsis. J Mol Med 85, 273–281 (2007). https://doi.org/10.1007/s00109-006-0123-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0123-8