Abstract
Endometrial carcinomas (EnCa) predominantly represent a steroid hormone-driven tumor initiated from prestages. The human endogenous retrovirus HERV-W envelope gene Syncytin-1 was significantly increased at the mRNA and protein levels in EnCa and prestages compared to controls. Steroid hormone treatment of primary EnCa cells and cell lines induced Syncytin-1 due to a new HERV-W estrogen response element and resulted in increased proliferation. Activation of the cAMP-pathway also resulted in Syncytin-1 upregulation, but in contrast to proliferation, classic cell–cell fusions similar to placental syncytiotrophoblasts occurred. Cell–cell fusions were also histologically identified in endometrioid EnCa tumors in vivo. Clonogenic soft agar experiments showed that Syncytin-1 is also involved in anchorage-independent colony growth as well as in colony fusions depending on steroid hormones or cAMP-activation. The posttranscriptional silencing of Syncytin-1 gene expression and a concomitant functional block of induced cell proliferation and cell–cell fusion with siRNAs proved the essential role of Syncytin-1 in these cellular processes. TGF-β1 and TGF-β3 were identified as main regulative factors, due to the finding that steroid hormone inducible TGF-β1 and TGF-β3 inhibited cell–cell fusion, whereas antibody-mediated TGF-β neutralization induced cell–cell fusions. These results showed that induced TGF-β could override Syncytin-1-mediated cell–cell fusions. Interactions between Syncytin-1 and TGF-β may contribute to the etiology of EnCa progression and also help to clarify the regulation of cell–cell fusions occurring in development and in other syncytial cell tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
EnCa is the most common invasive gynecologic cancer in Western industrialized countries, mainly occurring in postmenopausal women. In the USA, the EnCa incidence has been rising over the last 10 years from 41% in 1994 to currently 50%, making EnCa the eighth most common cause of cancer deaths [1, 2]. Over 85% of all EnCa cases are histologically classified as endometrioid (type I), mainly express the estrogen receptor (ER) and progesterone receptor (PR) and most likely develop from endometrial hyperplasia driven by hormonal changes, like estrogen stimulation [3]. Two percent of complex hyperplasias, but 52% of atypical hyperplasias can progress into a carcinoma [4]. The rare nonendometrioid EnCa (type II) is more aggressive, often lacks ER and PR expression, and may develop from transformed endometrial surface epithelium [1]. Although unclear in the staging of EnCa, benign endometrial polyps can internally progress into hyperplasias (11–24%) but also malignancies (1–3%) [5, 6]. According to the International Federation of Gynecology and Obstetrics, EnCa is staged according to tumor location (Ia is endometrial to IVb with distant metastasis) and graded according to the growth pattern (G1-3). A causal relationship between hormone replacement therapies and EnCa has been previously established [7, 8]. An increase of relative EnCa risk with selective estrogen receptor modulators, like Tamoxifen used for breast cancer prevention and for adjuvant trials was found to be >twofold [9].
Genetic markers noted in EnCa, which show correlations in tumors with and without complex atypical hyperplasia are, e.g., microsatellite instability, DNA nondiploidy, mutations in PTEN, p53, β-catenin 1, K-ras, and amplifications in c-myc and INT2 [1]. PTEN mutations occurred equally in both early and advanced EnCa; however, c-myc amplification was more frequent in advanced tumors, supporting an earlier and later role for PTEN and c-myc, respectively. Loss of heterozygosity of BRCA1, p53, and TCRD genes in EnCa tumors was associated with tumor grade, younger age, and family cancer history, respectively [10].
Cell–cell fusions leading to multinucleated or syncytial cells represent a central and essential event during embryogenesis and morphogenesis. In humans, besides sperm cell–oocyte fusion, other cell–cell fusion events include mononucleated hematopoietic or myoblast precursors fusing to syncytial osteoclasts or myofibrils during bone and muscle differentiation, respectively [11, 12]. During the first 11 days of pregnancy, while the blastocyst implants into maternal endometrium, placental cytotrophoblasts fuse to multinucleated syncytiotrophoblasts forming the maternal–fetal cellular interface [13]. Syncytiotrophoblasts also represent the invasive front of the blastocyst, which have similarities to invasive tumors. The so-called giant syncytial cells have also been identified in cells infected with enveloped viruses and in certain tumors, like EnCa [11, 14]. However, with the exception of enveloped viruses, the molecular mechanisms of cell fusion and the function of these multinucleated cells in tumors are not understood.
Human endogenous retrovirus (HERV) families represent remnants of past retroviral infections of germline cells of human ancestors and embody 7.7% of the human genome [15]. HERVs have been classified according to their gag, pol, and envelope gene homology with exogenous retroviruses, like, e.g., HIV and HTLV [15, 16]. So far, over 200 HERV families are described in the human genome, with sequences on different chromosomes, where most elements produce no functional proteins due to deletions and mutations [15, 17]. A functional HERV-W envelope gene, also called Syncytin-1 locates along with inactive gag and pol genes and intact flanking LTRs at 7q21-22 (Fig. 1). It is interesting to note that Syncytin-1 has evolved as being essential for human placental morphogenesis, where Syncytin-1 mediates cell–cell fusions of cytotrophoblasts into syncytiotrophoblasts [17, 18]. Syncytin-1 is regulated in cytotrophoblasts by the transcription factor “glial cells missing homolog” (GCMa) and by CpG-methylation [18–20], and is expressed as an inactive protein precursor, processed through cellular subtilisin-like proteases into a predicted extracellular surface domain (SU) and a transmembrane domain (TM) (Fig. 1) [21, 22]. Results from exogenous retroviral envelope proteins showed that the TM and SU remained associated after cleavage, where the SU recognized the host cell receptor and the TM promoted virus-cell fusion [23]. Proposed membrane receptors for Syncytin-1 are the Na+-dependent neutral amino acid transporters ASCT1 and ASCT2 [24]. ASCT2 is described as a general retrovirus-receptor for simian immunosuppressive type D retroviruses [25].
Genomic analysis, transcriptional, and protein expression of Syncytin-1 at 7q21–q22 in benign endometrial tissues and EnCa. a HERV-W gene showing 10.2 kb of the LTRs, gag, pol, and env (Syncytin-1) and three inserts. Forward and reverse oligos (1–17) used for RT-PCR and sequencing. Syncytin-1 was sequenced in EnCa #497, 562, 731, a pre- (#1076) and a postmenopausal endometrium (#366). b HERV-W predicted protein structure from amino acid (AA) position 1–538 including the signal peptide, surface unit (SU), CKS immunosuppressive domain, the transmembrane domain (TM) with the cytoplasmic peptide (cyto). The CWIC and the CX6CC sites are predicted to form disulfide bonds between SU and TM. The K/R-X-K/R-R (RnkR) consensus site is presumably recognized by subtilisin/Kex2p-like endoproteases. Asterisks (*) represent predicted N-glycosylation sites (21). A polyclonal antibody against the SU-domain (AA: 21–215) was used in this study (18). Cellular localization of the domains is shown below. c Results of qPCR absolute Syncytin-1 copy numbers per nanogram total RNA for premenopausal control endometrium, matched myometrium and placenta in box plots. Inset shows the standard curve of the threshold cycle (C T) of serially diluted cloned Syncytin-1 against the log starting quantity of mRNA copy numbers (log C0). Below are RT-PCRs of Syncytin-1 (748 bp) and β-actin (382 bp) of premenopausal control endometrium (E), matched myometrium (M), and placenta (Pl). Syncytin-1 fold-increase is indicated below. M DNA marker. d Results of qPCR Syncytin-1 mRNA copies per nanogram total RNA for postmenopausal endometrium (PoEn), endometrial polyps (EnPo), hyperproliferative endometrium (hyEn), endometrial hyperplasia (EnHy), and EnCa in box plots. Below are Syncytin-1 (748 bp) and β-actin (382 bp) RT-PCRs of matched (underlined) control endometrium (E) with EnCa (EC), and placenta (Pl). Patient numbers are indicated above. Fold Syncytin-1 increase compared to matched controls is indicated. M DNA marker. e Syncytin-1 protein expression using the SU-domain specific antibody in lysates from a term placenta (Pl), matched controls (E), EnCa (EC), hyperproliferative endometrium (hyEn), endometrial hyperplasias (EnHy), a vaginal control (co), and a vaginal metastasis (met) biopsy with a previous EnCa. Syncytin-1 protein species (kD) are indicated. β-actin protein from the same immunoblot after stripping and core histones (Hs) stained with colloidal Coomassie after protein transfer are shown. f DNA from placenta (Pl), matched postmenopausal controls (C), and EnCa (EC), hyperproliferative endometrium (hyEn), endometrial hyperplasia (EnHy), and polyp (EnPo) were restricted with Bgl II, Southern-blotted, and then hybridized with the 748 bp Syncytin-1 cDNA probe. Number below the lanes represents the band intensity to placenta set as 1.0
Transforming growth factor-beta (TGF-β) proteins regulate proliferation, differentiation, and can induce transformation of various cell types. TGF-β has a dual effect in carcinogenesis as a tumor suppressor or activator depending on the cell type [26]. High levels of TGF-β can be a marker for poor prognosis in cancer of, e.g., breast, colon, brain, and prostate [27]. The TGF-β signaling pathway appears disabled in both endometrial hyperplasias and EnCa showing a loss of growth inhibition, e.g., the TGF-β antagonist Smad7 was found significantly upregulated in EnCa [28, 29]. In addition, steroid hormones were found to regulate TGF-β3 gene expression in ER positive cells [30]. Specifically for endometrial stromal cells, 17β-estradiol (E2) induced TGF-β1 and β3 transcription, but not TGF-β2 [31].
This report analyzed the gene expression of Syncytin-1 in endometrioid EnCa, control endometrium and other benign endometrial tissues. The role of Syncytin-1 and TGF-β in steroid hormone stimulated cell proliferation and in cAMP-induced cell–cell fusions unraveled pivotal control points in EnCa.
Materials and methods
Patient tissues
Handling of patients and tissues was approved by the Ethics Committee at the University of Erlangen-Nuremberg. All patients gave a written informed consent. Tissues were collected between 2003 and 2004, classified by the Institute of Pathology (Erlangen) and flash frozen in liquid nitrogen. EnCa tumors of endometrioid histology were collected from 24 patients (age 51–88; mean 66.3) and grouped according to stages (Ib–IVb) and growth patterns (G1-3): stage Ib/G1 (n = 5), Ib/G2 (n = 3), Ib/G3 (n = 2), Ic/G1 (n = 2), Ic/G3 (n = 1), IIb/G1 (n = 1), IIb/G2 (n = 7), IIIa/G2 (n = 1), IIIc/G2 (n = 1), IVb/G2 (n = 1). In addition, a colorectal carcinoma from an EnCa Ic diagnosed in 1997 (#771), a vaginal tumor from an EnCa Ic diagnosed in 2002 (#1972) and an endometrioid tumor at the abdominal wall from an EnCa diagnosed in 2000 (#2104) were included. Twelve patient-matched and 12 age-matched carcinoma-free endometrial tissues were used as controls (age 51–81; mean 65.8). Sixteen endometrial polyps were from postmenopausal patients (age 53–86; mean 61), including two patients, who also had endometrioid EnCa Ic/G3 and Ib/G1 and seven patients associated with proliferative endometrium (>8 mm). Hyperproliferative endometrium (14–23 mm) (n = 11) were from postmenopausal patients (age 47–74; mean 58.3). Endometrial hyperplasias from seven postmenopausal patients (age 47–69; mean 53.3) were classified as simple (n = 5) and complex hyperplasia (n = 2), with (n = 2) and without atypia (n = 5). Endometrium and myometrium were from 23 premenopausal patients between 28–50 years (mean 39) including 13 in proliferative and 10 in secretory phases of the menstrual cycle. Full-term placenta tissue samples (n = 6) were from healthy women between 28–32 years.
RNA isolation and RT-PCR
Frozen tissues (50–100 mg) were minced using a Mikro-Dismembranator (Braun Biotech) and solubilized in Trizol® (Invitrogen). Approx. 30 μg RNA was pretreated with 5 U DNAase I (Sigma-Aldrich) for Syncytin-1 PCRs. Syncytin-1 [high performance liquid chromatography (HPLC)-purified primer 1-17] and the β-actin gene (primer βAc-TF and βAc-BR) (Supplementary Table 1) expression in tissues and cell cultures were simultaneously assessed using the SuperScript One-Step RT-PCR kit (Invitrogen) with 300 ng RNA for 30 min at 45°C, 30 cycles for 15 s at 94°C, 1 min at 55°C, 2 min at 68°C, and 7 min at 72°C. PCR products were purified and quantified using the Kodak 1 D Analysis Software and Image Quant Analysis against β-actin.
DNA cloning and sequencing
RT-PCR products from control pre- and postmenopausal endometrium and EnCa tissues were cloned using the pCR2.1-TOPO vector (Invitrogen), sequenced using the DYEnamic Terminator Kit (Amersham Biosciences) and analyzed (ABI377). DNA analysis of the Syncytin-1 LTRs was performed using the HERV-W DNA sequence HSAC000064 from the 56,093 bp human Bac-clone RG083m05 on chromosome 7q21–q22 with Macvector 7.1.1 software (accelrys®).
Semi- and absolute quantitative real-time PCR
cDNA was generated with the high capacity cDNA Kit (Applied Biosystems). ERα (exon 3–4), ERβ (exon 4–5), the two isoforms PR A/B (exon 4–5), Ki-67 (exon 11–12), ASCT1 (exon7–8), ASCT2 (exon 1–2), TGF-β1 (exon 1–2), and TGF-β3 (exon 1–2) gene expression were analyzed using TaqMan-Assays (Applied Biosystems). Coamplification of 18s-rRNA and one patient cDNA were used as internal controls for a standard curve in semiquantitation analysis. Gene expression for GCMa was analyzed according to Hashemolhosseini and Wegner [19]. For quantitative real-time PCR (qPCR) of Syncytin-1, a QuantiTect Multiplex Assay (Qiagen) was designed: primer 1 (CCCTATGACCATCTACAC) and 2 (GCACTCCTGCTCCTATAACAAA) with FAM-labeled probe (ATCTAAGCCCCGCAAC) including a 18s-rRNA control. As an external standard, cloned Syncytin-1 DNA with known copy numbers was used to generate a standard curve with the C T value against the log of amount of standard. The C T of Syncytin-1 was calculated according to the standard curve.
Northern and Southern analysis
Thirty μg DNAase I-treated RNA was electrophoresed on 1% formaldehyde agarose gels and for Southern 10 μg DNA was analyzed on TBE-agarose gels. Gels were electroblotted onto GeneScreen Plus (NEN) and hybridized. The cloned Syncytin-1 cDNA 748 bp [32P]dCTP labeled fragment was used as a probe (Fig. 1).
Immunoblot analysis
Proteins were isolated from tissues using a microdismembranator and resuspended 10-fold of wet weight in: 0.125 M Tris–HCl pH 6.8, 2% sodium dodecyl sulfate (SDS), 5% glycerol, 2 mM EDTA, 0.1% TritonX100, 1 mM Na3VO4, 1.5 mM NaF, and PI-cocktail I: 1 mM PMSF, 10 μg/ml of each Pepstatin, Leupeptin, and Chymostatin. An amount of 44 μg of each cell lysate were resolved on a 7.5–12.5% acrylamide gradient SDS-gel according to Strick et al. [32], then transferred to a polyvinylidene fluoride membrane using a CAPS-transfer buffer (10 mM CAPS, 10% methanol, 0.01% SDS, pH 10.4) at 0.5 A at 4°C. Immunoblot analyses were done with a Syncytin-1 polyclonal antibody (1:500) (gift of Dr. Chen, Taiwan); β-actin monoclonal antibody (mab) (1:1,000) along with secondary peroxidase labeled antibodies and a blocking reagent (all Sigma-Aldrich). Antibodies were stripped off immunoblots with 2% SDS at 50°C for 20 min and then reprobed.
DNA binding analysis
Purified human ERα (Invitrogen) was incubated with either 32P-labeled Syncytin-1-ERE (5′CTAGCTGGGAAGGTGACCACGTCCACCTTTAAACACG) or control ERE from the Xenopus laevis A2 vitellogenin gene at position −306 to −342 (5′CTGTCCAAAGTCAGGTCACAGTGACCTGATCAAAGTT) and 80 mM NaCl, 10 mM Tris–HCl pH 7.5, 0.5 mM DTT, 0.2 mM EDTA, 5 μM ZnCl2, 5% glycerol and 30 ng/μl poly dI:dC for 15 min at 21°C. DNA protein complexes were fractionated on a 6% nondenaturing acrylamide gel at 150 V and RT, then the gel was dried and autoradiographed.
Cell culture
Primary EnCa tumor cultures and the cell lines RL95-2, Kle and HEC-1-A [human endometrial carcinoma (HEC)] derived from poorly and moderately differentiated EnCa and the BeWo cell line derived from a choriocarcinoma (ATCC) were grown in phenol red free RPMI with 5% charcoal treated serum (CTS) (HyClone). For steroid hormone treatment, cells were seeded at 200,000 cells and 48 h later incubated with 10–50 nM E2, 4-OH-E2, 2-OH-E2, estrone, estriol, or 500 nM progesterone (Sigma-Aldrich) for an additional 72 h in RPMI + 2% CTS and then growth curves determined (Celldyne, Abbott). For isolation and culturing of primary cytotrophoblasts, placentas were minced, incubated with 250 mg trypsin and 120 mg DNAase I per 150 mg of tissue for 45 min at 37°C and cytotrophoblasts isolated using a continuous Percoll gradient centrifuged at 18,000×g for 50 min.
Cell–cell fusion analysis
Cells were seeded at 230,000 and after 72 h, cell–cell fusions were induced with 40 μM Forskolin or the SP isomer of cAMP (SP-cAMP) (Sigma-Aldrich) or induced with 1 μg/ml mabs against TGF-β1 or -β3 (R&D) in the presence of steroid hormones and then incubated for an additional 72 h. Cells were analyzed for DNA ploidy by, (1) microscopy using a May–Gruenwald and Giemsa stain (Sigma-Aldrich), or using the plasma membrane stain Wheat germ agglutinin with Alexa 594 and the nuclear Hoechst 33342 stain (Molecular Probes), or by (2) fluorescence activated cell sorting (FACS). For inhibition of cell–cell fusions, 5 ng/ml purified human recombinant TGF-β1 or TGF-β3 proteins (R&D) or 20 nM E2 were added 1 day before addition of Forskolin or SP-cAMP. Cell supernatants from steroid hormone, Forskolin, SP-cAMP, RP-cAMP, TGF-β1, or β3 or anti-TGF-β1 or TGF-β3-treated cells were assayed for β-hCG with an Immulite2000 (DPC).
siRNA transient transfections and soft agar assays
For the posttranscriptional gene silencing of Syncytin-1 two different siRNAs were designed: (1) 5′TCGCCTGGTAAGCCTATTTAA, Syncytin-1-siRNA-1 in the SU-domain and the (2) 5′TCCGGAATCGTCACTGAGAAA, Syncytin-1-siRNA-2 in the TM-domain (Qiagen). For optimal loss of function effects, the Syncytin-1-siRNA 1 or 2 were titrated and analyzed for Syncytin-1 gene expression and cell toxicity. RL95-2 or BeWo cells were seeded at 200,000 cells in RPMI + 5% CTS and on day 3, 60 nM Syncytin-1-siRNA 1 or 2 were transfected at a 1:9 ratio with lipofectin for 5.5 h. Mock transfections using neg-siRNA-Alexa 488 (Qiagen) demonstrated >80% transfection efficiency. Twenty-four hours posttransfection, cells were trypsinized and set up for treatment with steroid hormones, SP-cAMP, or Forskolin using growth curves or soft agar assays. Nontransfected, mock and Syncytin-1-siRNA transfected cells were plated in triplicate with soft agar in RPMI + 5% CTS (bottom 0.33% agar, top 0.30% agar with cells) at 30,000 cells/well. After 7–12 days, cell colonies were scored and measured in six fields per well using a Leitz Axiovert microscope and photographed.
Statistical analyses
A two-tailed t test and in addition an ANOVA test between the groups were performed using SPSS13.0 (SPSS), with p < 0.05 as statistically significant. For each mean value, a standard error of the mean (SEM) was calculated using SPSS. The box-plot graphs were performed using SPSS13.0.
Results
Syncytin-1 overexpression in EnCa and prestages
Syncytin-1 mRNA was amplified, cloned, and sequenced from three EnCa, one control pre- and one postmenopausal endometrium. Results confirmed sequence identity with no premature stop codons or deletions and insertions to the HERV-W envelope gene (7q21-q22) from placenta (Fig. 1a). Using RT-PCR and comparative qPCR, Syncytin-1 expression was high in placental tissues (138.35 ± 16.37 Syncytin-1 copies/ng total RNA; p < 0.001), but lower in premenopausal endometrium (36.07 ± 8.84; p = 0.004), and even more reduced in myometrium (6.74 ± 2.58) thus demonstrating tissue specificity (Fig. 1c). Syncytin-1 expression was then compared between a collective of control, benign and malignant endometrial tissues, including placentas using qPCR, RT-PCR, Northern, and Western analysis. Results showed that Syncytin-1 was upregulated in all benign and malignant tissues with the highest expression in EnCa above controls including placenta (Fig. 1c,d). Syncytin-1 mRNA copies per nanogram of total RNA were determined for control postmenopausal endometrium with a mean (M) of 15.93 ± 2.25; endometrial polyps: M = 72.41 ± 6.26; hyperproliferative endometrium: M = 53.14 ± 3.57; endometrial hyperplasia: M = 124.5 ± 16.75 and for EnCa the values ranged from 109.1–824.5 with an M = 363.58 ± 47.14. All quantitatively determined Syncytin-1 mRNA values from benign and malignant tissues were statistically significant as compared with postmenopausal controls (p < 0.001). Northern and long-range RT-PCR analysis of control, benign, and malignant tissues also detected three Syncytin-1 transcripts similar to placenta [17]: a full-length 7.0, 3.0, and 1.0 kb, the last corresponding to the cytoplasmic domain (Fig. 2d).
Steroid hormone induction of Syncytin-1 due to a new HERV-W ERE. a Box plots from semiquantitative real-time PCRs of steroid hormone receptors ERα, ERβ, and progesterone receptors A/B (PGR) normalized against 18s-rRNA, and analyzed in matched control postmenopausal endometrium (PoEn), polyp (EnPo), hyperplasias (EnHy), and EnCa. The y-axis represents arbitrary units. b Exponentially growing RL95-2 cells were incubated with 10 nM E2 (closed circle), 500 nM progesterone (open square), 10 nM 4-OH-E2 (closed square), or 40 μM SP-cAMP (triangle) for up to 72 h. Controls are indicated by an open circle. Cell number increase is indicated on the y-axis. c RT-PCRs of Syncytin-1 (748 bp) and β-actin (382 bp) from tissues and cell lines. Primary cells from EnCa (#731 ERα+, PR+, and #1974 ERα+, PR−) and cell lines Kle (ERα−, PRA−), BeWo (ERα+, PR+), and RL95-2 (ERα+, PR+) with a different steroid hormone receptor status as determined by real-time PCR and uterine fibroblastoid cells from an EnCa (#562) were incubated with 10 nM E2 (E2), 500 nM progesterone (P) or 40 μM Forskolin (F) for 72 h and Syncytin-1 was analyzed (see also Supplementary Figs. S1 and S2a). Syncytin-1 expression from syncytiotrophoblasts (T) is shown as control. Note that in addition to Syncytin-1 induction with steroid hormones, Syncytin-1 expression was low in RPMI+2% CTS (−). M DNA marker. d On the left, long-range RT-PCR with HERV-W primers 3 and 17 (Fig. 1a) from total RNA of RL95-2 (1), postmenopausal control #2073 (2), EnCa #2074 (3), endometrial hyperplasia #2524 (4), and BeWo (5) and analyzed on a 1% agarose gel and quantitated against β-Actin. Three RT-PCR products with ∼6.6, ∼2.3, and 0.9 kb were detected and shorter due to the primer used. An RT-PCR product of ∼1.4 kb could not be attributed to the published spliced forms of HERV-W. Northern blot (right) was hybridized with the 748-bp Syncytin-1 cDNA probe. RL95-2 cells incubated for 72 h with 10 nM E2 (lane 2) or 40 μM SP-cAMP (lane 3) resulting in Syncytin-1 upregulation, whereas in RPMI+2% CTS alone, Syncytin-1 levels were low (lane 1). Below is HERV-W provirus from 2,567–13,230 including 2.5 kb 5′ of the 5′LTR and GCMa-binding sites (GBS) (18). The ERE locates at the 5′ and 3′ LTR at 2,626 and 12,614, and ATGs with splice acceptor (small black arrows) and donor (large arrows). Transcripts in placental tissues (17) were also identified with Northern blots and RT-PCRs using different primers (Fig. 1a) from endometrial control, benign, and malignant tissues. e Band shift assay showing DNA binding of increasing ERα concentrations with either HERV-W ERE or the X. laevis vitellogenin ERE. Arrows 1 and 2 demonstrate DNA-binding of ERα-monomers or ERα-dimers to DNA, respectively
Examining Syncytin-1 protein levels using an antibody against the SU domain, Western analysis demonstrated an upregulation of all Syncytin-1 protein species in benign and malignant tissues as well as in EnCa metastatic tumors, in contrast to patient matched control (Fig. 1e). Specific Syncytin-1 proteins corresponding to a full-length glycosylated preprotein of 66 kD (gPr66-env), a protein of 50 kD (gp50SU) harboring the SU domain, and a third protein of 92 kD (gp92SU-TM) consisting presumably of linked SU and TM domains were detected (Fig. 1b,e). Although the fully processed SU and TM units are probably linked over disulfide bonds, the gp92SU-TM was still detected even after a 5-min, 100°C treatment in the presence of 1–10% β-mercaptoethanol, but was not resistant against 100 mM DTT and 30 min at 100°C (data not shown). Although Syncytin-1 was aberrantly overexpressed at the RNA and protein level, Syncytin-1 was not amplified genomically in all tissues tested, supporting transcriptional regulation (Fig. 1f).
In addition, real-time PCR gene expression analyses was performed to address if the putative Syncytin-1 receptors ASCT1, ASCT2, the Syncytin-1 GCMa transcription factor, as well as the proliferation cell marker Ki-67 were also overexpressed in the collective tissues. Only Ki-67 showed a significant induction in EnCa tissues (p = 0.002) indicating increased tumor cell proliferation, whereas all other transcripts were not upregulated significantly (Tables 1 and 2).
Steroid hormone regulation of Syncytin-1
A strong association exists between steroid hormones and EnCa and its clinical prestages [3], therefore a possible link between Syncytin-1 overexpression and steroid hormone regulation was investigated. First, examining the steroid hormone receptor status using real-time PCR, a significant higher ratio of ERα (p = 0.010), but not ERβ or PR A/B was detected in EnCa compared to patient matched controls (Fig. 2a). Endometrial polyps (p = 0.017) and hyperplasia (p = 0.002) showed a significant increase of ERα, but in contrast to EnCa, a significant increase of PR A/B was found for both benign tissues (p < 0.001) as compared to postmenopausal endometrium. Growth curves of RL95-2 and BeWo cells following either 10 nM E2, 2-OH-E2, 4-OH-E2, or 500 nM progesterone treatments determined a two- to threefold stimulation of cellular proliferation above control cells on the third day demonstrating steroid hormone responsiveness (Fig. 2b, Supplementary Fig. S1). Addition of either 10 nM E2, 2-OH-E2, 4-OH-E2, or 500 nM progesterone to ER or PR A/B positive EnCa primary cells, RL95-2, and BeWo, showed an ∼20-fold induction of Syncytin-1 RNA on the third day of growth correlating with the peak of cellular proliferation (Fig. 2c, Supplementary Fig. S2a). In addition to a steroid hormone upregulation of Syncytin-1 detected by RT-PCR, the incubation of RL95-2 and BeWo cells with steroid hormones also demonstrated an upregulation of all three Syncytin-1 transcripts (Fig. 2c,d). In contrast, the addition of 17α-estradiol, the inactive form of E2, as well as the weak estrogens estrone and estriol to the same cells as above, had no stimulatory effect on Syncytin-1 gene expression (Supplementary Fig. S2a). No induction of cellular proliferation and Syncytin-1 expression were detected in the presence of steroid hormones with the ER-negative cell line Kle or with cultured uterine fibroblastoid cells from an EnCa tumor (Fig. 2c, Supplementary Fig. S1).
Further gene sequence analysis of HERV-W revealed an estrogen response element (ERE) with 85% homology in the U3 region of the 5′ and 3′LTR (Fig. 2d). DNA binding assays showed that purified ERα specifically bound to the Syncytin-1 ERE, however, when compared to the control ERE from the Xenopus laevis vitellogenin A2 gene promoter region, an ∼20-fold lower DNA binding affinity was observed (Fig. 2e). All of the above results support a specific regulation of Syncytin-1 via steroid hormones and receptors.
cAMP-induced cell–cell fusions of EnCa cells
The activation of cell–cell fusion involving placental cytotrophoblasts and BeWo cells has been shown previously to be stimulated by the activation of the cAMP-pathway [22]. Therefore, studies were initiated to analyse EnCa cell–cell fusion regulation. In contrast to steroid hormone studies, treatment of EnCa primary cells and cell lines with the adenylyl cyclase activator Forskolin or with SP-cAMP for 48 to 72 h did not induce cell proliferation, but stimulated the classic cell–cell fusion phenotype compared to BeWo cells or spontaneously fused syncytiotrophoblasts (Figs. 2 and 3b, Supplementary Fig. S1). Forskolin or SP-cAMP treated EnCa cells also demonstrated Syncytin-1 overexpression of up to 20-fold correlating with cell–cell fusions (Fig. 2c,d). In contrast, the inactive RP-isomer of cAMP (RP-cAMP) had no effect on cell–cell fusion or Syncytin-1 expression. When 2′5′-dideoxy-adenosine (ddA), an inhibitor of the adenylyl cyclase, was added to E2 or progesterone-treated RL95-2 or BeWo cells, both stimulated cell proliferation and Syncytin-1 overexpression was not inhibited (Supplementary Figs. S1 and S2). This finding proved that Syncytin-1 can be upregulated by two distinct cellular pathways, involving steroid hormones or cAMP, leading either to cell proliferation or cell–cell fusion, respectively.
Induced cell–cell fusions. a BeWo, RL95-2, and Kle cells were treated for 72 h with 10 nM E2 or 40 μM Forskolin, stained with May–Grunwald and Giemsa then analyzed. Mitotic cells increased from 2 to 8% in the presence of E2 in ER-positive cells. Cell–cell fusions >32n were noted in Kle cells treated with Forskolin. Bars 50 μm. b Primary cells from an EnCa tumor (patient 731) were cultivated for 72 h with or without 40 μM SP-cAMP. Note cells with three or six nuclei (6n–12n). Spontaneously fusing syncytiotrophoblasts were used as controls. Note a variety of nuclei ploidy including >32n. c FACS analyses of RL95-2 cells incubated with or without 40 μM SP-cAMP, 10 nM E2 plus 40 μM SP-cAMP, 5 ng/ml TGF-β3, 40 μM SP-cAMP plus 5 ng/ml TGF-β3 and with 40 μM SP-cAMP treatment of Syncytin-1-siRNA 1 transfected cells. Total cell number (y-axis) and cellular DNA content (n)
Quantitative FACS analysis of SP-cAMP-treated EnCa cells showed an increase in cell–cell fusions up to 22.5-fold, with a range of DNA content from 4n–32n (Fig. 3c). In addition, microscopic analysis of EnCa and BeWo cells also demonstrated cell–cell fusions >32n after cAMP-activation similar to syncytiotrophoblasts (Fig. 3a,b). Fluorescent microscopy of SP-cAMP treated RL95-2 with specific plasma membrane and nuclear stains confirmed single multinucleated cells (Fig. 4a). A secreted protein marker for cytotrophoblast cell–cell fusions in vivo and in vitro is the human chorionic gonadotropin hormone (β-hCG) where high levels indicate fusion [13]. After Forskolin or SP-cAMP treatment of primary cytotrophoblasts and BeWo cells, β-hCG levels peaked at 72 h, whereas in primary EnCa cells as well as Kle and RL95-2 no induction of β-hCG occurred, except for a threefold stimulation of HEC-1-A (Table 2, Supplementary Table 2). In addition, treatment of BeWo or RL95-2 cells with SP-cAMP or Forskolin did not result in induced gene expression of the putative Syncytin-1 receptors ASCT1 and ASCT2, however, GCMa was stimulated with SP-cAMP or Forskolin in BeWo, but not in RL95-2 cells indicating a GCMa-specific regulation of Syncytin-1 in choriocarcinoma and placenta (Table 2).
Cell–cell fusions in EnCa. a RL95-2 cells incubated with 40 μM SP-cAMP and then the plasma membrane of live cells was stained with wheat germ agglutinin (Alexa 594) (red) and the nuclear DNA with Hoechst 33342 (blue). Fused cells with over 10 nuclei (top) and with six nuclei (bottom) are shown. Bars 50 μm. b Two different endometrioid EnCa biopsies (stage Ic) embedded in paraffin were hematoxylin–eosin stained and then analyzed for cell–cell fusions or syncytia, also called giant trophoblastic cells (arrows). Note the presence of infiltrating granulocytes and glandular structures. Bars 50 μm
Importantly, histologically examining a series of eosin–haematoxylin stained endometrioid EnCa biopsies revealed cell–cell fusions or syncytial (trophoblast-like) cells (Fig. 4b). These findings implicate that cell–cell fusions are part of endometrioid tumors and as shown above, could be due to aberrant cAMP-activation.
Inhibition of cell proliferation and cell–cell fusion after siRNA silenced Syncytin-1 expression
The correlation of both steroid hormone-induced proliferation and cell–cell fusion with increased Syncytin-1 gene expression led to RNA interference-studies investigating, if Syncytin-1 is essential for both pathways. The posttranscriptional gene silencing of Syncytin-1 was performed with two independent siRNAs (Syncytin-1-siRNA 1 and 2), where each demonstrated silencing specificity. Steroid hormone-treatment of transfected RL95-2 cells with Syncytin-1-siRNA 1 or 2 resulted in an ∼90% reduction of Syncytin-1 gene expression and inhibition of induced cell proliferation after 72 h postset-up (Fig. 5b,d, and data not shown). In addition, Forskolin or SP-cAMP treatment of transfected RL95-2 and BeWo cells with Syncytin-1-siRNA 1 or 2 caused a >95% inhibition of Syncytin-1 gene and protein expression and cell–cell fusions (Figs. 3c and 5a,f). After 7 days post Syncytin-1-siRNA 1 or 2 transfection, Syncytin-1 RNA and protein amounts returned to normal and cell proliferation and cell–cell fusions could be induced with E2 and SP-cAMP, respectively, demonstrating a true siRNA rescue (Supplementary Fig. S2b).
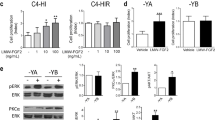
Loss of function analyses with Syncytin-1-siRNA and TGF-β regulation of cell proliferation and cell–cell-fusion. a Syncytin-1 and β-actin protein expression detected with Syncytin-1 SU or β-actin antibody in cell lysates of syncytiotrophoblasts (T) and RL95-2 cells transfected with 60 nM mock-siRNA or Syncytin-1-siRNA 1 with and without 40 μM SP-cAMP. b RL95-2 cells were transfected with mock-siRNA alone (closed circle), or mock-siRNA in the presence of 10 nM E2 (closed square), Syncytin-1-siRNA 1 alone (open square), or Syncytin-1-siRNA 1 in the presence of 10 nM E2 (triangle). Untransfected cells were also included (open circle which is covered by the open square). c RL95-2 cells were transfected with mock-siRNA or Syncytin-1-siRNA 1, then seeded in soft agar in the presence of 10 nM E2 or 4OH-E2, 500 nM progesterone or 40 μM SP-cAMP for 8 days. Top left and right panels shows mock-siRNA and Syncytin-1-siRNA 1 transfected RL95-2 cells without treatment. Colony number and size of Syncytin-1-siRNA 1 transfected cells in the presence of steroid hormones and SP-cAMP were similar to untreated nontransfected and mock-siRNA controls. Bars 200 μm. d RT-PCRs of the 3′ part of Syncytin-1 (748 bp) and β-actin (382 bp) from RL95-2 cells transfected with Syncytin-1-siRNA 1 (siRNA) then grown in the absence (−) or presence of steroid hormones (E2, P) or Forskolin (F) and caused Syncytin-1 inhibition (right). Co represents untreated mock-siRNA transfected cells. On the left, RL95-2 cells treated with 5 ng/ml purified human recombinant TGF-β3 grown in the presence or absence (−) of steroid hormones or Forskolin and resulted in no Syncytin-1 reduction. M DNA marker. e RT-PCRs of the 3′ part of Syncytin-1 (748 bp) and β-actin (382 bp) from primary placental trophoblasts cultivated for 72 h in the absence (−) or presence of 5 ng/ml TGF-β3 (TGF) or 40 μM Forskolin (F). Syncytin-1 expression in trophoblasts could be induced with Forskolin, but not with exogenous TGF-β3 after 72 h. M DNA marker. f Top left panel shows RL95-2 cells treated for 72 h with 100 μM nonactive RP-cAMP, 40 μM SP-cAMP, or 10 nM E2, and stained with May–Grunwald and Giemsa. Syncytin-1-siRNA 1 transfected RL95-2 cells treated with 40 μM SP-cAMP demonstrated no fusions (top right). A prior incubation of RL95-2 cells with 5 ng/ml purified human TGF-β1, then treatment with 40 μM SP-cAMP inhibited fusions (bottom panels). In contrast, RL95-2 cells treated with 10 nM E2 and then with 1 μg/ml of neutralizing TGF-β1 antibody showed cell fusions. Bar 50 μm. Far right shows cytotrophoblasts cultivated with 5 ng/ml purified human recombinant TGF-β 1 preventing fusions or cytotrophoblasts cultivated without TGF-β 1 leading to multinucleated syncytiotrophoblasts. Bar 50 μm
To analyze the role of Syncytin-1 in anchorage-independent colony growth, clonogenic soft agar experiments were performed with Syncytin-1-siRNA 1 or 2, mock-siRNA transfected or nontransfected RL95-2 cells in the presence or absence of steroid hormones, Forskolin or SP-cAMP (Fig. 5c). Nontransfected or mock-siRNA transfected RL95-2 cells treated with either E2, 4-OH-E2 or progesterone and seeded in soft agar, demonstrated a 37.8–41.6% increase of colony number and an increase of colony size above controls after 7 days (n = 3). In contrast, when RL95-2 cells were transfected with Syncytin-1-siRNA 1 or 2, the steroid hormone induction of colonies was blocked resulting in colony number and size similar to transfected, untreated cells. Treatment of mock-siRNA transfected RL95-2 cells with SP-cAMP or Forskolin resulted in an expansion of the average colony size with a fusion-like appearance. This colony fusion effect of SP-cAMP or Forskolin was repressed after Syncytin-1-siRNA 1 or 2 transfection of RL95-2 cells (Fig. 5c). These experiments showed that Syncytin-1 is also involved in anchorage-independent colony growth and fusion depending on steroid hormones or cAMP-activation.
Regulation of cell proliferation and cell–cell-fusion by TGF-β1 and TGF-β3
A complex regulation of cell proliferation and cell–cell fusion in primary EnCa cells and cell lines, as well as in primary syncytiotrophoblasts and BeWo cells was further unraveled between steroid hormones, TGF-β1 and TGF-β3 and the activated cAMP-pathway (Figs. 5d–f and 6, Supplementary Fig. S3). TGF-β1 and TGF-β3 gene expression analyses of endometrial hyperplasia, EnCa and placenta showed an increase of both gene products in comparison to matched control endometrium, supporting a role of TGF-β in these tissues (Table 1). Treatment of RL95-2 and BeWo cells with either 4-OH-E2, 2-OH-E2 or E2 also induced TGF-β1 protein levels significantly and correlated with Syncytin-1 upregulation during cell proliferation (Table 2, Fig. 2, Supplementary Figs. S1 and S2a). In contrast, TGF-β1 protein levels were not induced with Forskolin or SP-cAMP (Table 2). A monoclonal antibody-directed neutralization of TGF-β1 or TGF-β3 in E2-treated RL95-2 or BeWo cells resulted in cell–cell fusions similar to cAMP-activation, thus reversing the E2-induced cell proliferation (Fig. 5f, Supplementary Fig. S3). These results suggested that TGF-β has an inhibitory role in cell–cell fusion even in the presence of high Syncytin-1 levels. Indeed, addition of purified TGF-β1 or TGF-β3 proteins alone or before SP-cAMP or experiments with E2 addition before Forskolin or SP-cAMP treated primary EnCa, RL95-2 or BeWo cells inhibited the cell–cell fusion activity by >80% as determined by FACS and microscopy as well as decreasing β-hCG levels for BeWo (Figs. 3c and 5f, Table 2, Supplementary Fig. S3), however, Syncytin-1 levels remained high (Fig. 5d).
Summarized interactions of Syncytin-1 and TGF-β in cell proliferation and cell–cell fusions. Flowchart summarizes the results of this study using steroid hormones or cAMP-signaling pathway activators with primary EnCa cells and cell lines, primary trophoblasts and the choriocarcinoma cell line BeWo. Steroid hormones induce Syncytin-1 and TGF-β1 and TGF-β3 gene expressions resulting in stimulating cell proliferation, whereas neutralizing TGF-β1 or TGF-β3 with antibodies cause cell–cell fusion. Stimulating the cAMP-signaling pathway with SP-cAMP or Forskolin induce Syncytin-1 gene expression and cell–cell fusion, while addition of purified TGF-β1 or TGF-β3 proteins inhibit cell–cell fusion and results in cell proliferation
For comparison, the TGF-β regulatory action on cell–cell fusion was examined with cytotrophoblasts. Addition of TGF-β1 or TGF-β3 proteins blocked spontaneous fusion of cytotrophoblasts at 72 h, as analyzed by microscopy and decreasing β-hCG levels, with Syncytin-1 levels unchanged (Fig. 5e,f, Supplementary Table 2). Endogenous TGF-β levels were also analyzed. At day 3 of culturing, the cell–cell fusion peak of syncytiotrophoblasts showed low TGF-β1 gene expression (\( 2^{{ - \Delta \Delta {\text{ct}}}} \):0.02 ± 0.01 by real-time PCR), whereas on day 8, when cell–cell fusion activity decreased, TGF-β1 levels had risen to over 10-fold (\( 2^{{ - \Delta \Delta {\text{ct}}}} \):0.22 ± 0.09). This rise of endogenous TGF-β1 protein levels between days 3–8 could also be confirmed using enzyme-linked immunosorbent assay (ELISA).
In summary, TGF-β1 and TGF-β3 represent dominant negative regulators of cell–cell fusion, especially during cell proliferation due to steroid hormone induction of Syncytin-1 in EnCa, whereas Syncytin-1 in the absence of TGF-β contributes to cell–cell fusions.
Discussion
Models similar to the multistep evolution of colorectal carcinoma by Fearon and Vogelstein [33] have been proposed for EnCa [34, 35]. This report determined that Syncytin-1 increased in transcript copy number and protein levels from benign endometrial tissues to EnCa stages and metastasis, and was not due to genomic amplification (Fig. 1). We therefore propose that Syncytin-1 in addition to other genes, like K-ras, c-myc, and PTEN is important in the multistep genetic evolution to EnCa.
A strong connection exists between endometrioid EnCa and its prestages and steroid hormones or unopposed estrogen therapy; however, steroid hormone receptor expression demonstrated differences. In all tissues, the expression of ERβ was equally low, but ERα expression was the highest in EnCa (Fig. 2a). It is interesting to note that PR-expression was the lowest in EnCa possibly due to silencing of PR-B by hypermethylation [36]. In addition, the ratios of PR A/B and ERα demonstrated differences between the stages, a 2:1 ratio in hyperplasia, compared to 0.06:1 ratio in EnCa, thus, supporting progestin for treatment of hyperplasias. It has previously been demonstrated that EnCa tumors have 58% higher than normal E2 hormone levels compared to matched controls [37]. The ERE-based upregulation of Syncytin-1 by steroid hormones along with induced cell proliferation shed light on the connection between steroid hormones and EnCa growth. This clinical connection was further supported by experiments using primary EnCa cell lines with and without ER expression, high Ki-67 levels and especially with Syncytin-1-siRNA (Figs. 2 and 5, Tables 1 and 2, Supplementary Fig. S1). Taken together, these findings establish Syncytin-1 as an essential gene for steroid hormone-induced cell proliferation in EnCa staging.
Other examples of steroid hormone-induced envelope genes of endogenous retroviruses are HERV-K, which was also inducible up to 10-fold in breast cancer cells when treated with steroid hormones and the endogenous Jaagsiekte sheep retrovirus (enJSRV), which is highly related to the exogenous lung oncogenic JSRV retrovirus [38, 39]. The enJSRV was expressed in the endometrium epithelia of the ovine uterus and detected in trophoectoderm syncytia. Like Syncytin-1, the enJSRV plays a role in early placentogenesis and could be induced with progesterone, whereas JSRV was not inducible [38]. Recently, Syncytin-1 was found upregulated in ovarian carcinoma, which also could involve steroid hormone induction [40]. The correlation of Syncytin-1 with EnCa in this report and other published associations of HERV genes with human diseases, like the upregulation of HERV-K envelope transcripts in most breast tumor tissues support a connection of HERVs with tumors and tumor progression [39].
In addition to the essential role of cell–cell fusions in development, syncytia are also more commonly found in tumors, e.g., in renal cell carcinoma and in mamma adenocarcinoma [41, 42]. Multinucleated, syncytial or so-called trophoblast-like cells are described in EnCa tumors of serous papillary histology, but are also found in endometrioid EnCa (Fig. 4b). EnCa cases with trophoblastic cells were described as very aggressive with an unusually rapid and progressive clinical course [43]. This report supports that cAMP-activated Syncytin-1 is responsible for cell–cell fusions in EnCa. Cell–cell fusions of EnCa cells induced by the cAMP-signaling pathway were quantitated by FACS and verified with specific membrane stains demonstrating multinuclei surrounded by one cellular membrane (Figs. 3 and 4), in contrast to the phenomenon of endomitosis, as in megakaryocytes demonstrating a single polylobulated nucleus with >2n ploidy. EnCa as well as BeWo cell–cell fusions were mediated by Syncytin-1 because an inhibition of cell–cell fusion was achieved with Syncytin-1-siRNA 1 or 2 (Fig. 5). Other publications have showed that Syncytin-1 transfected BeWo cells increased cell–cell fusions, which could be inhibited with Syncytin-1 antibodies [22].
This report also identified TGF-β1 and TGF-β3 as key regulators of cell proliferation and cell–cell fusions in EnCa, choriocarcinoma, and placenta (Fig. 6). Treatment of EnCa, BeWo cells, or primary cytotrophoblasts with purified TGF-β1 or TGF-β3 proteins even in the presence of Forskolin or SP-cAMP inhibited cell–cell fusion and β-hCG expression (Figs. 3c and 5e,f; Tables 1 and 2). Steroid hormones, but not SP-cAMP treatment of EnCa and BeWo cells induced endogenous TGF-β1 expression resulting in cell proliferation and inhibiting cell–cell fusion. For example, noninduced RL95-2 endogenous TGF-β1 levels were >twofold when compared to BeWo cells and which significantly increased upon E2 treatment (Tables 1 and 2). Although, TGF-β can inhibit growth in some cell types, it is possible that higher endogenous EnCa TGF-β1 levels like in RL95-2 prevent an inhibition of cell proliferation even after addition of exogenous TGF-β1 proteins. These findings are supported by others using various HEC cell lines [44]. In addition, primary cytotrophoblasts showed an increase of endogenous TGF-β1 or TGF-β3 with a decrease of cell–cell fusion activity after 8 days of culturing. Other examples of the TGF-β-inhibitory activity of cell–cell fusions have been found with osteoclasts and myoblasts [45–47]. In contrast, an antibody mediated neutralization of TGF-β1 or TGF-β3 in steroid hormone treated EnCa cells or cell lines led to cell–cell fusions due to the steroid hormonal induction of Syncytin-1 (Figs. 5f and 6, Supplementary Fig. S3). Thus, high Syncytin-1 levels, but low TGF-β resulted in cell–cell fusions, whereas high Syncytin-1 and high TGF-β resulted in cell proliferation. One proposal for the above regulation of cell proliferation and cell–cell fusion by TGF-β could be explained by the capability of TGF-β to increase the cholesterol efflux from cellular membranes and therefore modifying the membrane location and function of Syncytin-1 [48]. For example, cholesterol depletion of host cells resulted in resistance against syncytia formation by HIV-1 and HTLV1 [49]. It is interesting to note that first experiments in our laboratory have shown that Syncytin-1 was located in a specific area of the cell membrane, the lipid raft domain, enriched in cholesterol (Strick and Strissel, unpublished results).
In summary, this study showed an overexpression of Syncytin-1 in EnCa and prestages linked with steroid hormone-induced cell proliferation and cAMP-stimulated cell–cell fusions. As a key regulatory gene in mediating both cell phenotypes in EnCa and placental cells, the steroid hormone inducible TGF-β1 and TGF-β3 were identified. Especially, the posttranscriptional knock-down of Syncytin-1 gene expression and concomitant functional silencing of induced cell proliferation and cell–cell fusion with two different siRNAs directed against the SU and TM unit proved the essential role of Syncytin-1 in these cellular processes. Although the fate or function of multinucleated cells in tumors is presently unknown, recently, it was shown that bone-marrow-derived cells fused with, e.g., hepatocytes and contributed to the development and maintenance of these cells [50]. Spontaneous cell–cell fusions between mouse tumor and normal cells in vivo, led to the hypothesis that these cells play a role in tumor progression and emergence of metastatic cells [51]. In addition, cell–cell fusions between human fibroblasts were found to occur due to a monkey retrovirus and oncogene expression, and resulted in transformed cells [52]. It is therefore plausible that cell–cell fusions between tumor cells and normal cells could change the phenotype and potency of normal cells to a cancer phenotype, which could be called “trans-carcinogenesis”. Syncytin-1 mediated cell–cell fusions between EnCa cells could also be involved in silencing the immune response, which was proposed for syncytiotrophoblasts protecting the fetus [17]. Escaping the immune response was shown for the envelope proteins of exogenous retroviruses, HERVs [15] and mouse tumor cells expressing MMLV envelope [53]. The immunosuppressive activity of the HERV-W envelope Syncytin-1 could involve modulation of the cytokine synthesis, inhibition of lymphocyte proliferation and natural killer cell action as shown with the related MMLV-envelope protein ([53] and references therein). Therefore, in addition to cell proliferation and cell–cell fusion, a role for Syncytin-1 in promoting tumor growth could also include controlling the immune system.
References
Ellenson LH, Wu TC (2004) Focus on endometrial and cervical cancer. Cancer Cell 5:533–538
Jemal A, Tiwari RC, Murray T et al (2004) Cancer statistics, 2004. CA Cancer J Clin 54:8–29
Sherman ME (2000) Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 13:295–308
Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J (2004) Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer 14:348–353
Anastasiadis PG, Koutlaki NG, Skaphida PG, Galazios GC, Tsikouras PN, Liberis VA (2000) Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol 21:180–183
Ben-Arie A, Goldchmit C, Laviv Y et al (2004) The malignant potential of endometrial polyps. Eur J Obstet Gynecol Reprod Biol 115:206–210
Emons G, Huschmand-Nia A, Krauss T, Hinney B (2004) Hormone replacement therapy and endometrial cancer. Onkologie 27:207–210
Smith DC, Prentice R, Thompson DJ, Herrmann WL (1975) Association of exogenous estrogen and endometrial carcinoma. N Engl J Med 293:1164–1167
Cuzick J, Powles T, Veronesi U et al (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Niederacher D, An HX, Camrath S et al (1998) Loss of heterozygosity of BRCA1, TP53 and TCRD markers analysed in sporadic endometrial cancer. Eur J Cancer 34:1770–1776
Chen EH, Olson EN (2005) Unveiling the mechanisms of cell–cell fusion. Science 308:369–373
Ogle BM, Cascalho M, Platt JL (2005) Biological implications of cell fusion. Nat Rev Mol Cell Biol 6:567–575
Potgens AJ, Schmitz U, Bose P, Versmold A, Kaufmann P, Frank HG (2002) Mechanisms of syncytial fusion: a review. Placenta 23:S107–S113
Bradley CS, Benjamin I, Wheeler JE, Rubin SC (1998) Endometrial adenocarcinoma with trophoblastic differentiation. Gynecol Oncol 69:74–77
Bannert N, Kurth R (2004) Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci USA 101:14572–14579
Benit L, Dessen P, Heidmann T (2001) Identification, phylogeny, and evolution of retroviral elements based on their envelope genes. J Virol 75:11709–11719
Mallet F, Bouton O, Prudhomme S et al (2004) The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc Natl Acad Sci USA 101:1731–1736
Yu C, Shen K, Lin M et al (2002) GCMa regulates the Syncytin-mediated trophoblastic fusion. J Biol Chem 277:50062–50068
Hashemolhosseini S, Wegner M (2004) Impacts of a new transcription factor family: mammalian GCM proteins in health and disease. J Cell Biol 166:765–768
Matouskova M, Blazkova J, Pajer P, Pavlicek A, Hejnar J (2006) CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp Cell Res 312:1011–1020
Cheynet V, Ruggieri A, Oriol G et al (2005) Synthesis, assembly, and processing of the Env ERVWE1/Syncytin human endogenous retroviral envelope. J Virol 79:5585–5593
Mi S, Lee X, Li X et al (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789
Johnston ER, Radke K (2000) The SU and TM envelope protein subunits of bovine leukemia virus are linked by disulfide bonds, both in cells and in virions. J Virol 74:2930–2935
Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D (2002) The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol 76:6442–6452
Sommerfelt MA, Williams BP, McKnight A, Goodfellow PN, Weiss RA (1990) Localization of the receptor gene for type D simian retroviruses on human chromosome 19. J Virol 64:6214–6220
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Gold LI (1999) The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog 10:303–360
Parekh TV, Gama P, Wen X et al (2002) Transforming growth factor beta signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res 62:2778–2790
Dowdy SC, Mariani A, Reinholz MM et al (2005) Overexpression of the TGF-beta antagonist Smad7 in endometrial cancer. Gynecol Oncol 96:368–373
Yang NN, Venugopalan M, Hardikar S, Glasebrook A (1996) Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science 273:1222–1225
Arici A, MacDonald PC, Casey ML (1996) Modulation of the levels of transforming growth factor beta messenger ribonucleic acids in human endometrial stromal cells. Biol Reprod 54:463–469
Strick R, Strissel PL, Gavrilov K, Levi-Setti R (2001) Cation–chromatin binding as shown by ion microscopy is essential for the structural integrity of chromosomes. J Cell Biol 155:899–910
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Beckmann MW, Niederacher D, Bender HG (1998) Mechanisms of steroid hormone action and resistance in endometrial and breast cancer. Eur J Cancer Prev 7:S25–S28
Lalloo F, Evans G (2001) Molecular genetics and endometrial cancer. Best Pract Res Clin Obstet Gynaecol 15:355–363
Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S, Dahiya R (2001) Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer. Cancer Res 61:97–102
Berstein LM, Tchernobrovkina AE, Gamajunova VB et al (2003) Tumor estrogen content and clinico-morphological and endocrine features of endometrial cancer. J Cancer Res Clin Oncol 129:245–249
Palmarini M, Mura M, Spencer TE (2004) Endogenous betaretroviruses of sheep: teaching new lessons in retroviral interference and adaptation. J Gen Virol 85:1–13
Wang-Johanning F, Frost AR, Johanning GL et al (2001) Expression of human endogenous retrovirus k envelope transcripts in human breast cancer. Clin Cancer Res 7:1553–1560
Menendez L, Benigno BB, McDonald JF (2004) L1 and HERV-W retrotransposons are hypomethylated in human ovarian carcinomas. Mol Cancer 3:12
Berzal Cantalejo F, Sabater Marco V, Alonso Hernandez S, Jimenez Pena R, Martorell Cebollada MA (2004) Syncytial giant cell component. Review of 55 renal cell carcinomas. Histol Histopathol 19:113–118
Vicandi B, Jimenez-Heffernan JA, Lopez-Ferrer P et al (2004) Fine needle aspiration cytology of mammary carcinoma with osteoclast-like giant cells. Cytopathology 15:321–325
Pesce C, Merino MJ, Chambers JT, Nogales F (1991) Endometrial carcinoma with trophoblastic differentiation. An aggressive form of uterine cancer. Cancer 68:1799–1802
Boyd JA, Kaufman DG (1990) Expression of transforming growth factor beta 1 by human endometrial carcinoma cell lines: inverse correlation with effects on growth rate and morphology. Cancer Res 50:3394–3399
Florini JR, Roberts AB, Ewton DZ, Falen SL, Flanders KC, Sporn MB (1986) Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem 261:16509–16513
Karsdal MA, Hjorth P, Henriksen K et al (2003) Transforming growth factor-beta controls human osteoclastogenesis through the p38 MAPK and regulation of RANK expression. J Biol Chem 278:44975–44987
Lafyatis R, Lechleider R, Roberts AB, Sporn MB (1991) Secretion and transcriptional regulation of transforming growth factor-beta 3 during myogenesis. Mol Cell Biol 11:3795–3803
Panousis CG, Evans G, Zuckerman SH (2001) TGF-beta increases cholesterol efflux and ABC-1 expression in macrophage-derived foam cells: opposing the effects of IFN-gamma. J Lipid Res 42:856–863
Niyogi K, Hildreth JE (2001) Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J Virol 75:7351–7361
Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM et al (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425:968–973
Kerbel RS, Lagarde AE, Dennis JW, Donaghue TP (1983) Spontaneous fusion in vivo between normal host and tumor cells: possible contribution to tumor progression and metastasis studied with a lectin-resistant mutant tumor. Mol Cell Biol 3:523–538
Duelli DM, Hearn S, Myers MP, Lazebnik Y (2005) A primate virus generates transformed human cells by fusion. J Cell Biol 171:493–503
Mangeney M, Heidmann T (1998) Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc Natl Acad Sci USA 95:14920–14925
Acknowledgments
The authors are especially grateful to the patients who participated in this study and to the Department of Gynaecology, Erlangen. The authors wish to thank Prof. Dr. Papadopoulos at the Institute for Pathology, University of Erlangen for the histology of the tissue samples, Prof. Dr. C-M Becker (Institute for Biochemistry, University of Erlangen) for the use of the isotope laboratory, Mrs. Wenzel (Institute for Biochemistry) for cloning and sequencing, Mrs. Staerker and Toborek for hCG determinations, and Mrs. Oeser and Stiegler (Department of Gynecology) for their expert technical assistance. This study was partially supported by a grant from the DFG (#555/2-1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Supplemental Table 1
(DOC 21 kb)
Supplemental Table 2
(DOC 22 kb)
supplemental Fig. S1
Growth curves of BeWo, Kle, and RL95-2 cells in the presence of different steroid hormones, ddA or Forskolin. Open circle control, open triangle 10 nM E2, closed triangle 10 nM 4-OH-E2, closed square 1 μM estrone, cross 10 nM 2-OH-E2, closed circle 1 μM Estriol, closed diamond 10 mM ddA plus 10 nM E2, open square 500 nM progesterone, open diamond 40 μM Forskolin (JPEG 125 kb)
supplemental Fig. S2
a RT-PCR of Syncytin-1 (748 bp) and β-actin (382 bp) with RNA isolated from RL95-2 cells treated with 10 nM of either E2, 4OH-E2 (4E2), or 2OH-E2 (2E2), 1 μM estrone (E1), and 10 nM E2 plus 10 mM ddA. M DNA marker. b RT-PCR of Syncytin-1 (748 bp) and β-actin (382 bp) with RNA isolated from Syncytin-1 transfected RL95-2 cells at 4 days post transfection (c1). After 4 days post transfection the cells were cultivated for an additional 3 days (c), and treated with 10 nM E2 or 40 μM Forskolin (F) (JPEG 82 kb)
supplemental Fig. S3
Cultured BeWo cells were incubated with 10 nM E2 (E2), 40 μM SP-cAMP (SP-cAMP), 40 μM SP-cAMP plus 5 ng/ml TGF-β1 (SP-cAMP + TGF-β1), and 10 nM E2 plus 1 μg/ml anti-TGF-β1 (E2 + anti-TGF-β1), then stained with May–Grunwald and Giemsa (JPEG 211 kb)
Rights and permissions
About this article
Cite this article
Strick, R., Ackermann, S., Langbein, M. et al. Proliferation and cell–cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-β. J Mol Med 85, 23–38 (2007). https://doi.org/10.1007/s00109-006-0104-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0104-y