Abstract
It is perhaps rare to encounter among the various immunologically competent receptor–ligand pairs that a single cell surface determinant unleashes both a hidden suppressive function and costimulation. 4-1BB, an activation-induced tumor necrosis factor receptor family member chiefly viewed as a powerful T-cell costimulatory molecule, is one such example. Accumulated evidence in recent years uncovered an unknown facet of in vivo 4-1BB signaling (i.e., “active suppression”). Although in vitro signaling via 4-1BB is shown to support both CD4+ and CD8+ T-cell responses, the same induces a predominant CD8+ T-cell response suppressing CD4+ T-cell function when applied in vivo. How, when, and why such dual immunoregulatory effect of anti-4-1BB monoclonal antibody (MAB) comes into play is currently the focus of intense research. Existing data, although not complete, uncover several important aspects of in vivo 4-1BB signaling in the amelioration or exacerbation of various immune disorders. Despite minor disagreements, a majority agree that upregulation of interferon (IFN)-γ is critical to anti-4-1BB MAB therapy in addition to immune modulators such as interleukin 2, transforming growth factor β, and indolamine 2,3-dioxygenase5, all of which contribute greatly to the success of anti-4-1BB MAB-based immunotherapy. Anti-4-1BB MAB-mediated expansion of novel CD11c+CD8+ T cells is additional weaponry that appears critical for its in vivo suppressive function. These CD11c+CD8+ T cells express high levels of IFN-γ, become effective killers, and mediate selective suppression of CD4+ T cells. In this review, we discuss the dual nature (costimulatory and suppressive) of 4-1BB-mediated immune regulation, its current status, future direction, and its impact on the immune system, with special reference to its immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
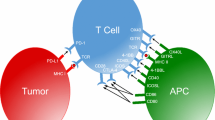
Optimal activation of T cells to clonally expand requires at least two distinct biological signals: one is generated by the interaction of the T-cell antigen receptor (TCR) with peptides bound to major histocompatibility complex (MHC) molecules. The other signal(s) is (are) generated by a functionally defined event called the costimulatory pathway. The nature and inventory of biological molecules with costimulatory properties have rapidly increased in the last decade. The molecules concerned with costimulation are predominantly confined to either B7 superfamily [1] or tumor necrosis factor (TNF) superfamily [2] (Fig. 1). Each of these known costimulatory receptor–ligand pairs provides a distinct yet important signal for either promoting positive immune regulation or supporting negative immune response [1, 2].
Members of B7 and TNF superfamily with immune regulatory function. Except for a few, most molecules are activation-induced. Once expressed, signaling through these receptor–ligand pairs transmit either costimulatory or suppressive signals. Although the expression patterns of various receptor–ligand pairs on T cells and APCs are represented here, evidence available suggests such expression was not exclusive to represented cell types but can be found on a variety of immune competent cells
From the ever-growing list of biological molecules with costimulatory properties, it is perhaps rare to find receptors that have two distinct and opposing features: activation and suppression (in that order). The case in point is a member of TNF receptor (TNFR) superfamily termed 4-1BB (CD137) [2, 3]. Although such dual immunoregulatory actions were also described for another TNFR member, receptor activator of nuclear factor kappa B (RANK)–RANK ligand (RANKL) [4–6], an in-depth understanding of molecular events underlying such actions is not as exhaustive as that available for 4-1BB. Rarely has such intense interest been generated among the currently characterized costimulatory molecules as does 4-1BB chiefly due to its dual nature of immunoregulatory features.
Discovery of 4-1BB, early characterization, and alternate names
4-1BB was first detected in screens for receptors on mouse concanavalin A (ConA)-activated helper and cytotoxic T-cell lines [7]. 4-1BB was also termed “induced by lymphocyte activation” (ILA) in humans [3, 8]. The entire gene [accession numbers AL009183, AY438976 (human), and U02567 (mouse)] spans approximately 13 kb of mouse chromosome 4 [7]. A 4-1BB cDNA, initially isolated from activated murine T cells by a modified differential screening procedure [7], was categorized as an early activation gene because the protein synthesis inhibitor, cyclohexamide, blocked formation of its transcription [9]. 4-1BB is a 30-kDa glycoprotein [accession numbers BC006196, L12964, U03397 (human) and AK019885, BC028507, J04492, NM011612 (mouse)] and exists both as a 30-kDa monomer and a 55-kDa homodimer on the T-cell surface [10].
Distribution, expression, regulation, and characteristics of 4-1BB
With few exceptions, the expression of 4-1BB is activation-dependent. 4-1BB is not detected (<3%) on resting T cells or T-cell lines. However, when T cells are activated by a variety of agonists [plate-bound anti-CD3, ConA, PHA, interleukin (IL)-2, IL-4, anti-CD28, phorbol myristoyl acetate (PMA), and ionomycin alone or in various combinations], in the presence of antigen-presenting cells (APCs), 4-1BB is stably upregulated [9–11]. To date, expression of 4-1BB is noted on both non-T and T cells [2]. Induction of 4-1BB is inhibited by cyclosporine A [11] and cyclohexamide [9]. Incubation with actinomycin D halved 4-1BB transcript levels in activated lymphocytes within 30 min, pointing to a relatively short half-life [12]. 4-1BB is also upregulated in human peripheral blood mononuclear cell (PBMC) by DNA-damaging agents such as anti-cancer drugs or γ-irradiation [13]. Using 5′-deletion constructs of the 4-1BB promoter in luciferase assays, Kim et al. [14] demonstrated that the transcriptional elements mediating the 4-1BB upregulation are located between approximately 0.9 and 1.1 kb of the translational start site. Further characterization of these sites revealed that NF-κB and activating protein 1 (AP-1) are involved. Also, MEK and c-Jun N-terminal kinase 1 are required for activation-dependent 4-1BB upregulation [14].
4-1BB and clinical importance
The expression of 4-1BB was detected in several human subjects with various clinical histories closely correlating with disease severity. 4-1BB, along with caspases, was induced by thyroid hormone stimulation [15]. Furthermore, 4-1BB expression was also detected in Crohn’s disease [16]. As with certain members of TNF superfamily, soluble forms of 4-1BB were found in sera of humans with rheumatoid arthritis (RA) where levels of circulating 4-1BB increased with increasing disease severity [17]. Expression of 4-1BB was observed in both hepatocellular carcinoma and nontumor regions of the liver [18], as well as at tumor sites [19]. Furthermore, the peripheral blood of liver-transplant patients not only contained 4-1BB, but its expression was also correlated with clinical severity [20]. In addition, 4-1BB, along with 4-1BBL, was detected in human neurons, astrocytes, and microglia, as well as peripheral blood samples from chronic heart failure patients [21]. Interestingly, Lim et al. [22] demonstrated 4-1BB-like molecule in the islet-infiltrating mononuclear cells and gray matter of the brain, but the significance of such expression is unclear. Taken together, the above-referred studies clearly imply a significant role for 4-1BB in various clinical disorders.
In vitro effects of 4-1BB signaling
Since its discovery, the studies that followed established 4-1BB as a potent costimulatory molecule [2]. To date, signaling via 4-1BB by agonistic monoclonal antibodies (MABs) or soluble 4-1BBL or cell lines expressing 4-1BBL in the presence of anti-TCR ABs has been shown to cause T-cell expansion, cytokine induction, upregulation of antiapoptotic genes, and prevention of activation-induced cell death [2, 23, 24]. Based on the delayed expression pattern, signaling through 4-1BB was thought to support intermediate and late immune responses [11]. There is evidence to suggest that 4-1BB signaling differentially activates CD4+ and CD8+ T cells. Kinetic studies and expression analyses revealed that, although 4-1BB expression is comparable among CD4+ and CD8+ T cells, the CD8+ T cells show more robust proliferative potential compared with CD4+ T cells [25]. A similar trend can be seen in vivo [26]. In accordance, a transgenic mouse strain, made to express 4-1BB protein under the control of the proximal lck promoter on CD4+ T cells, showed initial enhanced TCR-driven proliferative ability that did not sustain beyond 2 days compared with level of proliferation achieved by CD8+ T cells obtained from the same transgenic mouse [14]. The reasons for such differential costimulatory ability of anti-4-1BB are still not completely understood; hence, available data strongly proclaim that 4-1BB is a bona fide CD8+ T-cell-activating molecule [25]. However, the 4-1BB- and 4-1BBL-null mice show no apparent defects in CD4+ vs CD8+ T-cell ratios [27, 28].
Functional 4-1BB expression has also been observed on certain non-T cells. Natural killer/natural killer T (NK/NKT) cells express 4-1BB in an activation-dependent manner [29, 30] (Fig. 2). Experiments revealed that signaling through NK 4-1BB receptor augments IL-2 production, but addition of agonistic anti-4-1BB MAB during in vitro cytotoxic assays has no effect on cytotoxic T lymphocyte (CTL) responses [29, 30]. 4-1BB was also found on monocytes where cross-linking of 4-1BB by anti-4-1BB MAB amplified IL-8 and TNF-α and decreased IL-10 producing abilities of these cells [31]. In the same study [31], activation of monocytes by anti-4-1BB MAB caused B-cell death presumably due to the action of secretory components of monocyte/anti-4-1BB MAB reaction. Heinisch et al. [32] observed that 4-1BB activation abrogates granulocyte-macrophage colony-stimulating-factor-mediated antiapoptosis in neutrophils. To date, CD4+CD25+ T cells and dendritic cells (DCs) are shown to express 4-1BB in a constitutive manner, which increases further following activation [33, 34]. Ligation of CD4+CD25+ regulatory T cells by anti-41BB plus anti-TCR ABs has been shown to result in cellular activation [35]. On the other hand, Choi et al. [36] suggested that 4-1BB signaling did not result in CD4+CD25+ T-cell proliferation but significantly inhibited their suppressive function. The relevance of constitutive 4-1BB expression on DCs was studied by Wilcox and colleagues [34] to demonstrate that IL-12 production by DCs significantly enhanced when signals are relayed via 4-1BB receptor (Fig. 2). Unpublished data from our laboratory observed that in vivo administration of agonistic anti-4-1BB MAB alone or in conjunction with relevant antigens (Ags) causes rapid deletion of NK but not NKT cells and shows significantly reduced in vivo cytotoxicity against class-I-deficient tumors. The basis of such NK-cell-depleting ability of agonistic anti-4-1BB MAB is currently being investigated. Recent observations from our laboratory suggested that 4-1BB-deficient mice showed reduced NK/NKT numbers and function [37], perhaps hinting at a possible relation between 4-1BB and its expression on NK/NKT cells. These and other effects of in vitro 4-1BB signaling [reviewed in 2, 23, 24] collectively establish an important role for 4-1BB in the immune regulation.
In vitro responses of anti-4-1BB signaling. Agonistic anti-4-1BB MAB either alone (in the case of non-T cells) or together with anti-TCR (in the case of T cells) is shown to enhance activation and differentiation of several leukocyte populations. In the context of anti-TCR or appropriate Ag, the addition of anti-4-1BB MAB to cultures, although shown to support both CD4+ and CD8+ T-cell activation, is more partial to the latter than to the former. There is evidence to suggest that anti-4-1BB MAB also supports CD4+CD25+ regulatory T-cell activation, but whether such treatment also affects their suppressive potential is still far from clear. Likewise, stimulation with anti-4-1BB MAB results in cytokine induction in NK/NKT cells but has no effect on the cytolytic potential of these cells. Functional 4-1BB receptor is also reported on non-T cells. Studies thus far suggest that monocytes, when stimulated with 4-1BBFc and anti-Fc, leads to the induction of immune modulators in these cells. Similarly, dendritic cells, when triggered with anti-4-1BB MAB, leads to the activation and induction of IL-12. 4-1BB is also detected on follicular dendritic cells where its ligation by anti-4-1BB MAB is reported to propel B-cell activation
In vivo 4-1BB effects
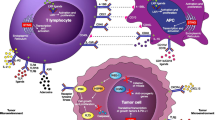
The in vivo effects of anti-4-1BB are much more dramatic than those seen in vitro (summarized in Fig. 3). Unarguably, in vivo anti-4-1BB MAB administration kills/suppresses more cell types than costimulating them and still offers protection against various clinical disorders, including autoimmunity, cancer, and transplantation [38–42]. Such dual immunoregulatory nature of 4-1BB signaling continues to remain enigmatic.
Novel immunoregulatory pathways of in vivo 4-1BB triggering. In vivo ligation of agonistic anti-4-1BB MAB in the context of antigen-specific T-cell triggering among others reduces the onset of autoimmune diseases by a selective amplification of CD11c+CD8+ T cells. These novel immunoregulatory T cells by their actions deplete autoreactive CD4+ T cells in an IFN-γ- and IDO-dependent manner
The foregoing account will briefly summarize the various in vivo effects of anti-4-1BB MAB therapy, study the pitfalls, make an effort to understand the molecular basis of such phenomena, and finally conclude the discussion by identifying future goals.
Anti-4-1BB MAB therapy of autoimmune diseases
There have been reports on the unprecedented increase in the occurrence of autoimmune diseases. A recent review on the epidemiology and significance of autoimmune diseases in health care suggests that about 20% of the human population suffer from at least one form of autoimmune diseases [43]. Autoreactive T cells (chiefly CD4+ T cells) produce inflammatory cytokines, help B cells produce autoantibodies, activate macrophages, and collectively are the main perpetuators of severity of autoimmune conditions. Among others, protocols directed at altering autoreactive T-cell function either by MAB therapy or by its deletion are some of the currently available treatment modalities.
To date, the role of 4-1BB signaling has been studied in several autoimmune processes, including RA [40], experimental autoimmune encephalomyelitis [38], and systemic lupus erythematosus [39, 40]. These studies showed significant protection against the autoimmune disorders investigated thus far unraveling several novel aspects of in vivo 4-1BB signaling. As pointed out by the work of Sun et al. [41], administration of anti-4-1BB MAB, at the time of preinduction of autoimmune lupus, is characterized by significant loss of autoreactive CD4+ T cells and autoantibody-producing B cells in an event involving elevated levels of IFN-γ because its neutralization reversed much of the anti-4-1BB MAB effect [41]. In an analogous study using NZB X NZW F1 mice, Foell et al. [39] suggested anti-4-1BB MAB-mediated protection against lupus involves reduced DC function and an inability to fully prime autoimmune T cells, thereby leading to immune suppression. In addition, unpublished data from our laboratory suggested that when AS.W (H-2s) mice are exposed to mercuric chloride (a prototypic Th2 autoimmunity causing agent), the associated autoimmune lesions were significantly reversed when treated with anti-4-1BB MAB even after the manifestation of autoimmune lesions. These above studies suggested the administration of anti-4-1BB MAB either at the predisease condition or at the onset of clinical symptoms or even after the establishment of disease is still effective, suggesting that time and dose of anti-4-1BB may not appear to be critical in some of the models tested.
However, a few things stand out as far as the operative mechanism underlying anti-4-1BB MAB therapy is concerned. A majority agrees that enhancement of Th1-type cytokines, notably IFN-γ, is critical for anti-4-1BB MAB therapy, resulting in the deletion of autoreactive CD4+ T cells [41], whereas others reported no such loss [39].
Anti-4-1BB therapy also severely affects B-cell numbers and function, resulting in the dampening of overall humoral immunity so that autoantibody production is haltered [41]. It is still not clear as to what causes anti-4-1BB MAB-mediated functional loss in B cells, but it is suspected that B cells are susceptible to stand-alone anti-4-1BB MAB treatment even in the absence of Ag stimulation [44]. Sun et al. [41] demonstrated that loss of B-cell function in anti-4-1BB MAB treatment of lupus-prone mice is significantly restored after in vivo IFN-γ is neutralized. Although systemic anti-4-1BB MAB therapy was not employed, Sytwu et al. [45], using transgenic nonobese diabetic mice (NOD) overexpressing membrane-bound agonistic single-chain anti-4-1BB Fv in pancreatic B cells, showed more severe diabetes than their nontransgenic littermates. Although the basis for such exacerbation of diabetic processes by 4-1BB signaling is not clear, it suggests that in vivo ligation of 4-1BB by agonistic MABs appears to differ from that of physiologic cross-linking seen in the above study.
Using an autoimmune RA model, our laboratory has provided a comprehensive account as to the basis of anti-4-1BB MAB-mediated protective effects. Seo et al. [40] made an interesting observation regarding anti-4-1BB MAB-mediated amelioration of RA. These authors [40] demonstrated that following anti-4-1BB MAB treatment, autoreactive CD4+ T cells are deleted in an IFN-γ and indoleamine 2,3-dioxygenase (IDO)-dependent manner. Although many cell types are targeted in anti-4-1BB MAB-mediated immune protection, DCs and CD11c+CD8+ T cells are singled out for their proven regulatory function [40, 46]. It was thought that anti-4-1BB MAB treatment upregulates IDO in macrophages and DCs, and interaction of such IDO+CD11b+ macrophages or IDO+CD11c+ DCs with autoreactive CD4+ T cells was responsible for the elimination of autoreactive CD4+ T cells [40] (Fig. 3). These authors [40] further made a seminal observation that anti-4-1BB MAB treatment expands a CD8+ T-cell type that coexpresses CD11c molecule among others. These CD11c+CD8+ cells are believed to be a subset of CD8+ T cells based on their ability to express CD3, perforin, and their inabilities to express CD4, CD40, MHC II, CD11b, B220, CD205, and 33D1 [40], and are distinct from the regulatory DCs that are described mainly because of their non-T-cell origin [47–49]. These CD11c+CD8+ T cells show limited mitotic activity, express IFN-γ, have normal migratory properties, are potent immunosuppressors, and are exclusive to 4-1BB signaling [40]. The adoptive transfer of CD11c+CD8+ T cells into arthritis-susceptible DBA/1 mice significantly retarded the progression of arthritis [40]. However, unfractionated splenocytes from mice previously treated with Ag and anti-4-1BB MAB, when recultured in vitro in the presence of same Ag, cellular proliferation was not overtly affected, perhaps due to the rapid death of CD11c+CD8+ T cells in ex vivo in spite of the presence of DCs in cultures (Vinay et al., manuscript in preparation). This demonstrates a clear demarcation between in vitro and in vivo effects of anti-4-1BB signaling. Taken together, these data suggest that IFN-γ, DCs, and CD11c+CD8+ regulatory T cells all play crucial parts in anti-4-1BB MAB-mediated immunotherapy in the models tested. Our laboratory is currently studying the origins, signaling requirements, and the basis of immunosuppressive function associated with CD11c+CD8+ T cells. These studies are hoped to shed additional light on 4-1BB-mediated signaling and open new avenues to target autoreactive T cells and help cure the autoimmune disorders.
Anti-4-1BB MAB-mediated antiviral immunity
The role of 4-1BB signaling was evaluated by several investigators and were equivocal in proposing that anti-4-1BB MAB treatment is by far one of the efficient means to enhance antiviral immunity [50]. In most cases, protection against viruses is dependent on CD8+ T cells, and it makes sense that 4-1BB, a strong activator of CD8+ T cells, is beneficial for viral clearance. Indeed, Halstead et al. [51] showed that in vivo 4-1BB stimulation enhances primary CD8+ T-cell response to influenza type A viral infection in mice. Similarly, Bertram et al. [52] have demonstrated that treatment of influenza-virus-infected mice with agonistic anti-4-1BB MAB during priming but not initial phase is effective in restoring the defective secondary CD8+ T-cell responses in CD28−/− mice because these mice exhibited defective secondary CD8+ T-cell responses. On the other hand, the 4-1BB−/− mice exhibit normal primary CD8+ T-cell responses but do not sustain secondary responses, suggesting that 4-1BB signaling is crucial for late antiviral immune responses. Maus et al. [53] elegantly demonstrated that in ex vivo coculture system, long-term proliferation and survival of virus-specific human CD8+ T cells were achieved in the presence of 4-1BB signals. These findings established that naive CD8+ T cells initially activated by TCR/CD28 signals require 4-1BB costimulation to sustain their responses. This approach can provide an invaluable tool for growing virus- or tumor-specific CD8+ T cells ex vivo. In a recent report, Arribillaga et al. [54] demonstrated that anti-4-1BB MAB administered after immunization of mice with recombinant adenovirus encoding hepatitis C virus increased cytotoxic potential. As seen in the case of autoimmune RA model [40], anti-4-1BB MAB therapy was shown to be effective against both primary and reinfection of herpes simplex virus type 1 (HSV1) by a mechanism involving massive expansion of CD11c+CD8+ T cells [55]. The study also showed that CD11c+CD8+ T cells were maintained in memory CD8+ T-cell pool [54] as evidenced by the observation that 50 days after initial exposure to HSV-1 and/or rat immunoglobulin (Ig)G or anti-4-1BB MAB when restimulated with HSV-1, only mice previously treated with anti-4-1BB MAB but not rat IgG showed accelerated reemergence of CD11c+CD8+ T cells. Collectively, these results clearly underscore the importance of anti-4-1BB MAB therapy in the treatment of viral infections presumably by boosting the immunity at priming and secondary phases of viral infection.
Anti-4-1BB MAB-mediated antitumor immunity
Suppression of tumor growth or its eradication has long remained a daunting task for clinicians. Treatment modalities generally become more difficult when dealing with nonimmunogenic or poorly immunogenic tumors that normally evade immune actions. Among the various animal models studied to date, 4-1BB signaling was the most investigated in tumor models. Melero et al. [56] were the first to demonstrate that anti-4-1BB MAB treatment of tumor-bearing mice is effective even in the eradication of established tumors. Tirapu et al. [57] showed that administration of agonistic anti-4-1BB MAB together with semiallogeneic DCs was associated with potent antitumor immune responses both spontaneous and established poorly immunogenic MC38 colon tumors. Similarly, the poorly immunogenic B16-F10 melanoma-bearing mice, when treated with anti-4-1BB and IL-12, enhanced the function of tumor-specific T cells and retarded the growth of subcutaneously inoculated tumors and increased the survival of tumor-bearing mice by 50% [58]. In the same study [58], using a pulmonary metastatic model, authors reported that in vivo depletion of NK or CD8+ but not CD4+ T cells eliminated the anti-4-1BB MAB-/IL-12-associated protective activity.
Tumor–lysate pulsed DC-based vaccination has attracted considerable attention in recent years [59, 60]. Ito et al. [61] demonstrated that mice bearing established pulmonary and subcutaneous tumors when treated with anti-4-1BB MABs significantly regressed tumor growth and markedly increased survival of mice. Closer examination revealed that the antitumor activity of anti-4-1BB MAB and DC vaccine was dependent more on intact CD8+ T and, to a lesser extent, on CD4+ T or NK cells, signifying the importance of CD8+ T cells in anti-4-1BB MAB-protective effects. Likewise, treatment of mice carrying hepatic MCA26 colon carcinoma with anti-4-1BB, although it did not increase survival, resulted in significant reduction (50%) of tumor growth [58]. In a recent study, Ju et al. [62] observed that 4-1BB+/+ mice inoculated with B16F10 melanoma cells survived longer compared with 4-1BB−/− mice. However, survival in 4-1BB+/+ mice was significantly pronounced when treated with anti-4-1BB in a CD8+ T-cell- and IFN-γ-dependent manner [62].
One of the side effects of anti-4-1BB MAB therapy seems to be due to loss of B-cell function [41]. Although anti-4-1BB MAB-mediated loss of humoral immunity appears to be beneficial in discrete animal models where containment of autoantibody production is crucial (e.g., RA and systemic lupus erythematosus; 39–41), lack of humoral immunity may prove detrimental in other models especially in viral models where intact B-cell function is necessary to neutralize viral responses. To circumvent some of the side effects associated with anti-4-1BB MAB therapy, Ye et al. [63] devised an attractive strategy in which mice were inoculated with tumor cells that were transfected to express anti-4-1BB single-chain Fv. Administration of such “engineered tumor cells” were shown to exert strong antitumor immune response but with reduced side effects. However, such treatment may have some limitations, as observed by Sytwu et al. [45], who showed enhanced incidence of diabetes in NOD mice made to express anti-4-1BB single-chain Fv in pancreatic B cells. Although certain issues need to be fine-tuned, the above studies offer an attractive strategy in the form anti-4-1BB MAB therapy to treat cancer and autoimmune disorders but must be cautiously exercised given the side effects. For example, Sabel et al. [64] demonstrated that in vivo administration of antihuman 4-1BB MAB into severe combined immunodeficiency (SCID) mice coinjected with tumor and peripheral blood lymphocytes failed to support lymphocyte-mediated tumor suppression, and tumors grew progressively. Failure to offer protection in the above experiment was suspected to have resulted from the activity of NK cells that may have killed the adoptively transferred peripheral blood lymphocytes by antibody-dependent cellular cytotoxicity [64]. When NK cells were eliminated by treatment by antiasialo GM1-specific antibodies, the antihuman 4-1BB MAB effects, however, became obvious. Incidentally, addition of antihuman 4-1BB MAB to one-way mixed lymphocyte reaction supported both CD4+ and CD8+ T-cell proliferation. These contrasting in vitro and in vivo roles of anti-4-1BB in the treatment of tumors are some of the best examples highlighting the dual nature of anti-4-1BB MAB therapy.
Other anti-4-1BB MAB-mediated in vivo effects
In vivo 4-1BB ligation suppresses B-cell function
Mittler et al. [65] were the first to make an important observation that administration of anti-4-1BB causes loss of humoral activity against T-dependent Ags. Closer examination revealed that loss of humoral activity in anti-4-1BB MAB-administered mice correlated with anergic CD4+ T cells in these mice [65]. On the other hand, Sun et al. [41] suggested loss of B-cell numbers and function in lupus-prone mice was associated with loss of CD4+ T cells and upregulation of IFN-γ because neutralization with anti-IFN-γ MAB treatment significantly restored the defect. Recently, Sun et al. [44] suggested that anti-4-1BB MAB-mediated treatment specifically deletes follicular dendritic cells (FDCs), thus preventing subsequent events leading to germinal center formation and AB production. These authors [39] further observed that anti-4-1BB MAB causes loss of B-cell function even in the absence of antigenic stimulation in a poorly understood phenomenon. Anti-4-1BB MAB-mediated effects on B-cell function in mice were substantiated in experiments dealing with nonhuman primates. Hong et al. [66] have demonstrated that treatment with humanized anti-4-1BB MAB suppresses humoral responses to T-dependent Ag (ovalbumin) in nonhuman primates. Collectively, these experiments suggest that loss of B-cell function by anti-4-1BB MAB is an integral component of events leading to protective immunity seen in some disease models. The exact nature of molecular events leading to loss of B-cell function, however, is not completely understood and remains an attractive field to explore.
CD11c+CD8+ T cells are responsible for the dual roles of anti-4-1BB
We found that a massive expansion of CD11c+CD8+ T cells occurred when anti-4-1BB was administered with Ag not only in the collagen-induced arthritis model [41] (Fig. 3) but also in experimental autoimmune uveitis (unpublished observations), melanoma (manuscript in preparation), viral infection models such as HSV1 [55], and vesicular stomatitis virus (unpublished observations). The CD11c+CD8+ T cells in the viral infection and tumor models were highly cytotoxic and contained a majority of viral Ag-specific CD8+ T cells [55]. Therefore, we propose that seemingly contradictory dual roles for anti-4-1BB—an enhanced function and a suppressor function—are mediated by this novel CD11c+CD8+ T-cell population. In other words, the CD11c+CD8+ T cells themselves become the primary killer cell in cancer and viral infection, the IFN-γ produced by these cells induces IDO in DCs, and the induced IDO then becomes a suppressor of Ag-specific CD4+ T cells in the autoimmune disease model. The only exception to this is the case of mice administered with super Ags such as staphylococcal enterotoxin A along with anti-4-1BB. Although this super Ag produced CD11c+CD8+ T cells when it was coadministered with anti-4-1BB, the result was not suppression of CD4+ T cells but rather an increase in their numbers as CD8+ T-cell numbers increased (unpublished observations).
Tryptophan depletion by anti-4-1BB MAB as an explanation to its suppressive effects
Indoleamine 2,3-dioxygenase is a potent immunoregulatory enzyme. Expression of IDO is upregulated with IFN-γ, which in turn enables certain macrophages and DCs to inhibit T-cell proliferation in vitro and in vivo [67, 68]. IDO degrades the essential amino acid tryptophan, which is needed for progressive T-cell proliferation [67, 68]. Seo et al. [41] observed that anti-4-1BB MAB treatment enhances IFN-γ, which in turn upregulates IDO in macrophages and DCs and hypothesized that interaction between IDO+ macrophages and DCs and partnering T cells suppresses or deletes T-cell activity. The anti-4-1BB MAB-controlled IDO-mediated immune suppression can substantially be eliminated when treated with 1-methyl tryptophan (a pharmacological inhibitor of IDO) [41]. Protection against experimental autoimmune uveitis syndrome and tumor-bearing mice by anti-4-1BB is shown to involve tryptophan depletion by increased IFN-γ and IDO expression, which in turn suppresses autoreactive CD4+ T-cell function (unpublished observations). These new concepts of anti-4-1BB MAB-associated protective effects open additional avenues for the treatment of various clinical disorders and explain the diverse roles of 4-1BB signaling.
Summary and future directions
4-1BB is an important T-cell molecule concerned with costimulation and T-cell survival. There is a clear demarcation between the in vitro and in vivo roles of 4-1BB signaling. In vitro signaling leads to activation of predominantly CD8+ T cells and, to a lesser extent, CD4+ T cells. In vivo 4-1BB signaling is quite complex and unique among the currently characterized costimulatory molecules in that in vivo administration of agonistic anti-4-1BB MAB causes massive expansion of CD8+ T cells, suppresses/deletes CD4+ T-cell numbers and function, retards B-cell function, upregulates IDO and IFN-γ, and expands unique CD11c+CD8+ T cells. These CD11c+CD8+ T cells are potent anticancer and antiviral effectors, yet induce IDO in DC by their production of IFN-γ. All above mentioned features of anti-4-1BB MAB therapy play an important role in the extent to which protection against various clinical disorders is achieved.
However, utmost caution is needed when contemplating the use of anti-4-1BB MAB as a therapeutic agent. Given its suppressive effects of CD4+ T-cell function, it suggests that such therapy may not be desirable when using in models where intact CD4+ T-cell function plays a major role in the protective processes. Likewise, anti-4-1BB MAB-mediated loss of humoral immunity may be beneficial in autoimmune processes but, when viewed in conjunction with viral models, do not seem to be a viable alternative especially in light of the fact that antibody production is much needed to neutralize viral infection. Although alternative approach of using anti-4-1BB single-chain Fv, proposed by Ye et al. [63], seems to work in tumor models, results demonstrated by Sytwu et al. [45] seem to contradict it as evidenced by increased incidence of diabetes in NOD mice transfected to express anti-4-1BB single-Fv in pancreatic B cells. Much of future research may be directed toward the understanding of properties of CD11c+CD8+ T cells. As to how the CD11c+CD8+ T cells alter the disease course, their side effects, their longevity in vivo, and signaling requirements must be fully understood. Nonetheless, in vivo ligation of 4-1BB by anti-4-1BB MAB offers a potent therapeutic means to counter the progression of various clinical conditions such as cancers, viral infection, and autoimmune diseases.
Abbreviations
- IDO:
-
indoleamine 2,3-dioxygenase
- HSV-1:
-
herpes simplex virus type 1
- TCR:
-
T-cell receptor
- APC:
-
antigen-presenting cell
- ConA:
-
concanavalin A
- PHA:
-
phytohemagglutinin
References
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548
Watts TH (2005) The TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 23:23–68
Vinay DS, Kwon BS (2006) Immunotherapy targeting 4-1BB and its ligand. Int J Hematol 83:23–28
Wong BR, Josien R, Lee SY, Sauter B, Li H-L, Steinman RM, Choi Y (1997) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186:2075–2080
Williamson E, Bilsborough JM, Viney JL (2002) Regulation of mucosal dendritic cell function by receptor activator of NF-κB (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol 169:3606–3612
Kim N, Takami M, Rho J, Josien R, Choi Y (2002) A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 195:201–209
Kwon BS, Weissman SM (1989) cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA 86:1963–1967
Schwarz H, Tuckwell J, Lotz M (1993) A receptor induced by lymphocyte activation (ILA); a new member of the human nerve growth factor/tumor necrosis factor receptor family. Gene 134:295–298
Kwon BS, Kim CS, Prystowki MB, Lancki DW, Sabath DE, Pan JL, Weissman SM (1987) Isolation and initial characterization of multiple species of T cell lymphocyte subset cDNA clones. Proc Natl Acad Sci USA 84:2896–2900
Pollok KE, Kim YJ, Zhou Z, Hurtado JC, Kim KK, Pickard RT, Kwon BS (1993) Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol 150:771–781
Garni-Wagner BA, Lee ZH, Kim YJ, Wilde CE, Kang CY, Kwon BS (1996) 4-1BB is expressed on CD45 RAhi ROhi translational T cells in humans. Cell Immunol 169:91–98
Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M (1995) ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood 85:1043–1052
Kim KM, Kim HW, Kim JO, Baek KM, Kim JG, Kang CY (2002) Induction of 4-1BB (CD137) expression by DNA damaging agents in human T lymphocytes. Immunology 107:472–479
Kim JO, Kim HW, Baek KM, Kang CY (2003) NF-κB and AP-1 regulate activation-dependent CD137 (4-1BB) expression in T cells. FEBS Lett 541:163–170
Yamada-Okabe T, Satoh H, Yamada-Okabe H (2003) Thyroid hormone induces the expression of 4-1BB and activation of caspases in thyroid hormone receptor-dependent manner. Eur J Biochem 270:3064–3073
Maerten T, Geboes K, De Hertogh G, Shen C, Cadot P, Bullens DM, Van Assche G, Penninckx F, Rutgeerts P, Cueppens JL (2004) Functional expression of 4-1BB (CD137) in the inflammatory tissue in Crohn’s disease. Clin Immunol 112:239–246
Jung HW, Choi SW, Choi JI, Kwon BS (2004) Serum concentrations of soluble 4-1BB and 4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp Mol Med 36:13–22
Wan YL, Zheng SS, Zhao ZC, Li MW, Jia CK, Zhang H (2004) Expression of co-stimulator 4-1BB molecule in hepatocellular carcinoma and adjacent non-tumor liver tissue, and its possible role in tumor immunity. World J Gastroenterol 10:195–1999
Zhang H, Merchant MS, Chua KS, Khanna C, Helman LJ, Telford B, Ward Y, Summers J, Toresky J, Thomas EK, June CH, Mackall CL (2003) Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther 2:579–586
Wan YL, Zheng SS, Jia CK, Liang TB, Huang DS, Wang WL, Li MW, Zhao ZC (2003) Expression of 4-1BB molecule on peripheral blood T cells in liver transplanted patients and its clinical implication. Hepatobiliary Pancreat Dis Int 2:38–43
Yndestad A, Damas JK, Ger Eiken H, Holm T, Hauh T, Simonsen S, Froland SS, Gullestad, Aukrust P (2002) Increased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc Res 54:175–182
Lim HY, Kim KK, Zhou FC, Yoon JW, Hill JM, Kwon BS (2002) 4-1BB-like molecule is expressed in islet-infiltrating mononuclear cells and in the gray matter of the brain. Cell Biol Int 26:271–278
Croft M (2003) Costimulatory members of TNFR family: keys to effective T-cell immunity. Nat Rev Immunol 3:609–620
Croft M (2003) Costimulation of T cells by OX-40, 4-1BB, and CD27. Cytokine Growth Factor Rev 14:265–273
Takahashi C, Mittler RS, Vella AT (1999) 4-1BB is a bona-fide CD8 T cell survival signal. J Immunol 162:5037–5040
Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS (1997) 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell response. J Exp Med 186:47–55
DeBenedette MA, Wen T, Bachman MF, Ohashi PM, Barber BH, Stocking KL, Peschin JJ, Watts TH (1999) Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol 163:4833–4841
Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmyer HE, Vinay DS (2002) Immune responses in 4-1BB (CD137)-deficient mice. J Immunol 168:5483–5490
Melero I, Johnston JV, Shuford WW, Mittler RS, Chen L (1998) NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol 190:167–172
Wilcox RA, Tamada K, Strome SE, Chen L (2002) Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol 169:4230–4236
Kienzel G, von Kempis J (2000) CD137 (ILA/4-1BB), expressed by human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int Immunol 12:73–82
Heinisch IV, Daigle I, Knopfli B, Simon HU (2000) CD137 activation abrogates granulocyte-macrophage colony-stimulating factor-mediated anti-apoptosis in neutrophils. Eur J Immunol 30:3441–3446
McHugh RS, Matthew JW, Piccrillo CA, Young DA, Shevach EM, Collins M, Byrne MC (2002) CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311–323
Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, Tamada K, Mittler RS, Tsuchiya H, Pardoll DM, Chen L (2002) Expression of functional CD137 receptor by dendritic cells. J Immunol 168:4262–4267
Zheng G, Wang B, Chen A (2004) The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol 173:2428–2434
Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS (2004) 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol 75:785–791
Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS (2004) CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol 173:4218–4229
Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX (2002) Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol 168:1457–1465
Foell J, Strahotin S, O’Neil SP, McClausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi N, Hoffmann MK, Mittler RS (2003) CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZB F1 mice. J Clin Invest 111:1505–1518
Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS (2004) 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med 10:1088–1094
Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX (2003) Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med 8:1405–1413
Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, Kwon BS, Kwon B (2004) Stimulation with 4-1BB (CD137) inhibits chronic-graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood 105:2206–2213
Cervara R (2001) The epidemiology and significance of autoimmune diseases in health care. Scand J Clin Lab Invest 61:27–35
Sun Y, Blink SE, Chen JH, Fu YX (2005) Regulation of follicular dendritic cell networks by activated T cells: the role of CD137 signaling. J Immunol 175:884–890
Sytwu HK, Lin WD, Roffler SR, Hung JT, Sung HS, Wang CH, Cheng TL, Tsou SC, His SC, Shen KL (2003) Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun 21:247–254
Kwon B, Lee HW, Kwon BS (2002) New insights into the role of 4-1BB in immune responses: beyond CD8+ T cells. Trends Immunol 23:378–380
Homann D, Jahreis A, Wolfe T, Hughes A, Coon B, van Stipdonk MJB, Prilliman KR, Schoenbeger SP, von Herrath MG (2002) CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity 16:403–415
Chan CW, Crafton E, Fan H-N, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins M, Lanier LL, Pardoll DM, Housseau F (2006) Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med 12:207–213
Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Metivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzmann D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer, Zitvgel L (2006) A novel dendritic cell subset in tumor immunosurveillance. Nat Med 12: 214–219
Bertram EM, Dawicki W, Watts TH (2004) Role of T cell costimulation in anti-viral immunity. Sem Immunol (2004) 16:185–196
Halstead ES, Mueller YM, Altman JD, Katsikis PD (2002) In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol 168:5483–5490
Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH (2004) A switch in costimulation from a CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza virus in vivo. J Immunol 15:981–988
Maus MV, Thomas AK, Leonard DGB, Allman D, Addya K, Schlienger K, Riley JL, June CH (2002) Expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotech 20:143–148
Arribillaga L, Sarobe P, Arina A, Gorraiz M, Borras-Cuesta F, Ruiz J, Prieto J, Chen L, Melero I, Lasarte JJ (2005) Enhancement of CD4 and CD8 immunity by anti-CD137 (4-1BB) monoclonal antibodies during hepatitis C vaccination with recombinant adenovirus. Vaccine 23:3493–3499
Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS (2005) 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol 238:76–86
Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L (1997) Monoclonal antibodies against 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 3:682–685
Tirapu I, Arina A, Mazzolini G, Duarte M, Alfaro C, Feiji E, Qian C, Chen L, Prieto J, Melero I (2004) Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens. Int J Cancer 110:51–60
Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH (2005) The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gen Ther 12:1526–1533
Schuler G, Steinman RM (1997) Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med 186:1183–1187
Pilon-Thomas SA, Verhaegen ME, Mule JJ (2005) Dendritic cell-based therapeutics for breast cancer. Breast Dis 20:65–71
Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Tietz-Tennenbaum S, Chang AE (2004) Anti-CD137 monoclonal antibody administration augments the anti tumor efficacy of dendritic cell-based vaccines. Cancer Res 64:8411–8419
Ju SA, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, Suh JH, Cho HR, Kwon B, Kwon BS, Kim BS (2005) Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumor-infiltrating lymphocytes. Immunol Cell Biol 83:344–351
Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE (2002) Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med 8:343–348
Sabel MS, Conway TF, Chen FA, Bankert RB (2000) Monoclonal antibodies directed against the T-cell activation molecule CD137 (interleukin-A or 4-1BB) block human lymphocyte-mediated suppression of tumor xenografts in severe combined immunodeficient mice. J Immunother 23:362–368
Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK (1999) Anti-4-1BB monoclonal antibodies abrogate T-cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med 190:1535–1540
Hong HJ, Lee JW, Park SS, Kang YJ, Chang SY, Kim KM, Kim JO, Murthy KK, Payne JS, Yoon SK, Park MJ, Kim IC, Kim JG, Kang CY (2000) A humanized anti-4-1BB monoclonal antibody suppresses antigen-induced humoral immune response in nonhuman primates. J Immunother 23:613–621
Grohmann U, Fallarino F, Puccetti P (2003) Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 24:242–249
Mellor A, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774
Acknowledgements
This work was supported in part by US Public Health Service grants RO1EY013325 (B.S.K.), KRF-2005-201-E00008, and KRF-2005-084-E00001; Korea Health 21 R&D, A050260; International Collaboration Fund, 2005-0441; and SRC funds to the IRC, University of Ulsan, Korea, from Korea Science and Engineering Foundation and the Korean Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinay, D.S., Cha, K. & Kwon, B.S. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med 84, 726–736 (2006). https://doi.org/10.1007/s00109-006-0072-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0072-2