Abstract
Polyglutamine diseases, such as Huntington disease (HD) and spinocerebellar ataxia 1 and 3, are autosomal dominant neurodegenerative disorders. They are caused by CAG trinucleotide repeat expansions that are translated into abnormally long polyglutamine tracts. One of the pathological hallmarks in polyglutamine diseases is the formation of intranuclear inclusions of polyglutamine-containing proteins in the brain. Although causal relationships between polyglutamine aggregation and cellular toxicity are much debated, inhibition of the polyglutamine-mediated protein aggregation may provide treatment options for polyglutamine diseases. However, the extreme insolubility of expanded polyglutamines makes it difficult to prepare polyglutamine-containing proteins on a large scale and to search for aggregation inhibitors by in vitro high-throughput screening. To overcome this we developed a novel in vitro model system for polygltamine diseases using myoglobin as a host protein. We searched for small molecules that inhibit polyglutamine-mediated aggregation by in vitro screening with a mutant myoglobin containing a 35 polyglutamine repeat. The screening assay revealed that disaccharides have a potential to inhibit polyglutamine-induced protein aggregation and to increase survival in a cellular model of HD. Oral administration of trehalose, the most effective disaccharide in vitro, decreased polyglutamine aggregates in the cerebrum and liver, improved motor dysfunction and extended life span in a transgenic mouse model of HD. In vitro experiments suggest that the beneficial effects of trehalose result from its ability to bind and stabilize polyglutamine-containing proteins. The lack of toxicity and high solubility, coupled with its efficacy upon oral administration, make trehalose promising as a therapeutic drug or lead compound for the treatment of polyglutamine diseases. The stabilization of aggregation-prone proteins with small molecules is an attractive strategy because it can block the initial stage of the disease cascade. In addition, this therapeutic approach could be applied not only to polyglutamine diseases but also to a wide variety of misfolding-induced diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huntington disease (HD) is an autosomal dominant progressive neurodegenerative disorder with a generally midlife age at onset. HD is characterized by uncontrolled movement, personality changes, and dementia [1]. The mutation that causes HD is an expansion of a CAG repeat in the first exon of the gene encoding huntingtin, an approx. 350-kDa protein that is essential to embryonic development, neurogenesis, and intracellular trafficking [2, 3, 4]. Huntingtin is a widely expressed and predominantly cytoplasmic protein that is enriched throughout the brain. While normal individuals possess a polyglutamine length of 6–34 repeats, individuals with more than 36 repeats develop HD. There is a positive correlation between repeat length and severity of symptoms, but an inverse correlation exists between repeat length and age at onset. A direct causative pathway from the expansion of glutamine repeats in huntingtin to neuronal dysfunction and death has not been established. Insoluble aggregates of huntingtin protein are observed in vitro in mammalian cells, in transgenic animals, and in the brain tissues from HD patients [5, 6, 7, 8, 9]. Although causal relationships between the formation of huntingtin aggregates and HD pathology have been controversial [10, 11, 12, 13], the formation of insoluble huntingtin aggregates may be related to cellular dysfunctions underlying HD. Therefore small molecules that inhibit huntingtin aggregation could provide a treatment option for HD [14]. In particular, inhibition of protein aggregation by stabilizing causative proteins with small molecules is an effective therapeutic strategy for a neurodegenative disorder, transthyretin (TTR) amyloidosis. The native ligand thyroxine inhibits TTR fibril formation by stabilizing TTR tetramers against dissociation and thereby preventing subsequent conformational changes required for amyloid fibril formation [15]. A nonnative ligand 2,4,6-triiodophenol which binds to TTR with slightly increased affinity also inhibits TTR fibril formation by this mechanism. Screening and structure-based drug design identified several TTR amyloidosis inhibitors that function by increasing the kinetic barrier associated with misfolding through stabilization of the native tetrameric state [16, 17]. These results establish the importance of protein misfolding energetics in pathogenesis [18, 19]. Here, we review and discuss a therapeutic strategy for HD involving the stabilization of aggregation-prone polyglutamine-containing proteins by a small molecule trehalose.
Formation of polyglutamine aggregates and neuropathology in HD
A characteristic neuropathology of HD is the significant dysfunction and death of neurons, particularly medium spiny neurons of the striatum (Fig. 1a). The caudate, putamen, and globus pallidus also undergo progressive atrophy, and there are subtle changes in the cerebral cortex. Another hallmark of HD pathology is the formation of intranuclear inclusions of huntingtin protein in the brain (Fig. 1b). Neuropil and cytoplasmic inclusions are also found in affected individuals [20]. The formation of insoluble huntingtin aggregates appears to be involved in cellular dysfunctions leading to HD. For instance, expression of a dominant negative caspase-1 mutant slows the aggregate formation of huntingtin-exon1 protein along with disease progression in a transgenic mouse model of HD [21]. Congo red inhibits neuronal aggregates of huntingtin-exon1 protein and is associated with phenotypic improvement in HD mice [22]. Huntingtin aggregates recruit many essential proteins including molecular chaperones, proteasome subunits, transcription-regulating proteins, and ubiquitin-binding proteins [23, 24, 25, 26, 27, 28, 29, 30]. While the formation of insoluble aggregates of huntingtin with an expanded polyglutamine may be related to the pathogenesis of HD, a growing body of evidence suggests that oligomeric species (e.g., protofibril and microaggregates) are the cytotoxic entity and inclusion body formation plays protective roles in neuronal cells [31, 32, 33]. The formation of oligomeic states similar to those formed by α-synuclein and amyloid-β is observed for polyglutamine-bearing proteins [34, 35, 36, 37, 38], and their relevance to cellular toxicity has been suggested [22, 33, 37]. It is noted that inhibitors targeting aggregates are likely to block the formation of oligomeric species as well. Therefore the discovery of small molecules or proteins that inhibit the formation of huntingtin aggregates and/or oligomers may lead to an effective treatment for HD [14].
Characteristic striatal atrophy and intranuclear inclusions in the cerebrum of HD patients. a Striatal atrophy of the cerebrum for HD patients. Coronal sections of the cerebrum for a normal (left) and an affected (right) individual. b Intranuclear inclusions in the striatum of HD patients. Intranuclear inclusions (arrow) were visualized with an antibody to ubiquitin
Small molecules ameliorate polyglutamine-mediated pathology in a mouse model of HD
An R6/2 transgenic mouse model of HD has played a crucial role in evaluating the effects of small molecules on neuropathology in vivo [39]. The R6/2 mice overexpressing truncated huntingtin (exon 1) with a 145 glutamine repeat develop progressive ataxia and show pronounced neuronal intranuclear aggregates that contain truncated huntingtin and ubiquitin. The appearance of intranuclear inclusions and characteristic morphological changes in the striatum of R6/2 mice resemble those observed in HD patients [6]. Several types of small molecules including aggregation inhibitors have shown neuroprotective effects on the R6/2 mice.
A histone deacetylase inhibitor, suberoylanilide hydroxamic acid, was shown to alleviate motor impairment when orally administered with cyclodextrin in water [40]. Another histone deacetylase inhibitor, sodium butyrate, also improved body weight and motor performance and increased survival [41]. Furthermore, phenylbutyrate also attenuates gross brain and neuronal atrophy and extends life span when administered after the onset of symptoms [42]. These results are consistent with the observation that transcriptional deregulation is closely related to the pathogensis of polyglutamine diseases [25, 26, 43, 44, 45], and therapies aimed at modulating transcription may provide clinical benefits to HD patients.
Apoptosis inhibitors such as ZVAD-fmk, and YVAD-cmk together with DEVD-fmk delayed the development of pathology and mortality of R6/2 mice [21, 46]. A tetracycline derivative, minocycline, prevents disease progression by inhibiting caspase-1 and caspase-3 expression [46] as well as caspase-independent mitochondrial cell death pathways [47], although the results on the effects of minocycline are not consistent [48]. Treatment with a hydrophilic bile acid, tauroursodeoxycholic acid, reduces striatal atrophy and the number of apoptotic cells and results in fewer and smaller striatal intranuclear inclusions [49]. These results indicate that apoptosis contributes to the pathogenesis of HD and its inhibition ameliorates polyglutamine-mediated pathology.
Creatine decreases the formation of intranuclear aggregates, retards the progression of pathology, and delays the mortality presumably by buffering intracellular energy levels and compensating for mitochondrial dysfunction [50, 51, 52]. Dichloroacetate, which stimulates the pyruvate dehydrogenase complex, improves motor function, attenuates the development of striatal neuron atrophy, and increases survival, providing evidence for a role of energy dysfunction in HD pathogenesis [53]. Coenzyme Q10 and remacemide delay the development of motor deficits by boosting the lowered energy metabolism and decreasing N-methyl-d-aspartate mediated excitotoxicity, respectively [54, 55]. On the basis of these results, improvement in mitochondorial dysfunction can be a therapeutic strategy for HD.
As observed for the treatment with remacemide, a glutamate antagonist riluzole alleviates motor dysfunction and delays mortality [56]. Lithium chloride, which has protective properties against excitotoxicity, also ameliorates motor performance [57]. Thus excitotoxicity is an alternative target for intervention. Antioxidants such as α-lipoic acid and BN82451 were shown to increase survival of R6/2 mice by inhibiting oxidative damage and blocking microglial activation and induction of cyclooxygenase 2 activity, respectively [58, 59]. Recently, an antitumor antibiotic, mithramycin, has been shown to improve motor performance and significantly prolong survival [60]. Thus these therapeutic agents directed against oxidative stress, inflammation, and tumor appear promising. A rapamycin analog improves motor deficits and decreases aggregate formation by inducing autophagy through inhibition of mammalian target of rapamycin (mTOR) activity [61], indicating that the mTOR pathway has a critical role in the cellular dysfunction underlying HD and its regulation leads to a therapy for HD.
A transglutaminase inhibitor, cystamine, given intraperitoneally decreases aggregate formation, reduces associated tremor, and improves motor performance, suggesting that cross-linking of huntingtin by transglutaminase is involved in the HD pathogenesis [62, 63]. Intraperitoneal or intracerebral infusion of Congo red delays the disease progression, ameliorates motor impairment, and increases survival by inhibiting oligomerization of huntingtin [22]. These observations together with several results obtained by the above therapeutic strategies show that amelioration of motor deficits and extension of life span are correlated with a decreased level of huntingtin aggregation, suggesting inhibition of the aggregate formation as one of the therapeutic targets for HD. We thus designed a therapeutic strategy to search for aggregation inhibitors for polyglutamine-containing proteins initially through in vitro screening, followed by testing the efficacy of candidate molecules in the R6/2 transgenic mouse model of HD.
Design and characterization of molecular models for polyglutamine-containing proteins
Although the potential importance of small molecules that prevent the formation of polyglutamine aggregates is widely accepted [64, 65, 66], expanded polyglutamines render proteins insoluble in water and make it difficult to prepare such proteins on the large scale required for in vitro high throughput screening of aggregation inhibitors. Polyglutamine peptides can be employed for the screening but even a peptide of approx. 20 glutamines (nonpathological length) is insoluble in water [67], although treatment with trifluoroacetic acid and hexafluoroisopropanol helps dissolution of long polyglutamine (Gln44) peptides [68]. For the proteins carrying an expanded polyglutamine, proteolytic cleavage of glutathione S transferase (GST) from GST-exon 1 huntingtin (Gln51) fusion protein rapidly leads to the formation of insoluble aggregates [5]. Bevivino et al. [69] prepared maltose-binding protein (MBP) fused ataxin-3 protein with Gln78, albeit in low yield, and showed that an expanded glutamine repeat destabilizes native ataxin-3 structure and mediates formation of parallel β-fibrils. However, the MBP-fused ataxin-3 protein results in rapid aggregation upon proteolytic cleavage of MBP. These studies suggest that the natural causative proteins containing an expanded polyglutamine are too unstable to use for the in vitro screening. Another strategy to prepare polyglutamine-bearing proteins suitable for the in vitro screening is to insert a polyglutamine tract into a stable host protein. Mutant mice carrying an expanded polyglutamine introduced into a mouse hypoxanthine phosphoribosyltransferase protein developed a progressive late onset neurological phenotype similar to that of human polyglutamine diseases, indicating crucial roles of the expanded polyglutamine on inducing neurodegeneration, regardless of the host protein [70]. Stott et al. prepared a chymotrypsin inhibitor 2 (CI2) mutant containing an inserted 10 glutamine repeat and found that incorporation of glutamine repeats in CI2 induces oligomerization of the protein [71]. However, the 10 glutamine repeat in the CI2 mutant is much shorter than the pathological length for polyglutamine diseases, and its reactivity with an expanded polyglutamine-specific 1C2 antibody [72] has not been reported, although it would be a piece of key evidence whether the inserted polyglutamine forms a pathogenic conformation. We thus sought to develop a novel in vitro molecular model for polygltamine diseases which is stable enough for a large-scale preparation and has an expanded polyglutamine reactive to the 1C2 antibody.
Using sperm whale myoglobin (Mb) as a host protein, we established a large-scale preparation of Mb mutants containing glutamine repeats of various lengths (12, 28, 35, 50) as a molecular model for polyglutamine diseases (Fig. 2a, b) [73]. In the Mb mutants the 12 and 28 glutamine repeats are of a nonpathological length and the 50 glutamine repeat is a pathological length, while the 35 glutamine repeat is located at the border between pathological and nonpathological. The Mb mutants reacted with the 1C2 antibody in a polyglutamine-length dependent manner as do natural polyglutamine-containing proteins, indicating that an expanded polyglutamine inserted into Mb forms a pathogenic conformation similar to that in native proteins. The Mb mutants spontaneously formed amyloid fibers in a polyglutamine-length dependent fashion under the physiological condition (pH 7.0, 37°C) as observed for truncated huntingtin containing a 51 glutamine repeat; Mb-Gln50 formed amyloid fibers more rapidly than Mb-Gln35, whereas wild-type Mb and Mb-Gln12 did not form amyloid fibrils (Fig. 2c) [35, 74]. Denaturing experiments with guanidine hydrochloride (GdHCl) revealed that the insertion of polyglutamine into Mb destabilizes the host protein in a polyglutamine-length dependent manner. Small-angle X-ray scattering analysis showed that the radius of gyration for Mb-Gln50 increased to 27 Å from 17 Å for wild-type Mb, indicating a partially unfolded structure of Mb-Gln50 [35]. The denaturing assay with GdHCl revealed that the expanded glutamine repeat specifically unfolds a region at the surface of the Mb mutant protein [73]. The destabilizing effects of polyglutamine expansion are suggested for a natural causative protein for ataxin-3 [69].
Sperm whale myoglobin containing an inserted glutamine repeat as a molecular model for polyglutamine diseases. a X-ray crystal structure of sperm whale myoglobin. Arrow The C-D corner into which a glutamine repeat was inserted; stick model a heme group and a histidine legend. b Amino acid sequence showing the region of polyglutamine insertion. c An electron microscopic image of Mb-Gln50 amyloid fibers that were spontaneously formed under the physiological condition (pH 7.0, 37°C). Mb-Gln50 fibers were stained with 2% sodium phosphotungstic acid and observed by electron microscopy. Scale bar 100 nm. (Reproduced from [73])
Trehalose alleviates polyglutamine-mediated pathology in a mouse model of HD
Since Mb-Gln35 can be prepared with high yield and reacts with the 1C2 antibody, we screened for inhibitors of polyglutamine-mediated aggregation in vitro using Mb-Gln35 and evaluated candidate molecules in cellular and transgenic mouse models of HD [75]. In the initial screening in vitro, we focused on small molecules that are nontoxic and can be safely and orally administered. Through the screening in vitro we found that various disaccharides have a potential to reduce aggregation of Mb-Gln35. Among disaccharides trehalose showed the most inhibitory effects for the Mb-Gln35 aggregation. GdHCl-induced denaturing experiments revealed that trehalose increases the stability of Mb-Gln35 to a level comparable to that of Mb-Gln12, which contains a nonpathological length of glutamine repeat. Since Mb-Gln35 is partially unfolded due to the insertion of a long polyglutamine repeat [73], the increased stability of Mb-Gln35 by trehalose is consistent with the previous finding that trehalose stabilizes proteins in a partially unfolded state [76].
The effects of disaccharides were further investigated in stable mouse neuroblastoma Neuro2a cells, where expression of truncated N-terminal huntingtin (1–90 amino acids) containing 60 or 150 glutamines fused to an enhanced green fluorescence protein (EGFP) is induced by ponasterone A [77]. In the Neuro2a cells huntingtin-EGFP forms cytoplasmic aggregates, and cell death is induced upon expression of huntingtin-EGFP. Addition of disaccharides to the cell culture show a tendency to reduce huntingtin-EGFP aggregation, and this is correlated with a decrease in cell death. In the screening of disaccharides trehalose shows the most beneficial effects on the cellular model of HD. To confirm this result we transiently overexpressed Escherichia coli otsA and otsB, which produce trehalose intracellularly [78], in the Neuro2a cellular model. The overexpression of otsA and otsB reduced the huntingtin-EGFP aggregation and enhanced cell viability by more than 50%. This result verified the neuroprotective effects of trehalose in the cellular model of HD.
We then explored the possible beneficial effects of trehalose on R6/2 transgenic mice in which N-terminal truncated huntingtin with a 145 glutamine repeat is overexpressed [39]. When trehalose (2%) was added to drinking water for R6/2 mice, these mice spontaneously drank the trehalose-containing water. We found that the oral administration of trehalose reduced weight loss, ameliorated striatal atrophy, and inhibited the formation of truncated huntingtin aggregates in the cerebrum and liver by more than 30% (Fig. 3a). In addition, the trehalose treatment improved the associated motor dysfunction (Fig. 3b), delayed the onset and reduced the frequency of foot clasping, a cardinal phenotype of R6/2 mice (Fig. 3c), and extended life span by approximately 10% (Fig. 3d). In contrast, oral administration of glucose, a potential metabolite of trehalose, did not change the number of intranuclear inclusions, improve rotarod performance, influence the onset and frequency of foot clasping, or increase survival [75]. Thus we conclude that treatment with trehalose specifically ameliorates the polyglutamine-mediated pathology.
Beneficial effects of trehalose on polyglutamine-mediated pathology in R6/2 transgenic mice. a Number of intranuclear aggregates (in mm2) in motor cortex of R6/2 transgenic mice. Huntingtin aggregates in frozen sections of cerebrum were visualized with an antibody to ubiquitin. Black bars 8-week-old R6/2 transgenic mice; gray bars 12-week-old R6/2 transgenic mice. *P<0.01 vs. 0% R6/2 transgenic mice. b Latency time of rotarod performance (seconds). Transgenic mice were placed on a rotating rod (3.5 rpm) and the rotating speed was linearly increased to 35 rpm in 300 s (for 7- to 11-week-old mice) and continued at 35 rpm until 600 s (for 4-week old mice). Circle 0% trehalose mice; triangle 2% trehalose mice. *P<0.05 vs. 0% R6/2 transgenic mice. c Frequency of the feet-clasping behavior; 5- to 7-week-old R6/2 transgenic mice were suspended by the tail for 30 s, and frequency of the feet-clasping posture was scored as follows: 3 (>10 s), 2 (5–10 s), 1 (0–5 s), 0 (0 s). Circle 0% trehalose mice; triangle 2% trehalose mice. *P<0.05 vs. 0% R6/2 transgenic mice. d Survival curves of R6/2 transgenic mice. P=0.0015 by log-rank test. Circle 0% trehalose mice; triangle 2% trehalose mice. Throughout the experiment trehalose (2%) was given to R6/2 transgenic mice by oral administration starting at approx. 21 days of age and continuing until the date of killing. (Reproduced from [75])
The alleviation of polyglutamine-mediated pathology by trehalose is correlated with the decrease in the number of intranuclear inclusions. Whether intranuclear inclusions are crucial for pathophysiology is still much disputed [10]. Regardless of this, our present finding as well as previous results using mouse and Drosophila models of HD [21, 22, 61, 62, 79] verifies that the inhibition of huntingtin aggregation can be a therapeutic strategy [14]. The inhibitory effects of trehalose on polyglutamine-induced aggregation in two different contexts of proteins (Mb-Gln35 and truncated huntingtin) suggest that trehalose can bind to polyglutamine expansions shared by the two proteins. Trehalose reduced the aggregation of Mb-Gln35 by increasing stability of the partially unfolded Mb-Gln35 in vitro. In vivo we detected trehalose in the brain homogenates of trehalose-supplemented mice. It thus appears that the incorporated trehalose exerts beneficial effects by binding to and stabilizing truncated huntingtin in the brain, although we cannot completely rule out the possibility that the alleviation of neuropathology is related to the effects of trehalose outside the neuron or the brain. Piccioni et al. [80] recently reported that a cardiac glycoside class of drugs inhibits polyglutamine-mediated toxicity by preventing caspase-3 activation. Thus saccharide molecules may have broad functions that reduce cellular toxicity. It is noted here that saccharide molecules possibly have adverse effects on blood glucose levels when administered. R6/2 mice are known to develop diabetes, a feature that mimics the elevated diabetes rate in individuals with HD [81, 82]. Although oral administration of trehalose did not substantially affect blood glucose levels of fasting R6/2 mice, this will need to be assessed carefully in clinical tests for HD patients.
Some of trehalose that is orally administered can be enzymatically hydrolyzed into two glucose molecules, which may have some effects on R6/2 mice. When glucose was orally administered to R6/2 mice, it did not attenuate motor impairment or delay mortality of R6/2 mice, indicating that the treatment with glucose does not ameliorate the polyglutamine-induced pathology of the mice [75]. Thus it is most plausible that the beneficial effects on R6/2 mice results from trehalose, not from glucose metabolized. Furthermore, based on this result it would be unlikely that trehalose alleviates the pathology of R6/2 mice simply by boosting intracellular energy levels. We are currently investigating in more detail the mechanism of the trehalose-mediated improvement in neuropathology. We are attempting to knock-out a gene encoding trehalase, an enzyme that hydrolyzes trehalose into two glucose molecules, in R6/2 mice so that trehalose administered remains stable in the mice. An alternate method is to synthesize trehalose analogs that are not enzymatically metabolized by trehalase and can be used for oral administration. These strategies will not only reveal more detailed effects of trehalose but will also lead to the development of more practical therapeutic agents. Another question to be answered is whether trehalose, as with Congo red [22], inhibits formation of oligomeric species (or microaggregates) of polyglutamine-containing proteins. Although we could not detect oligomeric states of truncated huntingtin in the brain of R6/2 mice [75], we found that Mb-Gln50 forms microaggregate species in the early stage of the aggregate (amyloid) formation [35]. Thus we are addressing this issue by investigating effects of trehalose on the aggregation process of Mb-Gln50.
A general therapeutic strategy of stabilizing aggregation-prone proteins and its future prospects
Our in vitro and in vivo studies suggest that trehalose inhibits aggregation of polyglutamine-containing proteins by interacting with expanded polyglutamines and stabilizing the polyglutamine-containing proteins. The polyglutamine-containing proteins undergo proteolysis by caspases [21, 46, 77, 83, 84], calpain [85, 86], or a novel aspartyl protease [87]. Cleaved fragments such as truncated N-terminal huntingtin show a virtually unordered structure [5] whereas causative full-length proteins would be only partially unfolded due to the expansion of glutamine repeats [69, 73]. Because trehalose stabilizes proteins in a partially unfolded state [76], it is likely that trehalose stabilizes the full-length proteins (before proteolysis) more efficiently than the fully unfolded truncated forms. In contrast to the destabilizing effects of polyglutamine expansion indicated by our results [73] and others [69], no significant difference was observed in an acid-induced equilibrium or the kinetic unfolding/folding transition between ataxin-3 proteins carrying 15, 28, or 50 glutamine repeats [88]. Since the polyglutamine tract in ataxin-3 protein is located in a flexible domain different from an N-terminal folded Josephin domain, it is conceivable that release of a polygutamine-containing domain upon proteolytic cleavage enhances potential destabilizing effects of the polyglutamine expansion on the truncated protein. Thus trehalose may stabilize the truncated form of ataxin-3 protein efficiently.
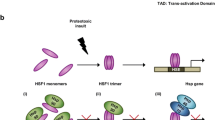
A previous study indicated that translocation of polyglutamine aggregates to the nucleus is essential to cytotoxicity [89]. A fibrillar form of a polyglutamine (Gln42) peptide caused dramatic cell death when directed to the nucleus but was not toxic when restricted to the cytoplasm. This translocation occurs after full-length polyglutamime-containing proteins undergo proteolysis. The translocation of truncated huntingtin can be inhibited by trehalose because the increase in stability of polyglutamine-containing proteins by trehalose would make the proteins more resistant to proteolysis, preventing eventual translocation to the nucleus. Therefore it is noteworthy that, apart from other types of inhibitors, trehalose has the potential to delay the disease progression at the initial stage of the disease cascade by stabilizing aggregation-prone polyglutamine-containing proteins and inhibiting their subsequent aggregation (Fig. 4).
A possible therapeutic strategy for polyglutamine diseases with chemical cheperones. Trehalose stabilizes aggregation-prone polyglutamine (polyQ) containing proteins and inhibits their aggregation (purple arrows). An increase in the protein stability by trehalose also could render the polyglutamine-containing proteins more resistant to proteolysis by various proteases and prevent subsequent translocation of the cleaved fragments containing expanded polyglutamines into the nucleus, where the truncated proteins interact with transcription factors and induce cellular dysfunction. This strategy of stabilizing aggregation-prone causative proteins targets the initial stage of the polyglutamine-mediated disease cascade (black arrows) and can be a general therapeutic approach for a wide variety of misfolding-induced diseases
Huntingtin aggregation can be suppressed by chemical compounds that activate a specific heat-shock response. Cell culture studies showed that treatment of mammalian cells with geldanamycin, a naturally occurring benzoquinone ansamycin that binds to heat-shock protein (Hsp) 90 and activates a heat shock response, induces the expression of Hsp40 and Hsp70, and inhibits huntingtin-exon 1 protein aggregation in a dose-dependent manner [90]. In the R6/2 mouse model of HD it has been proposed that a progressive decrease in HDJ1, HDJ2, Hsp70, and small glutamine-rich tetratricopeptide repeat domain proteins α and β levels in the brain contributes to the pathogenesis of HD [91]. Pharmacological induction of the heat-shock response with radicicol and geldanamycin in R6/2 mice could both maintain chaperone induction for at least 3 weeks and alter detergent insoluble properties of polyglutamine aggregates [91]. In addition to the protective effects on R6/2 mice, geldanamycin treatment prevents dopaminergic cell loss in a Drosophila model of Parkinson’s disease and inhibits α-synuclein aggregation and cytotoxicity in human H4 neuroglioma cells [92, 93]. While trehalose does not induce the Hsps [75], it could play similar roles of inhibiting huntingtin aggregation as a chemical chaperone.
Together with the observation for TTR amyloidosis [16, 17], the inhibition of unfolding and subsequent aggregation of aggregation-prone proteins by stabilization with small molecules could be a general strategy for various misfolding-induced diseases [18]. However, it is possible that nonspecific binding of small molecules induces adverse effects. Thus further efforts toward polyglutamine diseases including HD should be directed to search for chemical chaperones that selectively bind causative proteins. Since structural analysis of polyglutamine-containing proteins is difficult due to the poor solubility of these proteins, computational design cannot be applied to aggregation inhibitors for polyglutamine dieseases. High-throughput screening and subsequent investigation of candidate molecules in cellular, Drosophila and/or mouse models is thus a feasible way to search for aggregation inhibitors. Once an effective molecule is identified, it may be used as a lead compound to find more efficient inhibitors. For instance, trehalose is an appropriate lead compound because it is nontoxic and highly soluble in water. In addition, combinations of identified small molecules would be a practical therapeutic strategy to ameliorate the disease progression by targeting multiple pathways.
Abbreviations
- CI2 :
-
Chymotrypsin inhibitor 2
- EGFP :
-
Enhanced green fluorescence protein
- GdHCl :
-
Guanidine hydrochloride
- GST :
-
Glutathione S transferase
- HD :
-
Huntington disease
- Hsp :
-
Heat-shock protein
- Mb :
-
Myoglobin
- MBP :
-
Maltose-binding protein
- mTOR :
-
Mammalian target of rapamycin
- TTR :
-
Transthyretin
References
Zoghbi HY, Orr HT (2000) Glutamine repeats and neurodegeneration. Annu Rev Neurosci 23:217–247
White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, MacDonald ME (1997) Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet 17:404–410
Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A (1995) Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat Genet 11:155–163
Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F (2004) Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118:127–138
Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE (1997) Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90:549–558
Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548
DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993
Lunkes A, Mandel JL (1998) A cellular model that recapitulates major pathogenic steps of Huntington’s disease. Hum Mol Genet 7:1355–1361
Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM (1998) Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93:939–949
Michalik A, Van Broeckhoven C (2003) Pathogenesis of polyglutamine disorders: aggregation revisited. Hum Mol Genet 12:R173–R186
Mccampbell A, Fischbeck KH (2001) Polyglutamine and CBP: fatal attraction? Nat Med 7:528–530
Ross CA, Poirier MA, Wanker EE, Amzel M (2003) Polyglutamine fibrillogenesis: the pathway unfolds. Proc Natl Acad Sci U S A 100:1–3
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10[Suppl]:S10–S17
Bates G (2003) Huntingtin aggregation and toxicity in Huntington’s disease. Lancet 361:1642–1644
Miroy GJ, Lai Z, Lashuel HA, Peterson SA, Strang C, Kelly JW (1996) Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc Natl Acad Sci U S A 93:15051–15056
Hammarstrom P, Schneider F, Kelly JW (2001) Trans-suppression of misfolding in an amyloid disease. Science 293:2459–2462
Hammarstrom P, Wiseman RL, Powers ET, Kelly JW (2003) Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science 299:713–716
Cohen FE, Kelly JW (2003) Therapeutic approaches to protein-misfolding diseases. Nature 426:905–909
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ (1999) Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci 19:2522–2534
Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS, Li XJ, Stieg PE, Yuan J, Penney JB, Young AB, Cha JH, Friedlander RM (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399:263–267
Sanchez I, Mahlke C, Yuan J (2003) Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421:373–379
Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY (1998) Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet 19:148–154
Jana NR, Tanaka M, Wang GH, Nukina N (2000) Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet 9:2009–2018
Shimohata T, Nakajima T, Yamada M, Uchida C, Onodera O, Naruse S, Kimura T, Koide R, Nozaki K, Sano Y, Ishiguro H, Sakoe K, Ooshima T, Sato A, Ikeuchi T, Oyake M, Sato T, Aoyagi Y, Hozumi I, Nagatsu T, Takiyama Y, Nishizawa M, Goto J, Kanazawa I, Davidson I, Tanese N, Takahashi H, Tsuji S (2000) Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat Genet 26:29–36
Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, Krainc D (2002) Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science 296:2238–2243
Perez MK, Paulson HL, Pendse SJ, Saionz SJ, Bonini NM, Pittman RN (1998) Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J Cell Biol 143:1457–1470
Donaldson KM, Li W, Ching KA, Batalov S, Tsai CC, Joazeiro CA (2003) Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates. Proc Natl Acad Sci U S A 100:8892–8897
Nagaoka U, Kim K, Jana NR, Doi H, Maruyama M, Mitsui K, Oyama F, Nukina N (2004) Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J Neurochem 91:57–68
Doi H, Mitsui K, Kurosawa M, Machida Y, Kuroiwa Y, Nukina N (2004) Identification of ubiquitin-interacting proteins in purified polyglutamine aggregates. FEBS Lett 571:171–176
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298
Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81:678–699
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810
Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA (2002) Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem 277:41032–41037
Tanaka M, Machida Y, Nishikawa Y, Akagi T, Hashikawa T, Fujisawa T, Nukina N (2003) Expansion of polyglutamine induces the formation of quasi-aggregate in the early stage of protein fibrillization. J Biol Chem 278:34717–34724
Iuchi S, Hoffner G, Verbeke P, Djian P, Green H (2003) Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine. Proc Natl Acad Sci U S A 100:2409–2414
Schaffar G, Breuer P, Boteva R, Behrends C, Tzvetkov N, Strippel N, Sakahira H, Siegers K, Hayer-Hartl M, Hartl FU (2004) Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell 15:95–105
Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ (2004) Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol 11:1215–1222
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PS, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marksi PA, Bates GP (2003) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A 100:2041–2046
Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM (2003) Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci 23:9418–9427
Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, Ryu H, Langley B, Ratan RR, Ferrante RJ, Beal F (2005) Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington’s disease. J Biol Chem 280:556–563
Steffan JS, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu YZ, Gohler H, Wanker EE, Bates GP, Housman DE, Thompson LM (2000) The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci U S A 97:6763–6768
Nucifora FC Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291:2423–2428
Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413:739–743
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM (2003) Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A 100:10483–10487
Smith DL, Woodman B, Mahal A, Sathasivam K, Ghazi-Noori S, Lowden PA, Bates GP, Hockly E (2003) Minocycline and doxycycline are not beneficial in a model of Huntington’s disease. Ann Neurol 54:186–196
Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A 99:10671–10676
Ferrante RJ (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci 20:4389–4397
Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF (2001) Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol Dis 8:479–491
Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, Ferrante RJ (2003) Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J Neurochem 85:1359–1367
Andreassen OA, Ferrante RJ, Huang HM, Dedeoglu A, Park L, Ferrante KL, Kwon J, Borchelt DR, Ross CA, Gibson GE, Beal MF (2001) Dichloroacetate exerts therapeutic effects in transgenic mouse models of Huntington’s disease. Ann Neurol 50:112–117
Schilling G, Coonfield ML, Ross CA, Borchelt DR (2001) Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neurosci Lett 315:149–153
Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF (2002) Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci 22:1592–1599
Schiefer J, Landwehrmeyer GB, Luesse HG, Sprunken A, Puls C, Milkereit A, Milkereit E, Kosinski CM (2002) Riluzole prolongs survival time and alters nuclear inclusion formation in a transgenic mouse model of Huntington’s disease. Mov Disord 17:748–757
Wood NI, Morton AJ (2003) Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington’s disease mutation. Brain Res Bull 61:375–383
Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF (2001) Lipoic acid improves survival in transgenic mouse models of Huntington’s disease. Neuroreport 12:3371–3373
Klivenyi P, Ferrante RJ, Gardian G, Browne S, Chabrier PE, Beal MF (2003) Increased survival and neuroprotective effects of BN82451 in a transgenic mouse model of Huntington’s disease. J Neurochem 86:267–272
Ferrante RJ, Ryu H, Kubilus JK, D’Mello S, Sugars KL, Lee J, Lu P, Smith K, Browne S, Beal MF, Kristal BS, Stavrovskaya IG, Hewett S, Rubinsztein DC, Langley B, Ratan RR (2004) Chemotherapy for the brain: the antitumor antibiotic mithramycin prolongs survival in a mouse model of Huntington’s disease. J Neurosci 24:10335–10342
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595
Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ (2002) Therapeutic effects of cystamine in a murine model of Huntington’s disease. J Neurosci 22:8942–8950
Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L (2002) Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med 8:143–149
Heiser V, Scherzinger E, Boeddrich A, Nordhoff E, Lurz R, Schugardt N, Lehrach H, Wanker EE (2000) Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implication for Huntingtin’s disease therapy. Proc Natl Acad Sci U S A 97:6739–6744
Hughes RE, Olson JM (2001) Therapeutic opportunities in polyglutamine disease. Nat Med 7:419–423
Heiser V, Engemann S, Brocker W, Dunkel I, Boeddrich A, Waelter S, Nordhoff E, Lurz R, Schugardt N, Rautenberg S, Herhaus C, Barnickel G, Bottcher H, Lehrach H, Wanker EE (2002) Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington’s disease by using an automated filter retardation assay. Proc Natl Acad Sci U S A 99:16400–16406
Sharma D, Sharma S, Pasha S, Brahmachari SK (1999) Peptide models for inherited neurodegenerative disorders: conformation and aggregation properties of long polyglutamine peptides with and without interruptions. FEBS Lett 456:181–185
Chen S, Wetzel R (2001) Solubilization and disaggregation of polyglutamine peptides. Protein Sci 10:887–891
Bevivino AE, Loll PJ (2001) An expanded glutamine repeat destabilizes native ataxin-3 structure and mediates formation of parallel beta-fibrils. Proc Natl Acad Sci U S A 98:11955–11960
Ordway JM, Tallaksen-Greene S, Gutekunst CA, Bernstein EM, Cearley JA, Wiener HW, Dure LS 4th, Lindsey R, Hersch SM, Jope RS, Albin RL, Detloff PJ (1997) Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell 91:753–763
Stott K, Blackburn JM, Butler PJ, Perutz M (1995) Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc Natl Acad Sci U S A 92:6509–6513
Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, Saudou F, Weber C, David G, Tora L, Agid Y, Brice A, Mandel JL (1995) Polyglutamine expansion as a pathological epitope in Huntington’s disease and four dominant cerebellar ataxias. Nature 378:403–406
Tanaka M, Morishima I, Akagi T, Hashikawa T, Nukina N (2001) Intra-and intermolecular β-pleated sheet formation in glutamine-repeat inserted myoglobin as a model for polyglutamine diseases. J Biol Chem 276:45470–45475
Tanaka M, Machida Y, Nishikawa Y, Akagi T, Morishima I, Hashikawa T, Fujisawa T, Nukina N (2002) The effects of aggregation-inducing motifs on amyloid formation of model proteins related to neurodegenerative diseases. Biochemistry 41:10277–10286
Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, Kurosawa M, Nekooki M, Nukina N (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10:148–154
Singer MA, Lindquist S (1998) Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1:639–648
Wang GH, Mitsui K, Kotliarova S, Yamashita A, Nagao Y, Tokuhiro S, Iwatsubo T, Kanazawa I, Nukina N (1999) Caspase activation during apoptotic cell death induced by expanded polyglutamine in N2a cells. Neuroreport 10:2435–2438
Guo N, Puhlev I, Brown DR, Mansbridge J, Levine F (2000) Trehalose expression confers desiccation tolerance on human cells. Nat Biotechnol 18:168–171
Kazantsev A, Walker HA, Slepko N, Bear JE, Preisinger E, Steffan JS, Zhu YZ, Gertler FB, Housman DE, Marsh JL, Thompson LM (2002) A bivalent huntingtin binding peptide surpresses polyglutamine aggregation and pathogenesis in Drosophila. Nat Genet 30:367–376
Piccioni F, Roman BR, Fischbeck KH, Taylor JP (2004) A screen for drugs that protect against the cytotoxicity of polyglutamine-expanded androgen receptor. Hum Mol Genet 13:437–446
Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR (1999) Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes 48:649–651
Farrer A (1985) Diabetes mellitus in Huntington’s disease. Clin Genet 27:62–67
Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thornberry N, Pinsky L, Kakizuka A, Ross CA, Nicholson DW, Bredesen DE, Hayden MR (1998) Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem 273:9158–9167
Wellington CL, Ellerby LM, Gutekunst CA, Rogers D, Warby S, Graham RK, Loubser O, van Raamsdonk J, Singaraja R, Yang YZ, Gafni J, Bredesen D, Hersch SM, Leavitt BR, Roy S, Nicholson DW, Hayden MR (2002) Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington’s disease. J Neurosci 22:7862–7872
Gafni J, Ellerby LM (2002) Calpain activation in Huntington’s disease. J Neurosci 22:4842–4849
Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, Qin ZH, Aronin N, DiFiglia M (2001) Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington’s disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A 98:12784–12789
Lunkes A, Lindenberg KS, Ben-Haiem L, Weber C, Devys D, Landwehrmeyer GB, Mandel JL, Trottier Y (2002) Proteases acting on mutant huntingtin generate cleaved products that differentially build up cytoplasmic and nuclear inclusions. Mol Cell 10:259–269
Chow MK, Ellisdon AM, Cabrita LD, Bottomley SP (2004) Polyglutamine expansion in ataxin-3 does not affect protein stability: implications for misfolding and disease. J Biol Chem 279:47643–47651
Yang W, Dunlap JR, Andrews RB, Wetzel R (2002) Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet 11:2905–2917
Sittler A, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, Hartl FU, Wanker EE (2001) Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease. Hum Mol Genet 10:1307–1315
Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, Mestril R, Mahal A, Smith DL, Woodman B, Bates GP (2004) Progressive decrease in chaperone protein levels in a mouse model of Huntington’s disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet 13:1389–1405
Auluck PK, Bonini NM (2002) Pharmacological prevention of Parkinson disease in Drosophila. Nat Med 8:1185–1186
McLean PJ, Klucken J, Shin Y, Hyman BT (2004) Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro. Biochem Biophys Res Commun 321:665–669
Acknowledgements
We thank S. Collins and L. Osherovich for their comments about the manuscript. This study was partly supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology (M.T. and N.N.), and of Health, Labor, and Welfare (N.N.), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, M., Machida, Y. & Nukina, N. A novel therapeutic strategy for polyglutamine diseases by stabilizing aggregation-prone proteins with small molecules. J Mol Med 83, 343–352 (2005). https://doi.org/10.1007/s00109-004-0632-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-004-0632-2