Abstract
Tie2 is expressed predominantly in endothelial cells and is required for blood vessel formation and maintenance. A missense mutation resulting in an R to W substitution in the kinase domain of Tie2 co-segregates with an autosomal dominantly inherited form of vascular dysmorphogenesis, venous malformation (VM). The mechanism by which this activating mutation leads to vessel dysmorphogenesis in VM is not known. Here we examined Tie2 activation status in VM and found activated receptor in lesional and non-lesional vessels. To gain insight into functional effects of VM mutant Tie2, wild-type and R849W mutant receptor were expressed in cultured human venous endothelial cells. Mutant Tie2 was constitutively phosphorylated in endothelial cells in vivo and caused a marked suppression of apoptosis. The anti-apoptotic kinase Akt was constitutively activated in cells expressing mutant receptor. Dominant-negative Akt inhibited the pro-survival activity of mutant Tie2. Migration of smooth muscle cells induced by conditioned medium from cells expressing mutant receptor was similar to that from cells expressing wild-type receptor. These data suggest that a primary effect of R849W Tie2 in VM is to allow survival of mural cell poor vessels via ligand-independent Tie2 activation of Akt and endothelial survival, rather than to directly induce formation of dysmorphogenic vessels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular development is initiated by the process of vasculogenesis, in which endothelial precursor cells differentiate and organize into primitive vascular tubes in situ. Sprouting from these tubes via angiogenesis, involving endothelial cell migration and proliferation, in combination with intussusception, leads to growth and organization of the vascular tree [1, 2]. These processes are tightly controlled by a complex network of soluble and non-diffusible factors, including vascular endothelial growth factor and the angiopoietins, extracellular matrix proteins and angiostatic molecules [3, 4]. Although there have been major advances in uncovering critical controlling aspects of vasculogenesis and angiogenesis, understanding the process of vessel assembly and maintenance still remains a significant challenge. In particular, the signalling pathways controlling vessel stability, maintenance and remodelling in postnatal vasculature are poorly understood.
Inherited diseases of the vasculature can provide insights into the process of vessel formation. Importantly, where these anomalies are not developmentally lethal, they can also illuminate mechanisms involved in postnatal vascular maintenance and remodelling. Venous malformations (VM) are common vascular anomalies manifesting as a vascular mass composed of dilated channels lined by endothelial cells [5, 6]. Vessels of VM have an abnormal ratio of endothelial to smooth muscle cells, with very few supporting smooth muscle cells [5, 7] leading to a functionally low resistance vessel. The majority of VM are sporadic, although the anomaly can be inherited [5, 6]. An autosomal dominantly inherited form of VM (designated venous malformations, multiple cutaneous and mucosal; OMIM number 600195) has been shown to segregate with the same C2545T transition in the coding sequence of the receptor tyrosine kinase Tie2 in two unrelated families [7]. Another independent study of inherited VM found a third unrelated family in which the disease co-segregated with the same mutation [8], making this transition the single most common mutation detected in inherited VM.
Tie2 is expressed predominantly by endothelial cells and is essential for vessel formation where it is involved in microvessel sprouting, integrity and maturation [9]. Amongst other effects, Tie2 activation in cultured cells promotes monolayer integrity and inhibits endothelial apoptosis [10, 11, 12, 13]. A family of ligands, designated the angiopoietins, have been identified for Tie2. The best characterized of these are Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2). Ang-1 is a stimulatory ligand expressed by mesenchymal cells and smooth muscle cells associated with vessels [14, 15], whereas Ang-2 can act as a naturally occurring antagonist [16].
The C2545T transition in the Tie2 coding sequence, which has been linked to VM, results in substitution of an arginine at position 849 for a tryptophan (R849W) in the kinase domain of the receptor. The effects of this mutant Tie2 on endothelial function are not known. Analysis of the VM mutant R849W Tie2 expressed in insect cells revealed it to be hyper-phosphorylated and have elevated tyrosine kinase activity compared with wild-type receptor [7]. If and how this results in the development of VM is yet to be defined, although the relative lack of abluminal support cells has led to the suggestion that endothelial expression of the mutant receptor leads to defective recruitment of and/or interaction with mural cells [7].
Materials and methods
Materials
cDNA encoding human Tie2 and R849W VM mutant Tie2 were obtained from the American Tissue Culture Collection and subcloned into the expression vector pCR3 (Invitrogen Life Technologies, Paisley, UK). An expression vector encoding dominant-negative Akt, K179A/T308A/S473A, was kindly provided by Dr. B.A. Hemmings [17]. Antisera against the extracellular domain of Tie2 was from R & D Systems (Abingdon, UK), anti-phosphotyrosine antibodies were from BD Transduction Laboratories (San Jose, Calif., USA) phospho-Akt (S473/T308) and Akt antibodies were from Cell Signaling Technology (Beverly, Mass., USA) and anti-phospho-Tie2 (Y1094/1102) was from Oncogene Research Products (San Diego, Calif., USA). Human umbilical vein endothelial cells were cultured and transfected as detailed previously [18]. Transfection efficiency, as judged by expression of green fluorescent protein was routinely greater than 40%. Human saphenous vein smooth muscle cells were obtained and cultured as described previously [19].
Immunohistochemistry
Biopsy material from VM was obtained with informed consent from one of the patients described previously with the mutation giving rise to R849W Tie2 [8]. Immunohistochemistry was performed on 5-µm sections essentially as detailed previously [20]. Primary antibodies were detected with biotin-conjugated secondary antibodies and the Chemmate/Envsion Detection System (Dako, Cambridge UK). Negative controls with non-specific primary antibodies were included.
Apoptosis and cell survival assays
Assessment of survival and apoptosis was performed in transfected cells as described previously [21]. Briefly, cells were transfected with control vector, wild-type Tie2 or R849W Tie2, together with green fluorescent protein, to identify transfected cells. Apoptotic index was determined in transfected cells following staining with 4′6-diamidino-2-phenylindole at 0.2 µg/ml. In parallel cultures cell survival was determined by counting numbers of viable transfected cells in ten designated areas in gridded tissue culture wells before and after growth factor deprivation. Cell viability was routinely determined by exclusion of 0.2% trypan blue.
Immunoprecipitation and western blotting
Immunoprecipitation and western blotting was performed as previously described [22]. Briefly, endothelial cells were washed in PBS and lysed at 4°C in 0.5 ml of lysis buffer (50 mM Tris, pH 7.4, containing 50 mM NaCl, 1% Triton X100, 1 mM sodium orthavanadate, 1 mM sodium fluoride, 1 mM EGTA and Complete protease inhibitor cocktail). For immunoprecipitation lysates were cleared by centrifugation (13,000 g for 10 min at 4°C). Immunoprecipitations were performed by adding 0.4 µg/ml antibody to the lysate and incubating with rotation at 4°C for 2 h. Pre-washed protein A-Sepharose was added to the protein-antibody complex and incubated for a further 2 h. Immunoprecipitates were washed four times with lysis buffer. Proteins were eluted by boiling in Laemmli sample buffer containing 100 mM dithiothreitol and resolved by sodium dodecyl sulphate gel electrophoresis. Proteins were electrophoretically transferred onto nitrocellulose membranes and probed with appropriate antibodies. Immunoreactive proteins were visualized with a peroxidase conjugated secondary antibody and a chemiluminescent detection system [23].
Smooth muscle cell migration
Monolayers of endothelial cells transfected with empty vector, wild-type Tie2 or R849W Tie2 were washed extensively with PBS and incubated for 48 h in serum-free Dulbecco’s modified Eagle’s medium containing 1 mg/ml BSA. Conditioned media and identical media that had not been conditioned were centrifuged at 2500 g for 15 min to remove any particulate material. Media was placed in the lower chamber of Transwell tissue culture wells containing 8-µm pore size inserts. Smooth muscle cells (1×105) suspended in serum-free Dulbecco’s modified Eagle’s medium were placed in the top chamber of the inserts and cells were allowed to migrate for 6 h at 37°C. Smooth muscle cells on both sides of the insert membrane were fixed in 70% ethanol at −25°C and cells stained with haematoxylin/eosin. Filters were mounted in glycerol and the numbers of cells migrating through the porous membrane were counted at ×400 magnification in ten high-power fields on the underside of each insert membrane.
Results
Tie2 is activated in VM lesional and non-lesional vessels
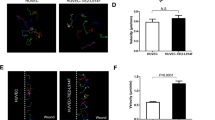
VM associated with R849W mutant Tie2 are characterized by enlarged and dilated vascular channels with a relative lack of mural cell support compared with non-lesional vessels (Fig. 1A, E). Segments of the lesional vessels are devoid of mural cells whereas in other areas mural cells are disorganized. The R849W mutant Tie2 has constitutively elevated kinase activity and increased receptor autophosphorylation in vitro which has been implicated in the pathogenesis of the vessel defects that characterize VM [7]. To examine the activation status of Tie2 in dysmorphogenic and non-dysmorphogenic vessels, sections were probed with an antibody that recognizes phosphorylated Tie2. Surprisingly, phospho-Tie2 was detected in the endothelial cells of both dysmorphogenic vessels and normal vessels and staining was of similar intensity in both normal and pathological vessels (Fig. 1B, F). Both Normal and VM vessels had similar levels of Tie2 immunopositivity (Fig. 1G, H). These observations suggest that the all-or-none activation of Tie2 as a result of the R849W mutation in VM vessels is not the basis of the vascular dysmorphogenesis.
Dysmorphogenic vessels in a patient with VM. Representative views are shown of dysmorphogenic (A–D) and non-dysmorphogenic (E–H) vessels from a patient with the mutation resulting in R848 W Tie2. Staining for the presence of smooth muscle cell actin reveals areas in which smooth muscle cells are disorganized (arrow) and areas with poor smooth muscle cover (asterisk) in the dilated lesional vessels (A) compared with uniform smooth muscle cell distribution (arrowhead) in non-dysmorphogenic vessels (E). Tyrosine phosphorylated Tie2 (B, F) and total Tie2 (C, G) is seen in both types of vessel. D, H Negative control sections. Scale bar 50 µm
R849W Tie2 constitutively suppresses endothelial cell death
To gain insight into the mechanisms by which mutation of Tie2 contributes to VM we investigated the functional impact of R849W mutant Tie2 in endothelial cells. Expression of R849W mutant Tie2 in human umbilical vein endothelial cells led to constitutively elevated receptor tyrosine phosphorylation (Fig. 2A). In comparison, over-expressed wild-type Tie2 showed relatively low levels of phosphorylation. Densitometric scanning of blots from three independent experiments and normalization of tyrosine phosphorylated Tie2 to Tie2 expression level revealed that R849W Tie2 exhibited a 4.4±0.24-fold higher level of tyrosine phosphorylation than wild-type Tie2. These results are consistent with previous studies in COS and Hek293 cells [8, 24] but demonstrate the effect in a more physiologically relevant endothelial background. In normal vessels Tie2 is maintained in an activated, phosphorylated, state by Ang-1 produced by mural cells [12, 25]. The present data suggest that even in the absence of mural cells to provide Ang-1 tyrosine phosphorylation of R849W mutant Tie2 would be able to occur.
VM mutant R849W Tie2 is tyrosine phosphorylated in endothelial cells. Human umbilical vein endothelial cells were transfected with plasmids encoding wild-type Tie2 (Wt), R849W mutant Tie2 (VM) or empty plasmid (Control). Cell lysates were resolved by sodium dodecyl sulphate gel electrophoresis and immunoblotted with antibodies recognizing phosphotyrosine. Membranes were stripped and Tie2 was detected by re-probing with antibodies recognizing Tie2 and β-actin as indicated. VM mutant Tie2 has increased levels of tyrosine phosphorylation
During new vessel formation nascent vessels require association with mural cells for survival; newly formed vessels that lack mural cell investment regress via endothelial apoptosis [26, 27]. Ang-1, expressed by the periendothelial cells, is thought to contribute to vessel stability via activation of Tie2 and suppression of endothelial apoptosis [12]. Thus it is surprising that although vessels of VM have a paucity of mural cells, the vessels do not regress. Therefore we hypothesized that the constitutive signalling that occurs as a result of the R849W mutation in Tie2 leads to endothelial survival in the absence of ligand. To examine this possibility endothelial cells were co-transfected with vectors encoding R849W Tie2 and green fluorescent protein. Two days post-transfection cells were switched to media lacking growth factors for 18 h, and the apoptotic index of the transfected cells was determined. In order to distinguish between the effects of mutant Tie2 and those of increased Tie2 expression per se parallel cultures of endothelial cells were transfected with wild-type receptor and green fluorescent protein and analysed similarly. Expression of R849W Tie2 significantly inhibited endothelial apoptosis (Fig. 3A), thus leading to markedly increased endothelial survival (Fig. 3B).
VM mutant R849W Tie2 inhibits endothelial cell death. Human umbilical vein endothelial cells were transfected with plasmids containing no insert (C), R849W VM mutant Tie2 (VM) or wild-type Tie2 (Wt). After 48 h cells were changed to serum-free medium for 18 h. The percentage of apoptotic cells and cell survival were determined in parallel cultures of transfected cells as described in the text. For comparison control transfected cells cultured with 10% serum (+S) are also shown. Data are presented as mean and SEM for at least three independent experiments. *P<0.05 (Student’s t test) vs. C. Expression of R849W VM mutant Tie2 significantly inhibits endothelial apoptosis and promotes endothelial survival
We also addressed the possibility that the R849W Tie2 leads to lack of mural cell investment as a result of decreased ability of endothelial cells to recruit smooth muscle cells. This was tested by examining the ability of serum-free medium conditioned for 48 h by endothelial cells expressing R849W Tie2 or wild-type Tie2 to induce migration of smooth muscle cells. We could find no difference in smooth muscle cell migration induced by medium conditioned by endothelial cells expressing R849W or wild-type Tie2 (data not shown).
R849W Tie2 signals via Akt to suppress endothelial death
Suppression of endothelial apoptosis following Ang-1 activation of wild-type Tie2 has been shown to involve the anti-apoptotic serine/threonine kinase Akt [12, 28]. The activation state of Akt was therefore examined in endothelial cells expressing R849W Tie2. Akt activation, as determined by immunodetection of phospho-S473/T308-Akt, was constitutively elevated in endothelial cells expressing the mutant receptor (Fig. 4). To test whether Akt is involved in the anti-apoptotic effect of R849W Tie2 a dominant-negative form of the kinase was co-expressed with the mutant receptor. Co-expression of the K179A/T308A/S473A dominant-negative Akt inhibited the anti-apoptotic and pro-survival effects of R849W Tie2 (Fig. 5A, B). These data indicate that R849W Tie2 constitutively activates Akt in endothelial cells and suggests that this serine/threonine kinase is required for the persistent pro-survival activity of the mutant receptor.
VM mutant R849W Tie2 activates Akt. HUVEC were transfected with plasmids containing no insert (C), wild-type Tie2 (Wt) or R849W VM mutant Tie2 (VM). After 48 h cells were changed to serum-free medium for 18 h and whole-cell lysates were prepared. Cellular proteins were resolved by sodium dodecyl sulphate gel electrophoresis and immunoblotted with antibodies recognizing phospho-S473/T308-Akt (P-Akt). Membranes were stripped and probed with antibodies recognizing and total Akt and Tie2. A Representative blot showing increased phospho-Akt in cells expressing R849W Tie2. B The relative levels of p-Akt/Akt were determined by densitometric scanning of blots from three independent experiments. The relative P-Akt/Akt for VM expressing cells is arbitrarily presented as 100. Data are shown as means and SEM for three independent experiments. *P<0.02 (Student’s t test) vs. control transfected cells. Activated phospho-Akt was significantly higher in endothelial cells expressing R849W VM mutant Tie2 compared with those expressing wild-type Tie2 and control transfected cells
Akt is required for the anti-apoptotic activity of VM mutant Tie2 in endothelial cells. Human umbilical vein endothelial cells were transfected with R849W mutant Tie2 (VM) and either control vector (CV) or K179A/T308A/S473A dominant-negative Akt (dn-Akt). After 48 h cells were changed to serum-free medium for 18 h. The percentage of apoptotic cells and cell survival were determined in parallel cultures as described in the text. For comparison the percentage apoptosis and cell survival of serum-deprived control transfected cells (C) are also shown. Data are presented as mean and SEM for at least three independent experiments. *P<0.05 (Student’s t test) vs. CV+VM. Expression of dominant-negative Akt inhibited the anti-apoptotic and pro-survival activity of R849W VM mutant Tie2
Discussion
In this study we have begun to explore the impact of mutant Tie2 (R849W) on endothelial functions with an emphasis on its potential role in the pathogenesis of VM. Although immunohistochemical analyses cannot detect subtle differences in the levels of expression or phosphorylation, our data indicate that Tie2 receptors are expressed and phosphorylated to similar levels in the endothelium of both normal and VM vessels. This suggests that receptor phosphorylation alone does not lead to the observed vessel defects. However, it should be remembered that immunohistochemical analysis was performed with an antibody recognizing Tie2 phsophorylated on tyrosines 1094 and 1102. As Tie2 undergoes phosphorylation on multiple tyrosine residues, it remains a possibility that the mutant receptor has increased phosphorylation on tyrosine residues other than those to which the immunohistochemical analyses was directed.
Based on our present observations we speculate that the mutant receptor circumvents the normal requirement for mural cell investment in vessel stabilization. Whereas mural cell-derived Ang-1 is needed to activate Tie2 and prevent apoptosis in endothelial cells of normal vessels, the mutant receptor is constitutively activated. The mutant receptor signals via Akt to suppress endothelial apoptosis. Thus the survival advantage conferred to endothelial cells expressing this mutant receptor may allow VM vessels to resist regression despite their lack of mural cell support. The lack of effect of R849W Tie2 on the ability of endothelial cells to chemoattract smooth muscle cells suggests that the relative lack of mural cells in VM vessels does not result from defects in recruitment of mural cells. In light of these data we speculate that the primary effect of R849W Tie2 is to allow the VM vessels to escape regression rather than to be directly responsible for the vascular defects. If this model is correct, an additional stimulus defect would be required for the initial formation of mural cell poor vessels. These vessels, if allowed to persist, would then develop into dilated vascular channels due to the lack of regulatory influence on size and structure normally mediated by mural cell interaction. The potential involvement of an additional stimulus defect may explain why in inherited VM the lesions are focal and often develop later in life. Finally, our observations also suggest that interference with the pro-survival activity of mutant Tie2, at the receptor level or downstream, may be a strategy for inducing regression of established VMs.
Abbreviations
- VM :
-
Venous malformation
References
Beck L, D’Amore PA (1997) Vascular development: cellular and molecular regulation. FASEB J 11:365–373
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Carmeliet P, Collen D (1998) Vascular development and disorders: molecular analysis and pathogenic insights. Kidney Int 53:1519–1549
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Mulliken JB, Young AE (1988) Vascular birthmarks: hemangiomas and vascular malformations. Saunders, Philadelphia
Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 69:412–420
Vikkula M, Boon LM, Carraway KLR, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR (1996) Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 87:1181–1190
Calvert JT, Riney TJ, Kontos CD, Cha EH, Prieto VG, Shea CR, Berg JN, Nevin NC, Simpson SA, Pasyk KA, Speer MC, Peters KG, Marchuk DA (1999) Allelic and locus heterogeneity in inherited venous malformations. Hum Mol Genet 8:1279–1289
Jones N, Iljin K, Dumont DJ, Alitalo K (2001) Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2:257–267
Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ (2001) Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J 20:5919–5928
Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA (2000) Angiopoietin-1 is an antipermeability and anti-Inflammatory agent in vitro and targets cell junctions. Circ Res 87:603–607
Kim I, Kim HG, So J-S, Kim JH, Kwak HJ, Koh GY (2000) Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Circ Res 86:24–29
Papapetropoulos A, García-Cardeña G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC (1999) Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest 79:213–223
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJC, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA (1997) Role of translocation in the activation and function of protein kinase B. J Biol Chem 272:31515–31524
Hughes DP, Marron MB, Brindle NPJ (2003) The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-κB inhibitor ABIN-2. Circ Res 92:630–636
White PJ, Kumari R, Porter KE, London NJ, Ng LL, Boarder MR (2000) Antiproliferative effect of UTP on human arterial and venous smooth muscle cells. Am J Physiol Heart Circ Physiol 279:H2735–H2742
Cotton JM, Thomas MR, Dunmore BJ, Salisbury J, Shah AM, Brindle NP (2002) Angiogenesis in chronically ischaemic human heart following percutaneous myocardial revascularisation. Heart 87:281–283
Tadros A, Hughes DP, Dunmore BJ, Brindle NPJ (2003) ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood 102:4407–4409
Marron MB, Hughes DP, Edge MD, Forder CL, Brindle NPJ (2000) Evidence for heterotypic interaction between the receptor tyrosine kinases TIE-1 and TIE-2. J Biol Chem 275:39741–39746
Matthews JA, Batki A, Hynds C, Kricka LJ (1985) Enhanced chemiluminescent method for the detection of DNA dot-hybridization assays. Anal Biochem 151:205–209
Korpelainen EI, Kärkkäinen M, Gunji Y, Vikkula M, Alitalo K (1999) Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene 18:1–8
Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG (1997) Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 81:567–574
Benjamin LE, Hemo I, Keshet E (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125:1591–1598
Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E (1999) Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103:159–165
Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC (2000) Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 275:9102–9105
Acknowledgements
This work was supported by grants from the Wellcome Trust (058695) to P.N.M. and N.P.J.B., Wellcome Trust Biomedical Collaboration Grant (061303/Z/00/Z) to N.P.J.B., grants from the Birth Defects Foundation (00/04) and Paton Masser Memorial Fund of the British Association of Plastic Surgeons to P.N.M., and a grant from the National Institutes of Health (EY05318) to P.A.D. P.A.D. is a Jules and Doris Stein Research to Prevent Blindness Professor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, P.N., Dunmore, B.J., Tadros, A. et al. Functional analysis of a mutant form of the receptor tyrosine kinase Tie2 causing venous malformations. J Mol Med 83, 58–63 (2005). https://doi.org/10.1007/s00109-004-0601-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-004-0601-9