Abstract
The present study provides a new concept for improving the properties of phenol-lignin-glyoxal (PLG) resins by lignin nanoparticles, a bio-nanomaterial. In the research work presented, lignin nanoparticles (10, 20, 30 and 40 wt%) were used instead of lignin for the phenol-lignin-glyoxal resin synthesis. The physicochemical and thermal properties of the prepared resins were then determined as well as the water absorption and mechanical properties (flexural modulus, flexural strength and internal bonding) of the particleboard panels bonded with them according to related standards methods. The addition of lignin nanoparticles appeared to change the physicochemical properties of the resins as the gelation time of the PLG resin was shorter and its viscosity increased. FTIR analysis indicated no differences in the chemical structure between the PLG resins with lignin and nanolignin. DSC analysis shows that the addition of lignin nanoparticles allows to decrease the curing temperature of the PLG resin from 170 °C to 159 °C. Based on panel analysis results, higher mechanical strength values and lower water absorption were obtained by progressively increasing the lignin nanoparticles proportion. The work presented here provides a new concept for the preparation of phenolic resins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phenol-formaldehyde (PF) resins are one of the most important types of wood adhesive for wood panel manufacturing. The advantages of PF resins as a wood adhesive have been reported by several researchers (e.g., Moubarik et al. 2009; Younesi-Kordkheili 2019; Pizzi and Mittal 2011). However, the health problems associated with the preparation of panels bonded with PF resins have led to increased attention being paid to developing ecofriendly adhesives of the same quality. In previous works, phenol-lignin-glyoxal (PLG) resins as wood adhesives of much lower toxicity and cost have been synthesized to bond wood panels in place of PF resins (Younesi-Kordkheili and Pizzi 2021; Younesi-Kordkheili and Pizzi 2018). Lignin has a much lower cost than phenol, and glyoxal has a much lower toxicity than formaldehyde. Hence, PLG resins are in general of lower toxicity and cost than PF resins. However, the mechanical properties and dimensional stability of panels bonded with PLG resins were not only lower than those bonded with PF resins but also needed a higher curing temperature. In industrial applications, higher curing temperature means that higher energy is needed, hence higher cost, which would render the adoption of such adhesives for industrial applications more difficult. Thus, the aim of the research work presented here is to improve the properties of PLG resins for bonding wood-based panels.
So far several methods have been proposed to improve the properties of wood-adhesives. One of the best methods so far proposed is the use of nanomaterials in wood adhesives. Previous research has shown that when reducing the materials to nanosize, strength as well as dimensional and thermal stability were significantly increased (Lei et al. 2010; Younesi-Kordkheili 2017). However, nanomaterials used in wood-based adhesives present the drawbacks of high cost and environmental problems. Hence, to find a new, high quality and inexpensive bionanomaterial for wood adhesives is of considerable interest.
Conversely, lignin as a main waste of the pulp and paper industry is just burnt to produce energy. The valorization of lignin nanoparticles and their nanocomposites have been extensively reviewed in recent years (Feldman 2016; Zhang et al. 2021). Lignin nanoparticles with different morphologies have been successfully synthesized by controlling the reaction conditions of solvent/anti-solvent, the lignin concentration, the temperature and pH of solution, etc. (Chen et al. 2019). Several researchers have inferred that lignin nanoparticles have potential applications in antioxidants, thermal/light stabilizers, reinforced materials and nano microcarriers owing to their advantages of no toxicity, environmental resistance, excellent thermal stability and biocompatibility. Chen et al. (2019) indicated that the board bonded with a nanolignin phenol formaldehyde resin, at a 40% by weight nanolignin substitution level of phenol could give a dry bond strength of 1.30 ± 0.08 MPa. This is 1.85 times higher than that of the requirements of the Chinese national grade 1 plywood standard (0.7 MPa) and the formaldehyde emission of 0.40 mg L− 1 also meets the GB/T 14732 − 2006 (E0, 0.5 mg L− 1) standard. Hence, in the present research, lignin nanoparticles were used to substitute a part of the lignin in PLG resins, and the properties of such resins and panels were investigated.
So far, extensive work has been conducted on the substitution of phenol by lignin to prepare LPF resins. However, there is no information in the literature on the effect of lignin nanoparticles on different properties of PLG resins. Thus, the aim of this research work was to investigate the influence of lignin nanoparticles on the properties of PLG resins and on the moisture resistance and mechanical strength of the panels bonded with them.
2 Materials and methods
2.1 Materials
Ethylene glycol, formaldehyde, phenol, and other chemical materials used in this experiment were all obtained from Merck & Co.
2.2 Methods
2.2.1 PF resin
The phenol-formaldehyde (PF) resin was prepared in a four neck round bottom flask equipped with stirrer, thermometer, condenser and dropping funnel according to a method already reported (Khan et al. 2004). Sufficient molten phenol, formaldehyde solution (formalin 37%) and each of distilled water and methanol were added to the reactor. The mixture was heated to 80 °C, then sodium hydroxide was added and the reaction was continued for 4 h at 80 °C. The molar ratio of formaldehyde to phenol was 2.2:1.
2.2.2 Lignin
Bagasse soda black liquor with pH = 13 and 40 wt% solid content as source of lignin was prepared by Pars Company, Haft Tepe, Iran.
2.2.3 Preparation and characterization of nanolignin
1.4 g lignin was dissolved in 50 mL ethylene glycol, the insoluble impurities were removed by filtration after 4 h stirring, and then hydrochloric acid (4.00 cm3, 0.025 mol L− 1) was added to the lignin filtrate solution at a rate of 4 drops per min. The solution was dialyzed in a 3 L beaker for three days with water changed three times a day. The lignin nanoparticles were recovered after precipitating in dilute HCl at pH 2, followed by centrifugation and ultrasonic cleaning to achieve neutral pH. The specific surface area and average pore diameter were measured at 200 °C by BET method.
2.2.4 Preparation of PLG and NPLG adhesives
A 500 ml flat bottom flask equipped with a condenser, thermometer and a magnetic stirrer bar was used for resin synthesis. Sufficient virgin lignin and lignin nanoparticles to substitute 0, 10, 20, 30 and 40 wt% of the phenol (according to pre-test results), phenol, NaOH, ethanol and water were mixed in a flask. Under stirring the flask was heated in a water bath preheated at 80 °C, when glyoxal (40% aqueous solution) was added dropwise. After 4 h of resinification reaction, the flask was cooled to room temperature to stop the reaction. The ethanol in the flask was removed by rotary evaporation at 60 °C under reduced pressure to produce an aqueous resin solution. Molar ratio of glyoxal/phenol (G/P) was 2.2: 1 in this research. The molar fraction of NaOH was 0.375. It should be noted that the only experimental parameter that was changed is the lignin content in the resin synthesis and all others parameters were not changed.
2.2.5 Physicochemical properties of synthesized resins
The solid content of the synthesized PF and PLG resins were determined according to ASTM standard D4426–01(2006). The viscosity of the resins was measured at 25 °C using a Ford Cup according to ASTM standard D1200–10 (2014). A hydrometer was used for measuring the densities of the resins prepared. The density of the resins was measured according to ASTM D1875–03 standard methods. Each physicochemical test was repeated three times. For gel time measuring, 5 g of adhesives was weighed in a 16 mm × 180 mm test tube and then a thin wire spring was placed in the tube. The test tube was placed in a constant temperature oil bath and the thin wire spring was moved gently up and down by hand until gelation. A stopwatch was used to record the gel time and the gelation test was done at 140 °C.
2.2.6 Differential scanning calorimetry analysis
The changes in curing temperature of the PLG resin containing nanoparticles compared to PF resin were determined by DSC using a NETZSCH DSC 200 F3Modelthermal analyzer. The DSC scans were recorded at a heating rate of 10 °C/min under nitrogen atmosphere with a flow rate of 60 ml/min. To determine the curing temperature of the resins, about 5 mg of freeze-dried sample was added to the aluminum pan. The samples were then heated from ambient temperature (25 °C) to 200 °C under a nitrogen atmosphere. The DSC cups were closed during the whole temperature range investigated.
2.2.7 Panel manufacturing
Particleboard panels were manufactured according to a method of Younesi-Kordkheili et al. (2016). A forming frame (35 × 35 × 1 cm) was placed on a stainless steel caul plate to control the thickness. The moisture of the wood chips used for the particleboard preparation was of 4 wt%. 10 wt% resin solids of the resins prepared (on dry weight of wood particles) was used as resin load. The nominal thickness and density of the manufactured panels were 16 mm and 0.6 g/cm3, respectively. Other details about panel manufacturing method were described in Younesi-Kordkheili et al. (2016).
2.2.8 Panel testing
Prior to panel testing, the prepared panels were conditioned at a temperature of 23 °C ± 2 °C and a relative humidity of 60%±5% for two weeks. All tests of the manufactured particleboards were carried out according to the relevant standard methods. The modulus of rupture (MOR) and modulus of elasticity (MOE) were determined according to EN 310. The internal bond (IB) strength was determined according to EN 319, and thickness swelling and water absorption according to EN 317.
2.2.9 Statistical analysis
Data for each test were statistically analyzed by SPSS software. The effects of the lignin nanoparticle content on the panel properties were evaluated by two-way analysis of variance (Two-way ANOVA) at 95% confidence level.
3 Results and discussion
3.1 FTIR analysis
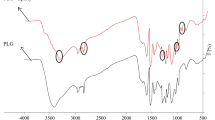
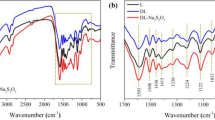
The FTIR spectra of the PLG resin with 30 wt% lignin and the nanolignin-phenol-glyoxal (NPLG) resins with 30 wt% nanolignin are shown in Fig. 1. The band assignments are listed in Table 1. It was found that there are no differences in the chemical structure between the resins with lignin and nanolignin. For example, the 2850 cm− 1band related to the C-H bonds in methylene linkages and the 1275 cm− 1 band related to the C-O stretching of phenolic C-OH and phenolic C-O are similar in the PLG and NPLG resins.
3.2 DSC analysis
Figure 2 shows the DSC curves of the PF, PLG resin and the PLG resin containing 30%wt of lignin nanoparticles. The curing process of the PLG resins is exothermic. The temperature peaks of the PLG resin with 30% lignin appeared at 145 and 170 °C while PLG resins with nanolignin exhibited two peaks at 139 and 159 °C. These peaks can be related to self-condensation reactions between the functional groups of a PLG resin. Figure 2 indicates that the addition of lignin nanoparticles had a marked influence on the curing behavior of the PLG resin. The DSC analysis confirms the gel time results as the PLG resins modified with lignin nanoparticles present shorter gel times than the unmodified PLG resin. The smaller size of nanolignin causes the –OH functional groups, which are surrounded by a highly crosslinked three-dimensional network in the lignin structure, to be more exposed on the nanolignin units, which makes nanolignin a remarkable crosslinking agent in the curing reaction (Kalami et al. 2018; Nair et al. 2014). Based on the findings of this research, the curing temperature of PLG resins was markedly higher than those of PF resins. In industrial applications, a higher curing temperature means higher energy and costs. Based on what was found in the present work, such a problem can be solved by addition of small proportions of lignin nanoparticles instead of lignin in the PLG resin formulation.
3.3 Physicochemical properties
Physicochemical properties of the synthesized resins are shown in Table 2. By incorporation of nanolignin into the PLG resin, the solids content increases and its gelation time is faster. The results showed that the PLG resin at a 30 wt% nanolignin substitution had the highest solid content (50%) and shortest gelation time (319 s). Conversely, the unmodified PLG resin with 10 wt% lignin had the lowest solids content (40%). Moreover, the longest gelation time (389 s) was related to PLG resin with 30% lignin. The faster gel time as a function of the increase in the proportion of lignin nanoparticles shows that cross-linking is noticeably faster, resulting in a greater level of cross-linking between phenol, nanolignin and glyoxal to form PLG condensates. Previous research has shown that PF resins present a faster gelation time than PLG resins (Younesi-Kordkheili and Pizzi 2020). The use of nanolignin instead of neat lignin can significantly improve the gelation time of the PLG resin (Table 2). Conversely, the physicochemical test results also indicated that with the progressive addition of higher proportions of lignin nanoparticles, the viscosity and the density of the resins increase. The PLG resins with 30%wt nanolignin added had higher viscosity and density. The viscosity of the resin could be one of the most important factors affecting the bond strength. Commonly, the viscosity of a resin is dependent on the size and shape of the adhesive molecules, the resin solids content. The molecular weight of nanolignin is larger than that of phenol. Thus, the addition of nanolignin increases the molecular weight and the internal frictional resistance of the NPLG resin. Hong and Park (2017) indicated that the reactivity of UF resins was improved as the viscosity increased. This was probably due to the fact that high viscosity UF resins contained much greater molecular weight oligomers than lower viscosity resins, resulting in a short time for the formation of network structures.
3.4 Properties of particleboard panels
Flexural modulus and flexural strength of the panels bonded with PLG resins containing 10, 20, 30 and 40%wt lignin nanoparticles compared to those made from unmodified PLG resin and PF resin are shown in Table 3. As shown in Table 3, the panels bonded with the PLG resin with 30%wt nanoparticles presented the highest flexural modulus (2723 MPa) and strength (19 MPa) while the control panels bonded with a PLG resin with 30%wt lignin had the lowest flexural modulus (2339 MPa) and strength (12.2 MPa) values, respectively. Conversely, statistical analysis showed that increasing lignin nanoparticles content from 10 to 40%wt has significant influence on the flexural properties of the panels prepared. While Younesi-Kordkheili et al. (2019) indicated in a previous work that the flexural strength of the panel bonded with PLG resins instead decreases with progressive addition of lignin to the PLG resins, the results of the current research indicate that higher flexural modulus and flexural strength can be obtained by increasing the addition of lignin nanoparticles from 10 to 40%wt. The addition of nanolignin increases the bond strengths of the adhesive because nanolignin is probably an excellent cross-linking agent for the synthesis of the PLG resins. As can be seen from Table 4, the specific surface area of lignin is 0.05371 m2 g− 1, which is only about one tenth of the specific surface area of nanolignin (6.1766 m2 g− 1). The pore volume of nanolignin is 0.0098 cm3 g− 1, which is 14 times the pore volume of lignin (0.0007 cm3 g− 1). The high specific surface area and porous structure of nanolignin increase the contact between nanolignin and other components, resulting in the enhanced reactivity of nanolignin. Moreover, the high specific surface area and porous structure of nanolignin increase the contact between nanolignin and formaldehyde, resulting in the enhanced reactivity of nanolignin. Chen et al. (2019) indicated that the bond strength of all the PLG adhesives modified by nanolignin is higher than that of the commercial PF resin.
The internal bond strength of the particleboards prepared is shown in Table 3. The highest IB strength (0.69 MPa) was achieved by the panels bonded with PLG resin containing 30 wt% lignin nanoparticles and the lowest by those bonded with the PLG resin with 30% virgin lignin (0.53 MPa). The greater mechanical strength of the panels bonded with PLG resins with nanoparticles when compared to those bonded with the control PLG resin is due to the void spaces that can be more filled by nanolignin, resulting in increasing strength of the glue line and thus, of the associated panels. For industrial production, the higher substitution rate of phenol with nanolignin means that less phenol will be used in the synthesis of PLG, which would facilitate the green production of wood adhesives and exert a profound and significant effect on the conservation of fossil resources.
The water absorption and thickness swelling of the panels after 24 h soaking in distilled water are shown in Table 3. The PLG resin without nanoparticles has the highest value water absorption and thickness swelling content whereas the addition of lignin nanoparticles decreased the water absorption and thickness swelling of the panels. The lower water absorption and thickness swelling can be attributed to the difference in the amount of bridges formed between the reactive sites. In addition, compared to lignin bonds, the bond strength of lignin nanoparticles to glyoxal is probably higher, hence by increasing the proportion of nanolignin the resistance to hydrolysis of the resins continuously improves. Younesi-Kordkheili and Pizzi (2019) showed that methylene bridges in PF resins have high resistance to hydrolysis while some bridges in PLG resins such as C-O bonds are not stable at the same level.
4 Conclusion
In the present work, nanolignin was used as the raw material to substitute phenol partially, and to combine with glyoxal to produce a new type of PLG wood adhesive. The following conclusions can be drawn from the results obtained:
-
The PLG resin containing 30%wt lignin nanoparticles has the shortest gelation time and the highest viscosity among all synthesized resins.
-
FTIR analysis indicated no differences in the chemical structure between the PLG resins with lignin and nanolignin.
-
DSC analysis indicated that the curing process of PLG resins with nanoparticles needs a lower temperature than pure PLG resin.
-
The mechanical strength (flexural modulus, flexural strength and IB strength) of the panels bonded with the modified PLG resins with nanolignin was higher than those made from pure PLG resin.
-
The use of nanolignin and good performance of PLG could reduce the energy and production costs, and promote the application of the nanolignin instead of fossil material phenol in the production of PLG resins.
-
The present study provides a new idea for the green production of phenolic resins with high quality.
References
ASTM standard D4426–01 (2006) Standard test method for determination of percent nonvolatile content of liquid phenolic resins used for wood laminating. ASTM, West Conshohocken, PA
ASTM D1200-10 (2014) Standard test method for viscosity by ford viscosity cup. ASTM, West Conshohocken, PA
Chen Y, Gong X, Yang G, Li Q, Zhou N (2019) Preparation and characterization of a nanolignin phenol formaldehyde resin by replacing phenol partially with lignin nanoparticles. RSC Adv 9:29255–29262
Feldman D (2016) Lignin nanocomposites. J Mac Sci Part A Pure Appl Chem 53(6):382–387
Hong MK, Park BD (2017) Effect of Urea-Formaldehyde Resin Adhesive Viscosity on Plywood Adhesion. J Korean Wood Sci Technol 45(2):223–231
Kalami S, Chen N, Borazjani H (2018) Comparative analysis of different lignins as phenol replacement in phenolic adhesive formulations. Ind Crops Prod 125:520–528
Khan MA, Ashraf SM, Malhotra VP, Lei H, Du G, Pizzi A, Celzard A, Fang Q, Pizzi A, Allal A, Charrier F, Charrier B (2004) (2009) Cornstarch and tannin in phenol–formaldehyde resins for plywood production. Ind Crop Prod 30(2009) 188–193
Nair S, Sharma S, Pu Y (2014) High Shear homogenization of lignin to Nanolignin and Thermal Stability of nanolignin-polyvinyl alcohol blends. Chem Sus Chem 7(12):3513–3520
Pizzi A, Mittal L (2011) Wood adhesives. CRC Press, New York, p 462
Younesi-Kordkheili H, Pizzi A (2021) Improving properties of phenol- lignin- glyoxal resin as a wood adhesive by an epoxy resin. Eur J Wood Prod 79(1):1–7
Younesi-Kordkheili H, Pizzi A (2020) Some of Physical and Mechanical Properties of Particleboard Panels bonded with phenol- lignin- glyoxal Resin. J Adhes 96(16):1385–1395
Younesi-Kordkheili H (2019) Ionic liquid modified lignin-phenol-glyoxal resin: a green alternative resin for production of particleboards. J Adhes 95(12):1075–1088
Younesi-Kordkheili H, Pizzi A (2018) Properties of plywood panels bonded with ionic liquid-modified lignin–phenol–formaldehyde resin. J Adhes 94(2):143–154
Younesi-Kordkheili H (2017) Improving Physical and Mechanical Properties of New Lignin- Urea- Glyoxal Resin by Nanoclay. Eur J Wood Prod 75(6):885–891
Younesi-Kordkheili H, Pizzi A, Niyatzade G (2016) Reduction of formaldehyde emission from particleboard by phenolated kraft lignin. J Adhes 92(6):485–497
Zhang Z, Terrasson V, Guenin E (2021) Lignin Nanoparticles and their nanocomposites. Nanomat 11(5):1–30
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Younesi-Kordkheili, H., Pizzi, A. Improving the properties of phenol-lignin-glyoxal as a wood adhesive by lignin nanoparticles. Eur. J. Wood Prod. 81, 507–512 (2023). https://doi.org/10.1007/s00107-022-01904-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-022-01904-5