Abstract
Recent and fossil woods from the genus of Sequoiadendron, as well as their milled wood lignins were investigated by means of FTIR spectroscopy and pyrolysis GC/MS technique. Though almost same in appearance the differences in band intensities of FTIR-spectra reveals the distinctly increased aromatic character and the oxidation in fossil specimens. More detailed information about the changes in old wood were obtained by analytical pyrolysis, which delivers degradation products with shortened side chains and more saturated units relating to fossil lignin.
Zusammenfassung
Recentes und fossiles Holz der Gattung Seqoiadendron giganteum, sowie daraus isoliertes Björkman-Lignin wurden mittels FTIR Spektroskopie und GC/MS Pyrolyse-Technik untersucht. Obwohl sich die Holzarten äußerlich sehr ähneln, zeigen die Unterschiede der Bandenintensität in den FTIR Spektren einen deutlich erhöhten, aromatischen Charakter und Oxidationsgrad in den fossilen Proben. Detailliertere Information über Veränderungen in den alten Holzarten wurden durch pyrolytische Analyse gewonnen, welche Abbauprodukte mit kürzeren Seitenketten und gesättigtere Einheiten des fossilen Lignin aufweisen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Two fossil trunks of sequoia tree were excavated in a coal mine located about 20 km west of Bosporus on the shore of Black sea. The wood was partly in such a good condition that it looked like as weathered recent wood. The 14C-method of age determination revealed, however, that their age was beyond the detectable limit. Accordingly, the samples are older than 41,000 years. The previous two publications on this topic dealt with the chemical composition and ultrastructure of these woods and their ethanol extractives, respectively. (Uçar et al. 1996, 1998). The barks of the same fossil specimen were also investigated (Staccioli et al. 1998, 2002).
It has been firmly established that the fossilization is accompanied with selective removal of polysaccharides leaving behind residues enriched in lignin, which can easily be determined as acid insoluble matter (Klason lignin). The acid insoluble part of fossil woods in this study is about two thirds of the total material. Therefore, in this paper the lignin moiety has been described in greater detail.
Usually, lignin analysis comprises time consuming degradation experiments. Therefore, in the experimental design for the present study the more rapid analytical pyrolysis approach combined with gas chromatography and mass spectrometry (Py-GC/MS) was preferred. The literature contains a large body on Py-GC/MS analysis of lignocellulosic materials, which confirms that in screening experiments this technique leads to useful data (Obst 1983, Saiz-Jimenez et al. 1987, Bremer 1991, Faix 1992, Meier and Faix 1992, Faix et al. 1992, Kleen 1993, Marques et al. 1994).
Two specimens belonging to the same fossil trunk of Sequoiadendron giganteum found on the site were studied: one light reddish brown and a dark colored—apparently more degraded—sample. The fossil woods and a recent S. giganteum wood for comparison such as the MWLs isolated hereof were submitted to analytical pyrolysis. Their FTIR-spectra have also been recorded.
2 Materials and methods
The light reddish brown fossil wood looked like weathered recent red wood and will be designated here as B (brown) and the dark colored one as D (dark). In case of the recent S. giganteum wood, the sapwood and heartwood were submitted to separate analyses.
The lignin content was determined on the previously acetone extracted material (extraction time 4 days) according to Runkel and Wilke (1951) using 72% H2SO4 and 40% HBr for the primary hydrolysis.
2.1 Isolation of milled-wood lignins
A modified Björkman procedure was applied for MWL preparation. Two successive Soxhlet extractions were conducted: pure acetone followed by a mixture of acetone-water (90/10, v/v) were used as solvents (48 h extraction time). Then the samples were thoroughly dried over P2O5 for a week and ball milled in toluene using a Retsch mill equipped with steel vessels and balls during three days. The extraction was done with dioxane-water (100/4, v/v) in an ultrasonic bath during 14 h according to Wegener and Stoll (1976) and Wegener and Fengel (1979).
The crude lignin obtained by evaporating the solvent were purified according to the standard procedure. First, the crude preparation was dissolved in 90% acetic acid and reprecipitated into water. The insoluble part was seperated by centrifugation and the acetic acid removed under reduced pressure. The further purification was performed by dissolving the product in a dichloroethane-ethanol mixture (2/1, v/v) and by precipitation in ethyl ether. The MWLs were dried in a desiccator 2–3 days under vacuum over P2O5. The yields of MWL’s were around 10% for fossil and between 6–7% for recent sapwood and heartwood based on wood extracted with acetone.
Carbohydrate contamination of MWLs was estimated by means of ion exchange chromatography of hydrolyzates obtained after TFA hydrolysis as described by Fengel and Wegener (1979).
2.2 FTIR spectroscopy
Spectroscopic measurements were performed in a Bio-Rad Excalibur series FTS 3000 spectrophotometer using the standard KBr method (300 mg KBr plus 1 mg lignin; resolution: 4 cm−1; 64 scans). The spectra were base line corrected at 3,700, 1,850 and 700 cm−1 and normalized to the highest band at 1,510 cm−1.
2.3 Pyrolysis GC/MS
Ball milled and acetone pre-extracted woods and MWLs were subjected to analytical pyrolysis in a CDS Pyroprobe 100 (coil filament) instrument. Before pyrolysis the samples (80–100 μg placed in an open quartz boat) remained for 30 s in the interface at 200°C. Each sample was pyrolysed in duplicate at 450°C for 10 s and from the results an average pyrogram was calculated. The temperature rising time was as short as possible (below 1 s). GC conditions: DB 1701 column (30 m×0.32 mm, 0.25 μm film, J&W Scientific); Injector and detector temperature: 250°C; Temperature program: 45°C, heating rate 4°C min, 280°C for 15 min, split 1/20; Carrier gas: helium; Product identification by quadrupol MS (HP 6890 Series, Mass Selective Detector). Product quantification by FID detector. The peak integrals are presented as %. The congruence indices were calculated according to Salo et al. (1989).
3 Results and discussions
It is documented in the literature that S. giganteum has a relative high Klason lignin contents (35,4% according to Leopold and Malmström 1952). Our results obtained by the Runkel-Wilke method confirm this yielding: 34% Klason residue after 6 h extraction with ethanol benzene and 35% residue after a 4 day extraction with acetone. Buried woods undergo selective removal of polysaccharides to leave lignin-enriched material. Thus the fossil woods gave rise to 66–72% Klason residues (dark sample) and 62–68% (light brown sample). The higher values are for the samples extracted with acetone. Obst et al. (1991) reported similar high lignin contents from Canadian Artic fossil softwoods. The lignin contents from woods of the Eocene and Paleocene were ranging from 66.5% to 85%, and those of Miocene (ca. 0.5 mill. yr-old) around 48%.
It is also well established that MWLs unavoidably contain polysaccharides, whose content rarely exceeds 2–3% when the lignin is properly prepared and carefully purified. The carbohydrate contents of the MWLs (Table 1) prepared both from recent and fossil woods are low.
As anhydroglucose occurs both in cellulose and glucomannane, it is not surprising that the yield of glucose (0.5%) is the highest. The absence of arabinose in fossil MWLs is also self explanatory in view of the low arabinose content in arabionoxylans and its easy hydrolysation during the millennia. Uçar et al. (1996) could not detect arabinose in fossil woods, either. The content of other sugars is also less in fossil MWLs in comparison to recent MWLs. This observation is consistent with the lower polysaccharide content of the fossil material.
3.1 FTIR spectroscopy
The FTIR spectra of all MWLs seem to be very similar in appearance. Concerning the interpretation of lignin FTIR spectra one should keep in mind the hint of Hergert (1971) : “..., the most common error is to conclude that two or more lignin products are identical because their infrared spectra are similar. Rather, the opposite must be true, i.e., if the spectra are not precisely identical, it must be concluded that products are not identical.” Thus, for a better comparability of small differences we present the band intensities of the fingerprint region as a bar diagram (Fig. 1) and also in Table 2.
Normalized band intensities of MWLs isolated from Sequoiadendron giganteum recent and fossil woods. Dotted box Sapwood, ⊟ Heartwood, grayed box Fossil B, ■ Fossil D
Normalisierte Bandenintensitäten von MWL aus rezentem und fossilem Holz der Gattung Seqoiadendron giganteum. Gepunkteter Balken Splintholz, ⊟ Kernholz, grauer Balken Fossil B, ■ Fossil D
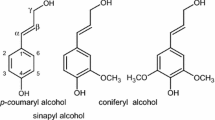
Both, the data in Table 2 and in Fig. 1 clearly display the typical features of a G lignin (1595 cm−1 <<1510 cm−1 dominant >>1463 cm−1; absorbance max. at 1140 cm−1; 1269 cm−1 second highest peak; 1032 cm−1 >1221 cm−1 two bands at 859 and 818 cm−1, according to Faix 1991).
The recent MWLs do have almost exact band frequencies, whereas small shifts were observed in fossil MWLs but only for two bands. The band of aromatic skeletal vibrations at 1595 cm−1 tends very slightly to become higher, as being between 1597 and 1600 cm−1 and the one at 1663 cm−1 changes to a shoulder at a bit higher wave number (1672 cm−1).
As can be seen from both presentations, the differences between the spectra are indeed very small. During interpretation of these results for the lignins as a whole one should have the low yield of MWLs (around 10%) in mind and the fact that a MWL represents the less changed moiety of a (fossil) lignin.
Compared to the spectra of recent MWLs, the fossil ones exhibit lower intensities at the majority of bands. Especially, the following regions show a pronounced decrease in absorbance:
-
1672 cm−1, shoulder instead of 1663 cm−1 (C=O stretch vibrations in conjugated para substituted aryl ketones). Coniferyl aldehyde alone absorbs at 1652 cm−1 and methylation increases the value to 1660 cm−1 (Hergert 1971).
-
1423 cm−1 (aromatic skeletal vibrations combined with C-H in plane deform.)
-
1327 cm−1 band appears in form of a shoulder but in higher intensities in fossil MWLs. As the interpretation of this band “syringyl ring breathing with CO stretching” does not apply for G lignins, it indicates condensation or substitution of aromatic ring at position 5 (Faix 1992). Obst et al. (1991) observed in the case of fossil white pine wood from the Miocene (Site “Resolute”; Klason residue 48% ) still a distinct maximum in this region. In samples with Klason residues exceeding 65% the band also merged into the 1270 cm−1 band.
-
1269 cm−1 (G ring plus C=O stretch)
-
The absorption maximum at 1140 cm−1 (C-H in plane deformations of guaiacyl units) instead of 1120 cm−1 is the indicator for the absence of syringyl units in lignins.
-
1032 cm−1 (C-H in plane deformations of guaiacyl rings assisted by C-O deformation in primary alcohols and unconjugated C=O stretch)
The decreased band intensities indicated above can be interpreted in general terms as a “loss” of guaiacyl units and secondary alcohols during fossilization. The splitting of aromatic methoxyl (or methyl) groups and terminal aliphatic CH2OH groups could account for these observations.
There are only three following IR-bands of MWLs isolated from fossil woods showing somewhat higher absorptions:
-
1595 cm−1 band (aromatic skeletal vibrations and C=O stretch)
-
1455 cm−1 (C-H deformations: asym. in -CH3 and -CH2-)
-
1367 cm−1 (aliphatic C-H stretch in CH3 but not OCH3 groups with phenolic OH groups)
The higher intensities of the aromatic skeletal vibrations are a consequence of the splitting of aliphatic side chains and the lower carbohydrate contents in fossil MWLs. The increase in the intensities at 1455 and 1367 cm−1 allows the assumption that more saturated shortened side chains were then created.
3.2 Pyrolysis gas chromatography / mass spectrometry
A complete, very long table of pyrolysis products of woods and MWLs could not be provided here. However, it is available from the corresponding author with an off print. In this table, the peaks and their relative areas are listed as % values according to their origin: (1) Peaks of mostly carbohydrate origin, (2) Phenols with one OH group, (3) Guaiacyl type degradation products, (4) Benzenes and phenols with two OH groups, and (5) Unidentified compounds.
In Table 3 the results are summarized based on the above mentioned categories, where the phenols and guaicacols are additionally subdivided into substances with saturated and unsaturated side chains.
The full interpretation of the typical carbohydrate type degradation products is not intended here. Nevertheless it must be mentioned that the most abundant substances in this category are hydroxyacetaldehyde, methylglyoxal, acetol (partly results of the reverse aldol condensation, i.e. the less specific splitting of pyranose rings) and levoglucosan (as a results of glucan chain splitting and the subsequent dehydration of glucose units in cellulose), Shafizadeh and DeGroot (1976), Byrne et al. (1966).
The fossil specimens deliver 2–3 times more levoglucosan than the recent ones. This observation has two explanations: first, the degree of crystallization in fossil woods is probably higher as a result of the preferred hydrolysis of the amorphous regions (Fengel and Wegener 1988). Second, earth alkali metals are known to promote the reverse aldol condensation and thus lowering the levoglucosane yield (Kleen 1993). Clearly, the probability is high that the Na+ and K+ content in fossil woods became low due to ion exchange with Ca2+ in the course of fossilization. In view of this, it is understandable that the (supposedly more degraded) dark brown specimen yielded more levoglucosan than the other one.
It has to be recalled that listing of several unspecific degradation products such as methanol, formic acid, acetic acid, etc. among the carbohydrate type degradation product is not cogent. Moreover, one has to consider that no individual response factors were used for quantitative evaluation. For this reason the sum of carbohydrate and lignin type pyrolysis products cannot truly reflect the real carbohydrate/lignin ratio in the samples. Hence these data should rather be interpreted as tendencies instead of absolute values.
As such, the data are consistent: all MWLs give rise mainly to guaicayl type phenols while the fossil ones also yield 9% (B sample) – 14% (D sample) phenols without methoxyl. This observation is undoubtedly the result of demethoxylation of G units during ageing. The sum of guiacyl units in pyrograms of recent woods (21–24%) and fossil woods (37–49%) also reflect properly the higher Klason residue of the fossil specimens.
The distribution of lignin related phenolics—sub-classified as 4-hydroxy-phenyl, guaiacyl, saturated and non-saturated products—are presented in two different ways in Figs. 2 and 3. These Figures illustrate again well the demethoxylation and the relative increment of saturated product as far as the fossil samples are concerned. The latter finding can be rationalized in the following way:
Recent samples contain “native” lignins. This means that they contain a lot of β-O-4 linkages with OH groups in the neighboring α-position. From these units water is easily split as accompanying reaction of the thermal rupture of the β-O-4 linkages. As a consequence, unsaturated products such as coniferyl alcohol, coniferyl aldehyde but also isoeugenol, vinylguaicacol, etc. can be observed in the pyrograms. On the other hand, in aged lignins more “condensed” units can be expected, where the α-position is frequently substituted by C-C bonds. These units, however, are lowering the overall yield and lead to phenols having shorter side chains, which also contain less double bounds.
Generally, it can be stated that with the ageing of wood distinctly increased yields are observed for phenol, cresols, 4-vinyl phenol, guaiacol, 4-methyl guaiacol, 4-ethyl guaiacol, isoeugenol, while the amounts of vanillin, acetoguaiacone, coniferyl alcohol and coniferyl aldehyde decrease. The decrease of the latter two compound is especially pronounced, which also explain to a great extent the diminished yield of unsaturated substances in fossil lignins.
3.3 Congruence indices
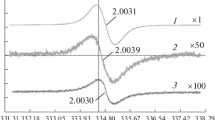
A pyrogram bears a lot of information which is not completely described in a simple manner as represented in Table 3 and Figs. 2 and 3. The approach of calculating congruence indices is another possibility to account for more complex similarities and differences between two or several samples (Salo et al. 1989; Faix et al. 1991). The reproducibility of pyrograms of identical samples is such that one can obtain pyrograms with similarity indices around 95%. In Fig. 4 the similarity indices are shown for relevant pairs of pyrograms, where woods and MWLs are compared.
Lignin related congruence indices between the relevant substances: R recent, F fossil, S sap, H heart, B light brown, D dark brown, W wood, L: lignin
Ligninverwandte Übereinstimmungsindices zwischen den betreffenden Substanzen. R jüngere, F fossile, S Saft, H Kernholz, B hellbraun, D dunkelbraun, W Holz, L Lignin
Recent sapwood and heartwood pyrograms show 89% similarity. The similarities between recent and fossil brown woods are surprisingly high (86% heartwood vs. fossil brown wood; 82% sapwood vs. fossil brown wood). Thus the pyrograms confirm the visual impression concerning the less degraded character of the light brown sample. The two fossil woods are also similar on the 84% level. On the other hand, the similarity indices between the more degraded fossil wood (dark brown) and recent woods are—as expected—lower (80–73%).
The pyrograms of the fossil MWLs are more similar (92%) than those from recent MWLs (86%). The comparison between recent and fossil MWLs distinctly reveal the differences caused by ageing, such as shortening of the side chains, decrement of unsaturated pyrolysis products and yield similarity indices between 71 and 64%.
4 Conclusions
A comparison of the FTIR spectra of MWLs obtained from recent and fossil woods and the pyrograms of the same specimens give a uniform picture of ageing of S. giganteum in buried state in a geological time scale. During a preferred hydrolytic removal of 50–60% of the polysaccharides, the lignin moieties suffer relative little, yet discernible structural changes, as far as the MWLs are concerned. However, it should be noted that the MWLs represent only 10% of the whole lignin content.
The darker fossil wood had changed more than the light brown wood. Analytical pyrolysis gives evidence of clear differences between the lignins in recent and fossil samples, especially when the congruence indices were taken for comparison. The pyrograms exhibit degradation products with shortened side chains and less double bonds. The FTIR spectra disclose more saturated groups, a higher aromaticity, and the presence of more condensed units in the case of fossil MWLs.
References
Bremer J (1991) Quantifizierung der Gerüstsubstanzen von Lignocellulosen durch analytische Pyrolyse-Gasschromatographie/Massenspektrometrie. Doctoral thesis, University of Hamburg, Department of Biology
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45(Suppl):21–27
Faix O, Bremer J, Schmidt O, Stevanovic T (1991) Monitoring of chemical changes in white-rot degraded beech wood by pyrolysis-gas chromatography and Fourier-transform infrared spectroscopy. J Anal Appl Pyrolysis 21:147–162
Faix O (1992) Fourier transform infrared spectroscopy. In: Lin SY, Dence CW (eds) Methods of lignin chemistry. Springer, Berlin Heidelberg New York, pp 83–109
Faix O, Bremer J, Meier D, Fortmann I, Scheyen MA, Boon JJ (1992) Characterization of tobacco lignin by analytical pyrolysis and FT-IR spectroscopy. J Anal Appl Pyrolysis 22:239–259
Fengel D, Wegener G (1979) Hydrolysis of polysaccharides with trifluoracetic acid and its application to rapid wood and pulp analysis. In: Brown RD Jr, Jurassek L (eds) Hydrolysis of cellulose; mechanisms of enzymatic and acid catalysis. Adv Chem Series no: 181. Am. Chem. Soc. Washington, pp 145–158
Fengel D, Wegener G (1988) Wood, chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin
Hergert HL (1971) Infrared spectra. In: Sarkanen KV, Ludwig CH (eds) Lignins, occurrence, formation, structure and reactions. Wiley, New York, pp 267–297
Kleen M (1993) Characterization of wood and pulp using analytical pyrolysis and multivariate data analysis. Doctoral thesis, Royal Institute of Technology
Lai YZ, Sarkanen KV (1971) Isolation and structural studies. In: Sarkanen KV, Ludwig CH (eds) Lignins, occurrence, formation, structure and reactions. Wiley, New York, pp 165–240
Leopold B, Malmström IL (1952) Acta Chem Scand 6:49
Marques A V, Pereira H, Meier D, Faix O (1994) Quantitative analysis of cork (Quercus suber L) and milled cork lignin by FTIR spectroscopy, analytical pyrolysis, and total hydrolysis. Holzforschung 48(special issue):43–50
Meier D, Faix O (1992) Pyrolysis-gas chromatography-mass spectrometry. In: Lin SY, Dence CW (eds) Methods of lignin chemistry. Springer, Berlin Heidelberg New York, pp 177–199
Obst JR (1983) Analytical pyrolysis of hardwood and softwood lignins and its use in lignin-type determination of hardwood vessel elements. J Wood Sci Technol 3:377–397
Obst JR, Mc Millian HJ, Blanchette RA, Christensen DJ, Faix O, Han CS, Kuster TA, Landucci LL, Newman RH, Pettersen RC, Schwandt VH, Wesolowski MF (1991) Characterization of Canadian arctic fossil woods. In: Christie RL, McMillan NJ (eds) Tertiary fossil forests of the geodetic hills, Axel Heiberg Island, Arctic Archipelago. Geological survey of Canada. Bulletin. 403, pp 123–146
Runkel ROH, Wilke KD (1951) Zur Kenntnis des thermoplastischen Verhaltens von Holz. II. Mittl. Holz Roh- Werkstoff 9:260–270
Saiz-Jimenez C, Boon JJ, Hedges JI, Hessels JKS, DeLeeuw JW (1987) Chemical characterization of recent and buried woods by analytical pyrolysis. Comparison of pyrolysis data with 13C NMR and wet chemical data. J Anal Appl Pyrolysis 11:437–450
Salo S, Niemelä S, Elomaa M, Lindberg JJ (1989) Identification of lignins by pyrolytical methods. Holzforschung 43:257–260
Staccioli G, Uçar G, Bartolini G, Koppi C, Mochi M (1998) Investigation on a fossil Sequoia bark from Turkey. Holz Roh- Werkstoff 56:426–429
Staccioli G, McMillan NJ, Meli A, Bartolini G (2002) Chemical characterisation of a 45 million year bark from geodetic hills fossil forest, Axel Heiberg Island, Canada. Wood Sci Technol 36:419–427
Uçar G, Staccioli CG, Stoll M (1996) Chemical composition and ultrastructure of a fossil wood from the genus of ancestral sequoia. Holz Roh- Werkstoff 54:411–421
Uçar G, Balaban M, Gören N (1998) Ethanol extractives of fossil sequoia wood. Holz Roh- Werkstoff 56:430–436
Wegener G, Stoll M (1976) Eine schnelle Isolierung von Milled-Wood-Lignin mit Hilfe von Ultraschall. Cellulose Chem Technol 10:611–616
Wegener G, Fengel D (1979) Rapid ultrasonic isolation of milled wood lignins. Tappi 62–3:97–100
Acknowledgment
G. Uçar expresses his thanks to DAAD for the scholarship of summer 1998.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uçar, G., Meier, D., Faix, O. et al. Analytical pyrolysis and FTIR spectroscopy of fossil Sequoiadendron giganteum (Lindl.) wood and MWLs isolated hereof. Holz Roh Werkst 63, 57–63 (2005). https://doi.org/10.1007/s00107-004-0530-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-004-0530-x