Abstract
Purpose
After laparoscopic cholecystectomy patients have moderate pain in the early postoperative period. According to several studies an erector spinae plane (ESP) block can be a valuable part of multimodal analgesia. Our intention was to evaluate how ESP block influences postoperative pain scores and opioid consumption after laparoscopic cholecystectomy.
Methods
This single-blinded, prospective, randomized study included 60 patients undergoing laparoscopic cholecystectomy to receive either bilateral ESP block at the Th 7 level (n = 30) with 20 ml of 0.25% levobupivacaine plus dexamethasone 2 mg per side, or standard multimodal analgesia (n = 30). Patients from the standard multimodal analgesia group received tramadol 100 mg at the end of the procedure. Postoperative analgesia for both groups was acetaminophen 1 g/8 h i.v. and ketorolac 30 mg/8 h. Tramadol 1 mg/kg was a rescue treatment for pain breakthrough (numeric rating scale/NRS ≥ 6) in both groups. Pain at rest was recorded at 10 min, 30 min, 2 h, 4 h, 8 h, 12 h and 24 h after surgery using NRS (0–10).
Results
An ESP block significantly reduced postoperative pain scores compared to standard multimodal analgesia after 10 min (p = 0.011), 30 min (p = 0.004), 2 h (p = 0.011), 4 h (p = 0.003), 8 h (p = 0.013), 12 h (p = 0.004) and 24 h (p = 0.005). Tramadol consumption was significantly lower in the ESP group 25.02 ± 56.8g than in the standard analgesia group 208.3 ± 88.1g (p < 0.001).
Conclusion
An ESP block can provide superior postoperative analgesia and reduction in opioid requirement after laparoscopic cholecystectomy
Zusammenfassung
Ziel
Nach einer laparoskopischen Cholezystektomie haben Patienten in der frühen postoperativen Phase mäßige Schmerzen. Mehreren Studien zufolge kann der Erector-spinae-plane-Block (ESP) zum wertvollen Bestandteil der multimodalen Analgesie werden. Ziel dieser Arbeit war es, zu untersuchen, wie der ESP-Block den postoperativen Schmerzscore und den Opioidverbrauch nach einer laparoskopischen Cholezystektomie beeinflusst.
Methoden
Die einzelverblindete, prospektive, randomisierte Studie schloss 60 Patienten ein, die sich einer laparoskopischen Cholezystektomie unterzogen, um entweder einen bilateralen ESP-Block in Höhe des Th 7 (n = 30) mit 20 ml 0,25 % Levobupivacain plus Dexamethason 2 mg pro Seite oder eine standardmäßige multimodale Analgesie (n = 30) zu erhalten. Die Patienten der multimodalen Standardanalgesiegruppe erhielten am Ende des Eingriffs Tramadol 100 mg. Die postoperative Analgesie für beide Gruppen war Paracetamol 1 g/8 h i.v. und Ketorolac 30 mg/8 h. Tramadol 1 mg/kg war eine Rescue-Therapie bei Schmerzdurchbruch (NRS ≥ 6) in beiden Gruppen. Der Ruheschmerz wurde 10 min, 30 min, 2 h, 4 h, 8 h, 12 h und 24 h nach der Operation aufgezeichnet, wobei eine NR-Skala (0–10) verwendet wurde.
Ergebnisse
Der ESP-Block reduzierte deutlich die postoperativen Schmerzscores (NRS) im Vergleich zur multimodalen Standardanalgesie nach 10 min (p = 0,011), 30 min (p = 0,004), 2h (p = 0,011), 4h (p = 0,003), 8 h (p = 0,013), 12 h (p = 0,004) und 24 h (p = 0,005). Der Tramadolverbrauch war in der ESP-Gruppe mit 25,02 ± 56,8 g deutlich niedriger als in der Standardanalgesie-Gruppe mit 208,3 ± 88,1 g (p < 0,001).
Schlussfolgerung
Der ESP-Block kann eine überlegene postoperative Analgesie und eine Reduzierung des Opioidbedarfs nach laparoskopischer Cholezystektomie bieten
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic cholecystectomy (LC) is a common day care surgery procedure with moderate intensity of pain in the early postoperative period. Known benefits of the early hospital discharge require efficient analgesia [1]. Multimodal analgesia which combines best effects of different analgesics has been advocated for these purposes. The combination of opioid and nonopioid analgesics is the core of the multimodal analgesia (MA). Regardless of the opioid and nonopioid combination some patients still experience moderate to severe pain and demand extra doses of opioids. These extra doses can potentially intensify very well-known adverse effects like nausea, vomiting and sedation. This can postpone hospital discharge and increase the treatment costs [1,2,3,4,5]. Nevertheless, Zhao et al. showed that a significant number of patients after LC needed oral opioid prescriptions after hospital discharge [6]. This inspired other authors to investigate the addition of other types of medication (ketamine, dexamethasone, gabapentinoids, lidocaine) and analgesia technique (intraperitoneal or incisional local anesthetic infiltration) to the multimodal analgesia regimen [2, 3]. The need for a more efficient preventive postoperative analgesia introduced in 2012 subcostal transversus abdominis plane block (STAP) as a new part of multimodal analgesia with a potential to decrease postoperative pain and reduce opioid consumption [7]. The initial results were promising but not convincing enough and the STAP block was presented in the latest PROSPECT (procedure specific postoperative pain management) recommendations only as a back-up option [2, 8].
Erector spinae plane (ESP) block was introduced in 2016 by Forero et al. Its potential effects on ventral and dorsal rami of the spinal nerves demonstrates the great potential of this block [9]. Several case studies have shown the efficiency of ESP block as an analgesia adjunct in the abdominal and laparoscopic surgery [10,11,12,13]. Its main advantage with respect to other truncal blocks (rectus sheath block, transversus abdominis plane block or quadratus lumborum block) is the potential for visceral pain relief [13, 14]. Beneficial analgesic effects of ESP block for laparoscopic cholecystectomy were obtained by Tulgar et al. and Altiparmak et al. but the medium strength of the effect and insufficient number of patients brought necessity for further investigations [15,16,17,18].
Their results inspired us to investigate implementation of ESP block in our hospital multimodal analgesia protocol for LC.
The aim of our study was to evaluate if ESP block would lower postoperative pain scores after LC and to what extent. Our secondary intention was to assess whether ESP block would decrease the need for postoperative opioid rescue analgesia.
Patients and methods
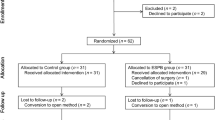
This single-blinded, prospective, randomized, study was performed in the Clinical Centre of Vojvodina, Novi Sad, Serbia between November 2019 and March 2020 with the approval of the ethics committee of the Clinical Centre of Vojvodina. Our study was registered with TCTR (registration no: TCTR20200207006), and CONSORT checklist was used for the patient enrolment and allocation (Fig. 1). Signed informed consent was obtained from all the patients.
The study included 60 patients with American Society of Anesthesiologists (ASA) classes I‑III, aged 20–65 years, who were scheduled for laparoscopic cholecystectomy. After analysis of the differences in postoperative opioid consumption in the previous studies we have used a power of 0.8 and significance level of 0.05 to calculate a sample size of 54 [15,16,17,18]. Considering the possibility of drop-out, 60 patients were included in the study.
The exclusion criteria were patient enrolment refusal, blood coagulation pathology, relevant drug allergy, pregnancy, alcohol or drug abuse, morbid obesity (BMI ≥ 35), severe liver or kidney disease, chronic opioid use or inability to understand the study protocol. The sealed envelope technique was used to randomize patients into groups. Randomization was performed by a senior anesthesiologist who did not participate in the study. Patients were randomly assigned to receive bilateral erector spinae plane (ESP) block with 20 ml of 0.25% levobupivacaine plus dexamethasone 2 mg per side (study group) or standard multimodal analgesia protocol (control group).
For the induction and maintenance of anesthesia propofol (2–2.5 mg/kg), fentanyl (3 μg/kg), rocuronium (0.6–0.8 mg/kg) and sevoflurane was used. At the end of the procedure, all the patients received acetaminophen 1 g i.v. and ketorolac 30 mg. Patients without ESP block received at the end of operation 100 mg of tramadol as part of our hospital multimodal analgesia protocol. Postoperative analgesia regimen in both groups was acetaminophen 1 g/8 h i.v and ketorolac 30 mg/8 h. All the patients with a significant postoperative pain (NRS ≥ 6) were administered tramadol 1 mg/kg on request. Intraperitoneal pressure was 10–12 mm Hg. After the surgery patients with vomiting, or patients who experienced vomiting shortly after the surgery received ondasetron 4 mg i.v.
The ultrasound (US)-guided ESP block was always performed before the surgical procedure by two experienced anesthesiologists. Patients were positioned in the right lateral decubitus. A US probe (Mindray M5 diagnostic ultrasound system) was placed longitudinally at the level of the Th7 spinal process, then moved 3 cm laterally from the midline. The ultrasound landmarks, Th7 transverse process, and overlying erector spinae muscle were identified. After the making of aseptic field a 100-mm, 20G Stimuplex (Stimuplex B Brown Medical) block needle was guided in-plane cranial to caudal until the tip contacted the Th7 transverse process. After using hydrodissection with 4 ml of isotonic saline to confirm the right needle position 20 ml of 0.25% levobupivacaine plus 2 mg of dexamethasone was administered below the erector spinae muscle. The same procedure was repeated on the other side.
Postoperatively, patients were monitored for 1h at the recovery unit, and after that transferred to the surgical ward. Pain at rest was recorded for each patient using a numeric rating scale (NRS 0–10) at defined postoperative time intervals (10 min, 30 min, 2 h, 4 h, 8 h, 12 h and 24 h). Pain scores were recorded by the attending recovery and ward staff, who were blinded to which group the patient belonged to. Postoperative rescue opioid requirements were also recorded. In addition, the occurrence of postoperative nausea and vomiting was noted.
All the other adverse effects in possible connection with ESP block and opioid analgesia were also recorded.
Statistical analysis
For the statistical analysis SPSS 20.0 was used. Differences between the groups were analysed by Student’s t test for normal distribution data and Mann-Whitney U-test for data without normal distribution. For nonparametric data the chi-square test was used. P < 0.05 was considered statistically significant.
Results
This study recruited 60 patients between November 2019 and March 2020. In both groups female patients were more prevalent (53% in ESP group and 56% in control group). Correlation between the patients’ demographics and intraoperative analgesia is shown in Table 1.
There were no statistically significant differences between groups. Concerning intraoperative analgesia (fentanyl) higher opioid consumption was documented in the ESP group (p = 0.21) but without significant difference.
Pain scores are shown in Table 2.
Patients administered the ESP block had significantly lower pain scores through the entire postoperative period.
Tramadol consumption (mg) was significantly lower in the ESP group 25.02 ± 56.8 (mg) than in the standard analgesia group 208.3 ± 88.1 (mg) (p < 0.001). Of the patients from the ESP group 6 received 100 mg of tramadol because of the significant postoperative pain (NRS ≥ 6) and 2 of the patients from the ESP group received 200 mg of tramadol because of the significant postoperative pain (NRS ≥ 6).
The incidence of nausea and vomiting was 10% (3 patients) in the ESP group and 16% (5 patients) in control multimodal analgesia group (p = 0.7). In each group 2 patients were administered ondansetron.
The ESP block adverse effects, such as bleeding, pneumothorax, local infection or anesthetic toxicity were not recorded. We did not obtain any other opioid-related adverse effect (apart from PONV) in the ESP group.
Discussion
Incisional pain after LC is very well covered by the components of multimodal analgesia but 13–27% of patients still have significant pain after 1 week [1, 15]. Since local anesthetic in the ESP block can reach spinal nerve roots and communicating branches thus potentially enabling visceral pain relief, ESP block emerged as a new and promising part of a multimodal analgesia regimen after LC. Our results confirmed potential benefits of the ESP block. Significantly lower pain scores were obtained through the entire early postoperative period (24 h) and we recorded significantly lower postoperative tramadol consumption. This is in congruence with both the studies of Altiparmak et al. and Tulgar et al. Interestingly, the latter study showed lower pain scores only in the first 3 h postoperatively [15,16,17].
Important dilemma is still present concerning ideal thoracic vertebral level and volume or concentration of local anesthetic when ESP block is administered for LC. We performed the block at the 7th thoracic level same as Chin et al. [13], Altiparmak et al. [16, 18], Hannig et al. [12] and Niraj et al. [19] but in the study of Tulgar et al. [15, 17] ESPB was performed at the 9th thoracic level. Lower level chosen for the ESP block performance in the study of Tulgar et al. could be the reason for less efficiency of ESP block as analgesic adjunct. When bilateral block is performed it is difficult to administer more than 20 ml of local anesthetic per side in order to avoid LA toxicity. With respect to optimal local anesthetic concentration for ESPB in abdominal and laparoscopic surgery the studies are lacking; there are different case series with various examples. For this reason future studies to evaluate the benefits of more or less concentrated levobupivacain (0.25%/0.375%) or ropivacain (0.35%/0.5%) are a necessity.
Obtained results are compatible with our study investigating subcostal TAP (STAP) block as part of a multimodal analgesia for LC and published in 2018 [20]. Study of Tulgar et al. did not show any advantage of ESP with respect to STAP block but the one of Altiparmak et al. from 2019 showed significant advantage of ESP block compared to STAP block for analgesia after LC [17, 18]. As STAP block represents the only regional anesthesia technique mentioned in the expert consensus, new comparable studies are strongly encouraged to define possible advantages of ESP block with respect to postoperative pain scores and opioid consumption [2].
Significantly lower postoperative consumption of tramadol documented after ESP block in our study is similar to the results of Altiparmak et al. and Tulgar et al. [15,16,17]. This is a potential benefit of the block which could contribute to less PONV and postoperative sedation occurrence. Our data did not show any difference between 2 groups in the PONV incidence. We also did not have any patients in the multimodal analgesia group with opioid-related adverse events which would postpone hospital discharge.
Although many expert panels recommend wound infiltration as a standard part of multimodal analgesia after LC, we did not use it in our study because it does not belong to our hospital analgesia protocol after LC. There is some evidence of its benefit as part of multimodal analgesia, according to Loizides et al. but is still inconclusive [21]. Meta-analysis from Guo et al. found significant advantage of TAP block to wound infiltration in postoperative analgesia after LC [22]. Easiness of wound infiltration and negligible adverse effects are strong arguments for its routine use but ESP block performed by an experienced anesthesiologist should be considered also, since it carries a minimal risk. Every study, including our own, reports negligible number of complications after ESP block but as a new technique we should be careful and recommend it only to anesthesiologists with experience in various ultrasound regional anesthesia techniques [10,11,12,13,14,15,16,17,18].
The presumed effects on visceral pain and possible influence on residual pain after LC are the most important advantages of ESP block [23]. Studies of Bisgaard et al. and Blichfeldt-Eckhardt et al. showed how severe postoperative pain after LC (especially visceral component) influences residual and chronic pain [24, 25].
It is important to note that our study has several limitations. We recorded only pain at rest, and did not have any patients who received placebo instead of LA. This limits the strength of our conclusions. Also, the influence of dexamethasone used as adjuvant for ESP block on PONV cannot be excluded. Absence of the group with LA wound infiltration also represents a significant limitation of our study. Our use of tramadol boluses instead of PCA can be also seen as a limitation. Unfortunately, there is a lack of PCA pumps in our hospital.
As a conclusion, bilateral ultrasound-guided ESPB performed at the beginning of LC significantly decreased analgesia requirements during the first 24 h, and improved the quality of multimodal analgesia compared to the standard multimodal analgesia in our study. Further studies are required to determine optimal level of ESP block and best concentration of local anesthetic.
References
Bisgaard T (2006) Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology 104:835–846
Barazanchi AWH, MacFater WS, Rahiri JI, Tutone S, Hill AG, Joshi GP (2018) Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth 121(4):787–803
Jesus RR, Leite AM, Leite SS, Vieira MC, Villela NR (2018) Anesthetic therapy for acute pain relief after laparoscopic cholecystectomy: systematic review. Rev Col Bras Cir 45(4):1885
Sinha S, Munikrishnan V, Montgomery J, Mitchell SJ (2007) The impact of patient-controlled analgesia on laparoscopic cholecystectomy. Ann R Coll Surg Engl 89(4):374–378
Lledó HB, Castro PB, Gavara IG, Cirión JLI, Andújar RL, Granero EG (2016) Twenty-five years of ambulatory laparoscopic. Cir Esp 94(8):429–441
Zhao J, Peters L, Gelzinnis S, Carroll R, Nolan J, Di Sano S, Pockney P, Smith S (2020) Post-discharge opioid prescribing after laparoscopic appendicectomy and cholecystectomy. ANZ J Surg 90:1014–1018. https://doi.org/10.1111/ans.15882
Tolchard S, Davies R, Martindale S (2012) Efficacy of the subcostal transversus abdominis plane block in laparoscopic cholecystectomy: comparison with conventional port-site infiltration. J Anaesthesiol Clin Pharmacol 28:339–343
Peng K, Ji FH, Liu HY, Wu SR (2016) Ultrasound-guided transversus abdominis plane block for analgesia in laparoscopic cholecystectomy: a systematic review and meta-analysis. Med Princ Pract 25:237–246
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ (2016) The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 41(5):621–627
Kot P, Rodriguez P, Granell M, Cano B, Rovira L, Morales J, Broseta A, Andrés J (2019) The erector spinae plane block: a narrative review. Korean J Anesthesiol 72(3):209–220
Kadam VR, Wahba M (2018) Use of erector spinae plane block in open abdominal surgery and cancer pain. J Anaesthesiol Clin Pharmacol 34(4):564–567
Hannig KE, Jessen C, Soni UK, Børglum J, Bendtsen TF (2018) Erector spinae plane block for elective laparoscopic cholecystectomy in the ambulatory surgical setting. Case Rep Anesthesiol 2018:5492527
Chin KJ, Malhas L, Perlas A (2017) The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med 42(3):372–376
De Cassai A, Bonvicini D, Correale C, Sandei L, Tulgar S, Tonetti T (2019) Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol 85(3):308–319
Tulgar S, Kapakli MS, Senturk O, Selvi O, Serifsoy TE, Ozer Z (2018) Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth 49:101–106
Altiparmak B, Korkmaz Toker M, Uysal AI, Kuşçua Y, Gümüş Demirbilek S (2019) Efficacy of ultrasound-guided erector spinae planeblock for analgesia after laparoscopiccholecystectomy: a randomized controlled trial. Braz J Anesthesiol 69(6):561–568
Tulgar S, Kapakli MS, Kose HC, Senturk O, Selvi O, Serifsoy TE et al (2019) Evaluation of ultrasound-guided erector spinae plane block and oblique subcostal transversus abdominis plane block in laparoscopic cholecystectomy: randomized, controlled, prospective study. Anesth Essays Res 13:50–56
Altıparmak B, Korkmaz Toker M, Uysal AI, Kuşçu Y, Gümüş Demirbilek S (2019) Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: Randomized, controlled trial. J Clin Anesth 57:31–36
Niraj G, Zubair T (2018) Continuous erector spinae plane (ESP) analgesia in different open abdominal surgical procedures: a case series. J Anesth Surg 5(1):57–60
Vrsajkov V, Mančić N, Mihajlović D, Milićević ST, Uvelin A, Vrsajkov JP (2018) Subcostal transversus abdominis plane block can improve analgesia after laparoscopic cholecystectomy. Rev Bras Anestesiol 68(2):149–153
Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR (2014) Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev 3:CD7049
Guo Q, Li R, Wang L, Zhang D, Ma Y (2015) Transversus abdominis plane block versus local anaesthetic wound infiltration for postoperative analgesia: a systematic review and meta-analysis. Int J Clin Exp Med 8(10):17343–17352
Kwon H, Kim D, Jeong S et al (2020) Does erector spinae plane block have a visceral analgesic effect?: A randomized controlled trial. Sci Rep 10:8389
Bisgaard T, Rosenberg J, Kehlet H (2005) From acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysis. Scand J Gastroenterol 40(11):1358–1364
Blichfeldt-Eckhardt MR, Ording H, Andersen C, Licht PB, Toft P (2014) Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain 155(11):2400–2407
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. Vrsajkov, N. Ilić, A. Uvelin, R. Ilić, M. Lukić-Šarkanović and A. Plećaš-Đurić declare that they have no competing interests.
Ethical standards.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Vrsajkov, V., Ilić, N., Uvelin, A. et al. Erector spinae plane block reduces pain after laparoscopic cholecystectomy. Anaesthesist 70 (Suppl 1), 48–52 (2021). https://doi.org/10.1007/s00101-021-01015-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00101-021-01015-5