Abstract
Purpose
Peripheral nerve blockade (PNB) is a useful tool for pain control in the perioperative period. However, there are significant concerns about the use of PNBs following acute orthopaedic trauma due to the theoretical risk of masking acute compartment syndrome (ACS). This study aims to systematically review the effects of PNBs on diagnosis of ACS following long bone fractures.
Methods
A systematic review of the literature was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Six studies, all of which were single-patient case reports, met criteria for inclusion in this review. Two studies reported a delay in diagnosis of ACS in the setting of PNB use, while four studies did not.
Conclusions
Due to the low incidence of ACS, there is a paucity of literature available on ACS following PNB use in the setting of orthopedic trauma. There is no consensus in the literature about the safety of PNB use in the setting of acute long bone fractures, and this review could draw no conclusions from the literature, as the level of evidence is limited to case reports. PNBs should be administered to orthopedic trauma patients only in strictly controlled research environments, and surgeons should be highly cautious about using PNBs for orthopedic long bone fractures, particularly in cases at increased risk for developing ACS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long bone extremity fractures are associated with significant pain [1] and an inherent risk for acute compartment syndrome (ACS). ACS is both a limb and life-threatening emergency that requires urgent surgical intervention to minimize irreversible damage and morbidity. ACS is caused by an elevation of interstitial pressure within the relatively inelastic osteofascial compartments that can lead to inadequate perfusion and subsequent ischaemic tissue damage [2,3,4]. Most common in diaphyseal long bone fractures of the distal extremities; the reported incidence of compartment syndrome is 3% in forearm diaphyseal fractures and 0.25% in distal radius fractures [5]. The prevalence of ACS in tibial shaft fractures ranges from 2.7 to 15%, making up almost one-third of all cases of ACS that occur secondary to fracture [5]. While uncommon, the sequelae associated with delayed or missed ACS can have profound effects on limb function and have become a common source of litigation against orthopedic surgeons [6,7,8,9,10]. The diagnosis of ACS relies on clinical examination, and therefore the surgeon must have a high index of suspicion for ACS in at-risk patients. Pain out of proportion to the injury and pain with passive stretch of the offending compartment have been shown to be more reliable markers of early ACS than the classic “six Ps” (pain, paresthesia, pallor, paralysis, poikilothermia, pulselessness), which more often signify the presence of irreversible tissue damage [11,12,13]. In the equivocal or the unexaminable patient, intracompartmental pressure measurement can guide diagnosis and treatment [3, 14,15,16].
Peripheral nerve blocks (PNBs) can be an effective anesthetic modality for managing pain associated with long bone fractures during the perioperative phase [17]. They are associated with increased patient satisfaction, decreased post-operative pain, reduced opioid use, and decreased nausea when utilized for total knee arthroplasty (TKA) [18, 19]. In the setting of orthopedic long bone fractures, PNBs can be delivered as a one-time dose, in long-acting formulations, or through a continuous infusion [20]. Local anesthetic blockade of peripheral nerves by PNBs can block impulse transductions, thereby limiting the perception of pain [21]. However, this poses a potential risk in the diagnosis of ACS [22]. Concerns regarding delayed or missed diagnosis of ACS due to decreased pain secondary to PNB use have led to significant debate regarding the use of regional anesthesia in the setting of patients at high risk for ACS [22]. Risk factors for the development of ACS include young age, high energy injuries of the forearm and femoral shaft, tibial fractures, polytrauma, and congenital/iatrogenic coagulopathies [5]. In addition to PNBs, epidural anesthesia and intravenous opioid patient-controlled analgesia (PCA) have been implicated in delayed diagnosis of ACS [22,23,24,25,26].
At the authors’ institution, regional anesthesia is routinely employed prior to surgery on long bone fractures, but there is little evidence to support this practice. The existing literature on PNB use and ACS in fractures is limited to case reports and expert opinion. Furthermore, a significant number of these case reports are confounded by concomitant utilization of epidural anesthesia or intravenous opioid PCA. This study aims to systematically review the available literature to determine the effect of PNB use on the diagnosis and subsequent management of ACS in long bone fractures.
Materials and methods
Search criteria
The MEDLINE/PUBMED and SCOPUS database were queried for publications utilizing keywords on acute compartment syndrome, regional anesthesia, and nerve blockade (Table 1). The search was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Inclusion and exclusion criteria
Studies that involved the following were included: (1) patients with extremity long bone fractures, (2) administration of regional anesthesia to the fractured limb, (3) development of compartment syndrome in the fractured limb. Studies that involved the following were excluded: (1) concomitant neuraxial anesthesia, (2) concomitant opioid intravenous patient-controlled analgesia (PCA) use, (3) non-English language publications, (4) review articles (5) comments/letters to the editor, (6) injury or procedure unrelated to extremity fracture. Neuraxial anesthesia and opioid PCA use were excluded due to evidence suggesting that these modalities may independently delay diagnosis of ACS.
Data collection
Two authors independently conducted the search. During initial review of the data, the following information was collected for each study: title, author, year published, study design, type and formulation of regional anesthesia, concomitant use of other anesthesia, type of fracture, type of fracture fixation, time to diagnosis of acute compartment syndrome (ACS), symptoms leading to diagnosis of ACS, intervention performed, and presence of residual symptoms. All authors compiled a list of articles that met inclusion and exclusion criteria. Discrepancies between the authors were resolved by discussion. The level of evidence for each article was independently assessed with the Oxford Centre for Evidence-based Medicine (CEBM) grading system [27].
Results
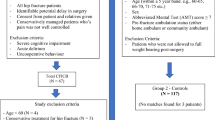
140 unique articles were identified using the search criteria. 49 of these articles met inclusion criteria. After applying the exclusion criteria, six articles remained and were included in this review (Fig. 1). 43 articles were excluded due to concomitant opioid PCA or neuraxial anesthesia use, non-orthopedic trauma-related injuries and procedures, or because they were review articles, comments, or letters to the editor. All six remaining studies were case reports and therefore Center for Evidence-Based Medicine (CEBM) level IV.
Delay in diagnosis of acute compartment syndrome
Two cases suggested a delay in diagnosis of acute compartment syndrome (ACS) after peripheral nerve block (PNB) (Table 2). Ganeshan et al. reported the case of a 75-year-old male who underwent open reduction and volar plating of a volarly displaced distal radius fracture. This procedure was performed 2 weeks after initial failure of closed reduction and percutaneous pinning and 5 weeks from initial injury [28]. An axillary brachial plexus block was placed pre-operatively and the patient was discharged the same day without significant pain or discomfort. The patient returned to the emergency department 24 h later due to swelling and blisters in his operative extremity, but he did not report significant resting pain at that time. He was noted to have median nerve paresthesias and pain with passive stretch of the digits, and compartment pressures were obtained and found to be elevated. He underwent emergent volar compartment fasciotomy and carpal tunnel release. At 12-month follow-up, he had 18% decreased grip strength compared to the contralateral, dominant arm.
Hyder et al. reported the case of a 28-year-old male who underwent intramedullary nailing of a tibial shaft fracture with pre-operative use of a triple nerve block (femoral nerve, obturator nerve, and lateral cutaneous nerve of the thigh) [29]. The patient remained pain-free post-operatively but had intermittent patchy paresthesias in the foot and an inability to extend the great toe. After 48 h of persistent symptoms, compartment pressures were measured and found to be elevated in the anterior compartment. Fasciotomy was performed, and the entire anterior compartment musculature was nonviable and debrided. At follow-up, the patient required an ankle–foot orthosis for ambulation due to foot drop.
No delay in diagnosis of ACS
Four cases reported timely diagnosis of ACS in patients receiving PNBs (Table 2). Rauf et al. reported on a 19-year-old male who underwent revision volar plating of a radial shaft fracture 12 days after initial failed open reduction internal fixation (ORIF) [30]. The patient was given a supraclavicular brachial plexus block pre-operatively. Twenty minutes after extubation, the patient began complaining of progressively worsening pain over the lateral forearm despite dense numbness in his hand distally. Significant swelling, loss of radial pulse, and prolonged capillary refill time were noted and he was taken back emergently for a rapidly expanding volar compartment hematoma. The hematoma was evacuated, and no full-length fasciotomy was required. Sensory and motor deficits from the PNB began resolving at 6 h, after which the patient had no residual paresthesias or deficits.
Aguirre et al. reported the case of a 47-year-old woman who underwent ORIF of a distal humerus fracture with pre-operative placement of a continuous infraclavicular brachial plexus PNB catheter [2]. Fourteen hours post-operatively, the patient began complaining of severe forearm pain despite complete motor and sensory blockade distally in the hand. The pain persisted despite an additional bolus through the PNB catheter, prompting measurement of compartment pressures, which were elevated in the dorsal compartment of the forearm. The patient underwent fasciotomy, and muscles of the dorsal compartment of the forearm were severely edematous but still viable. There were no long-term sequelae at 3-month follow-up.
Uzel et al. reported the case of a 26-year-old male who underwent intramedullary nailing of a closed femoral shaft fracture with a pre-operative femoral nerve block [31]. Eighteen hours post-operatively, the patient began reporting a severe increase in thigh pain without sensory or motor deficit. Significant swelling of the anterior compartment was noted and compartment pressures were elevated. The patient underwent emergent fasciotomy and the muscle of the anterior compartment was noted to be under tension but viable. The patient recovered full quadriceps strength relative to the contralateral side at 4 months.
Munk-Andersen et al. reported the case of a 12-year-old male who underwent external fixation of an open distal tibial shaft fracture with pre-operative and post-operative sciatic nerve block [32]. On post-operative day 1, the patient continued to have uncontrolled pain with an intact sensory and motor exam, and a continuous sciatic PNB catheter was placed with significant improvement in symptoms. Seven hours after catheter placement, the patient was noted to have a tense calf and pain with passive dorsiflexion of the ankle. After an additional hour, he began having severe calf pain and was taken for emergent four-compartment fasciotomy. The muscle was under tension in three compartments but viable in all four compartments, and the patient recovered with no sensory or motor deficits.
Discussion
While acute compartment syndrome can have devastating consequences to limb function, early diagnosis and treatment of ACS can lead to improved outcomes [33]. It is important to be able to identify patients who are at high risk of developing ACS. Duckworth et al. found that age less than 35 years, male gender, fractures involving the diaphysis of a long bone, polytraumas, and clotting disorders were associated with a higher rate of ACS [5]. Of note, tibial shaft fractures had the highest reported incidence of ACS, ranging from 2.7 to 15% [5]. Branco et al. noted an association between mechanism of injury and rates of ACS. They found that the following mechanisms were most likely to result in ACS, in order of weakest to strongest association: gunshot wounds, penetrating stab wounds, motorcycle accidents, and pedestrians struck by motor vehicles [34]. There is conflicting evidence on rates of ACS in open versus closed fractures; however, open fractures may represent higher energy trauma, which has been associated with a higher rate of ACS [5, 34].
The role of regional anesthesia in patients with orthopedic injuries continues to grow [33]. PNBs have been shown to significantly decrease pain, narcotic use, and hospital length of stay after orthopedic surgery [17,18,19, 35]. However, since increasing pain is considered the earliest sign of compartment syndrome, there is significant concern that PNBs may lead to a delay in the diagnosis of ACS. A review of the limited available literature demonstrated two cases in which the authors believed PNB use resulted in an ACS delay and worse overall outcomes. Ganeshan et al. describe an atypical case of a revision surgery significantly delayed from initial trauma [28]. While evidence suggests that distal radius fractures are a cause of 37.5% of forearm compartment syndromes, this patient was far out of the window for developing ACS from injury and had no significant risk factors for ACS [36]. The authors noted that this case changed their practice, and now patients are only discharged home after regaining distal sensory and motor function. Hyder et al. described the case of a young patient with a tibial shaft fracture that underwent intramedullary nailing. This patient could have been categorized preoperatively as having a high risk for ACS, given the location of the fracture and age of the patient [5, 29]. The surgical team elected to place a three-in-one regional PNB, and although pain was well controlled post-operatively, this likely led to a delay in diagnosis and a resultant residual foot drop. This case highlights the importance of risk-stratifying patients prior to initiating a dense sensory blockade.
The remaining studies included in this review failed to show an association between PNB use and ACS [2, 30,31,32]. In all four cases, severe pain was the presenting symptom despite PNB analgesia. Timely intervention was performed, and patients were left without residual deficits. While PNBs can reliably blunt the pain response, these findings suggest that the level of pain experienced during ACS is sufficient to break through the blockade provided by regional anesthesia. Some studies have even posited that PNBs may facilitate the diagnosis of ACS, as increasing pain in the setting of peripheral nerve blockade is an unusual finding and may sway the clinician towards diagnosing ACS and treating it in a timely fashion [37]. The authors believe that this phenomenon of sensing breakthrough pain despite peripheral nerve blockade is due to the local nerve endings still effectively transmitting a pain response for certain pain etiologies (i.e., ischemia) and pain level magnitudes. An alternative hypothesis is that breakthrough pain is detected by nearby un-blocked nerve endings sending excitatory signals to the central nervous system. This latter mechanism is less plausible as the primary mechanism, as one would expect that breakthrough pain would go unnoticed in cases utilizing epidural anesthesia, since the nearby nerve endings would also be under blockade.
To avoid confounders, this present review did not include epidural analgesia or concomitant opioid PCA use. With epidural anesthesia, similar to cases with PNBs, patients can sense breakthrough pain or discomfort as ACS begins to develop. Kontrobarsky et al. reported a case of gluteal compartment syndrome in a 70-year-old male following ankle arthrodesis and Patillo et al. reported leg compartment syndrome following a pilon fracture repair with perioperative epidural anesthesia [38, 39]. In both cases, breakthrough pain alerted the surgical team to the diagnosis, and appropriate intervention occurred with no sequelae of compartment syndrome were noted at the 12-month follow up. However, other cases demonstrate the effect of epidural anesthesia in masking the presence of compartment syndrome. Morrow et. al reported a delay in the diagnosis of leg ACS following femoral intramedullary nailing, diagnosed only by significant visible turgidity of the calf muscle and compartment pressure measurements [40]. Haggins et al. reported five cases of compartment syndrome following total knee arthroplasty with peri-operative epidural anesthesia that resulted in permanent muscle injury in at least one compartment [41, 42]. Additional case reports noted a delay in the diagnosis of lower extremity compartment syndrome with permanent sequelae in the setting of epidural use [43, 44]. Given the unpredictable effect of epidural analgesia on the diagnosis of ACS, these cases were excluded from this review.
Similar to epidural anesthesia, opioid PCA use in the post-operative period has been reported to independently delay the diagnosis of ACS following orthopedic procedures. Harrington et al. and O’Sullivan reported two cases of ACS in the lower limb following a tibial intramedullary nail in which an absence of pain led to delay in diagnosis of compartment syndrome [22, 23]. No long-term sequelae were noted. Richards et al. report a similar experience with four intramedullary nails for tibial shaft fractures [24]. In all four cases, the use of opioid PCA was thought to have contributed to a delay in ACS diagnosis, as all patients were relatively pain free, and fasciotomies were only performed once tense compartments with elevated pressures were noted. All cases ended with lasting neurologic dysfunction. It is important to note that pain out of proportion may not be the first sign of ACS in patients with excellent pain control. Recent literature notes that an overreliance of pain as an indicator of ACS could be a systemic error rather than a direct consequence from adequate pain control from opioid PCA [44]. Yang et al. propose that opioid PCAs may be a potential detection system for ACS [25]. The authors state that graphing trends in PCA use and serial physical examinations can lead to a diagnosis of a developing compartment syndrome [25].
At the authors’ institution, an urban level 1 trauma center, it has been commonplace to use PNBs prior to orthopedic surgery on long bone fractures. This has been standard practice for the past 4 years through extensive collaboration between the orthopedic surgery and anesthesia services. While orthopedic trauma patients are rarely denied regional anesthesia, those at particularly high risk of ACS are not given the option of a PNB. This high-risk subset of patients typically includes a combination of the following risk factors: tibial fractures, mid-shaft radius and ulna fractures, high-energy mechanism with supporting findings (i.e., segmental fracture), significant soft tissue injuries (i.e., high-grade open fracture or mangled extremity), crush injuries, and vascular injuries (at risk of compartment syndrome from ischemia–reperfusion injury). Surgery is then followed by a 24–48-h period of vigilant monitoring for the development of ACS. Using these general guidelines, there have been no cases of delayed ACS diagnosis when utilizing PNBs for long bone fractures at this institution.
The overall rate of ACS is low, with an estimated annual incidence of 7.3 per 100,000 men and 0.7 per 100,000 women [45]. There is a paucity of available literature on ACS following PNB use in the absence of neuraxial anesthesia or intravenous opioid PCA, both of which can serve as confounding variables in delay of ACS diagnosis. This review of the literature only found level IV evidence in the form of case reports, and there was no standardization of PNB type or dose and no comparative groups. Furthermore, there were conflicting findings among the case reports analyzed for this review. Given these limitations, no conclusions can be drawn about the safety of utilizing regional anesthesia in patients with long bone fractures. The great variability in patient demographics, injury mechanisms, and fracture location and morphology make it difficult to recommend the use of regional anesthesia in general practice, particularly when the potential consequences of missed ACS are so significant.
An understanding of these cases provides an initial framework for the use of PNBs in orthopedic trauma patients with long bone fractures. At this time, regional anesthesia should be administered to patients with long bone fractures only in a strictly controlled research environment. In this setting, the Idea, Development, Exploration, Assessment, Long-Term (IDEAL) study framework can be utilized. The IDEAL framework was developed to describe how evidence for a new interventional innovation could be optimally created, with emphasis on the stages of development, the characteristics of each stage, and the study design types that correspond to each stage [46]. Given the evidence analyzed for this systematic review, the use of PNBs for orthopedic long bone fractures is in the early development stage (2a), in which a single-center case series with described modifications would contribute significantly to furthering the understanding of the safety of this practice. If such a study were to be performed, it would be prudent to utilize compartment pressure measurements in addition to monitoring for clinical signs of ACS to avoid a delayed diagnosis in atypical presentations of ACS. There is precedent for this in the literature, as Hatz et al. have used compartment pressure measurements, either via the Stryker system or the arterial catheter method, to guide the need for operative fasciotomies in cases of ACS [47].
While further research should aim to perform a case series with compartment pressure monitoring in a controlled setting, there is presently insufficient evidence to support the safety of regional anesthesia in the setting of orthopedic long bone fractures at risk for ACS. If PNBs are utilized, the surgical team should be hypervigilant about atypical presentations of ACS and have a high index of suspicion for ACS should symptoms arise.
References
Minick P, Clark PC, Dalton JA, Horne E, Greene D, Brown M. Long-bone fracture pain management in the emergency department. J Emerg Nurs. 2012;38(3):211–7.
Aguirre JA, Gresch D, Popovici A, Bernhard J, Borgeat A. Case scenario: compartment syndrome of the forearm in patient with an infraclavicular catheter: breakthrough pain as indicator. Anesthesiology. 2013;118(5):1198–205.
Whitesides TE Jr, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43–51.
Matsen FA, 3rd. Compartmental syndrome. A unified concept. Clin Orthop Relat Res. 1975;(113):8–14.
Duckworth AD, McQueen MM. The diagnosis of acute compartment syndrome: a critical analysis review. JBJS Rev. 2017;5(12):e1.
Schmidt AH. Acute compartment syndrome. Orthopedic Clin. 2016;47(3):517–25.
Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93–7.
Lagerstrom CF, Reed RL 2nd, Rowlands BJ, Fischer RP. Early fasciotomy for acute clinically evident posttraumatic compartment syndrome. Am J Surg. 1989;158(1):36–9.
Hayakawa H, Aldington DJ, Moore RA. Acute traumatic compartment syndrome: a systematic review of results of fasciotomy. Trauma. 2009;11(1):5–35.
Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864–8.
Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625–32.
Cascio BM, Wilckens JH, Ain MC, Toulson C, Frassica FJ. Documentation of acute compartment syndrome at an academic health-care center. J Bone Joint Surg Am. 2005;87(2):346–50.
Mannion S, Capdevila X. Acute compartment syndrome and the role of regional anesthesia. Int Anesthesiol Clin. 2010;48(4):85–105.
McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99–104.
McQueen MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78(1):95–8.
White TO, Howell GE, Will EM, Court-Brown CM, McQueen MM. Elevated intramuscular compartment pressures do not influence outcome after tibial fracture. J Trauma. 2003;55(6):1133–8.
Clark L, Robinson M, Varbanova M. Role of regional anesthesia in orthopedic trauma. Anesthesiol Clin. 2014;32(4):789–808.
Paul JE, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113(5):1144–62.
Pagnotto MR, Pagnano MW. Multimodal pain management with peripheral nerve blocks for total knee arthroplasty. Instr Course Lect. 2012;61:389–95.
Wiederhold BD, Garmon EH, O'Rourke MC. Nerve Block Anesthesia. [Updated 2019 Jun 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431109/
Vadhanan P, Tripaty DK, Adinarayanan S. Physiollogical and pharmacologic aspects of peripheral nerve blocks. J Anaesthesiol Clin Pharmacol. 2015;31(3):384–93.
Klucka J, Stourac P, Stouracova A, Masek M, Repko M. Compartment syndrome and regional anaesthesia: Critical review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(3):242–51.
O’Sullivan MJ, Rice J, McGuinness AJ. Compartment syndrome without pain! Ir Med J. 2002;95(1):22.
Harrington P, Bunola J, Jennings AJ, Bush DJ, Smith RM. Acute compartment syndrome masked by intravenous morphine from a patient-controlled analgesia pump. Injury. 2000;31(5):387–9.
Richards H, Langston A, Kulkarni R, Downes EM. Does patient controlled analgesia delay the diagnosis of compartment syndrome following intramedullary nailing of the tibia? Injury. 2004;35(3):296–8.
Yang J, Cooper MG. Compartment syndrome and patient-controlled analgesia in children—analgesic complication or early warning system? Anaesth Intensive Care. 2010;38(2):359–63.
Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85(1):1–3.
Ganeshan RM, Mamoowala N, Ward M, Sochart D. Acute compartment syndrome risk in fracture fixation with regional blocks. BMJ Case Rep. 2015;pii: bcr2015210499.
Hyder N, Kessler S, Jennings AG, De Boer PG. Compartment syndrome in tibial shaft fracture missed because of a local nerve block. J Bone Joint Surg Br. 1996;78(3):499–500.
Rauf J, Iohom G, O’Donnell B. Acute compartment syndrome and regional anaesthesia—a case report. Rom J Anaesth Intensive Care. 2015;22(1):51–4.
Uzel AP, Steinmann G. Thigh compartment syndrome after intramedullary femoral nailing: possible femoral nerve block influence on diagnosis timing. Orthop Traumatol Surg Res. 2009;95(4):309–13.
Munk-Andersen H, Laustrup TK. Compartment syndrome diagnosed in due time by breakthrough pain despite continuous peripheral nerve block. Acta Anaesthesiol Scand. 2013;57(10):1328–30.
Via AG, Oliva F, Spoliti M, Maffulli N. Acute compartment syndrome. Muscles Ligaments Tendons J. 2015;5(1):18–22.
Branco BC, Inaba K, Barmparas G, Schnuriger B, Lustenberger T, Talving P, Lam L, Demetraides D. Injury. 2011;42(10):1157–63.
Lenart MJ, Wong K, Gupta RK, Mercaldo ND, Schildcrout JS, Michaels D, Malchow RJ. The impact of peripheral nerve techniques on hospital stay following major orthopedic surgery. Pain Med. 2012;13(6):828–34.
Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36(3):535–43.
Gadsden J, Warlick A. Regional anesthesia for the trauma patient: improving patient outcomes. Local Reg Anesth. 2015;8:45–55.
Kontrobarsky Y, Love J. Gluteal compartment syndrome following epidural analgesic infusion with motor blockage. Anaesth Intensive Care. 1997;25(6):696–8.
Patillo D, Della Rocca GJ, Murtha YM, Crist BD. Pilon fracture complicated by compartment syndrome: a case report. J Orthop Trauma. 2010;25(6):e54–e5757.
Morrow BC, Mawhinney IN, Elliott JR. Tibial compartment syndrome complicating closed femoral nailing: diagnosis delayed by an epidural analgesic technique—case report. J Trauma. 1994;37(5):867–8.
Haggis P, Yates P, Blakeway C, Fick D, Morgan DA, Holt M, Wood D. Compartment syndrome following total knee arthroplasty: a report of seven cases. J Bone Joint Surg Br. 2006;88(3):331–4.
Kumar V, Saeeed K, Panagopoulous A, Parker PJ. Gluteal compartment syndrome following joint arthroplasty under epidural anaesthesia: a report of 4 cases. J Orthop Surg (Hong Kong). 2007;15(1):113–7.
Pachecho RJ, Buckley S, Oxborrow NJ, Weeber AC, Allerton K. Gluteal compartment syndrome after total knee arthroplasty with epidural postoperative analgesia. J Bone Joint Surg Br. 2001;83(5):739–40.
Mannion S, Lee P, Taylor C. Cold Case Files: 15 years on, did patient controlled analgesia mask acute compartment syndrome? Ir Med J. 2017;110(7):625.
McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82(2):200–3.
McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105–12.
Hatz BA, Frima H, Sommer C. Selective fasciotomy for acute traumatic lower leg compartment syndrome: is it feasible? Arch Orthop Trauma Surg. 2019;139(12):1755–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Andrew Tran, Danny Lee, Safa Fassihi, Evan Smith, Ryan Lee, and Gautam Siram have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tran, A.A., Lee, D., Fassihi, S.C. et al. A systematic review of the effect of regional anesthesia on diagnosis and management of acute compartment syndrome in long bone fractures. Eur J Trauma Emerg Surg 46, 1281–1290 (2020). https://doi.org/10.1007/s00068-020-01320-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-020-01320-5