Abstract
Purpose
To evaluate feasibility and efficacy of Stereotactic Body Radiation Therapy (SBRT) for unresectable liver metastasis in oligometastatic patients.
Methods
Oligometastatic patients with up to three liver metastases of a maximum diameter of 6 cm were treated with SBRT. Total dose was 75 Gy in three consecutive fractions. Study endpoints were efficacy of this fractionation in terms of local control (LC), overall survival (OS), toxicity, and prognostic factors affecting OS and LC.
Results
Between February 2010 and December 2016, we enrolled 202 patients, with a total of 268 unresectable liver metastases. Median follow-up time from SBRT was 33 months (5–87 months). One-, 3‑, and 5‑year LC rates were 92%, 84%, and 84%, respectively. In univariate analysis, the primary histology and previous local ablative therapies were significant. Median OS was 21 months and the survival rates were 79%, 27%, and 15% at 1, 3, and 5 years after SBRT, respectively. At univariate analysis, sex, primary disease histology, intra-, and extra-hepatic progression were significant prognostic factors. This analysis confirmed the absence of late toxicity >G3.
Conclusion
This study confirms the efficacy and safety of SBRT for unresectable liver metastases. Selection of cases may improve survival and LC.
Zusammenfassung
Zielsetzung
Bewertung von Durchführbarkeit und Wirksamkeit von SBRT („stereotactic body radiation therapy“), für nichtresezierbare Lebermetastasen bei oligometastatischen Patienten.
Methoden
Oligometastatische Patienten mit bis zu 3 Lebermetastasen mit einem maximalen Durchmesser von 6 cm wurden mit SBRT behandelt. Gesamtdosis war 75 Gy in 3 Fraktionen hintereinander. Endpunkt der Untersuchung war die Wirksamkeit der Fraktionierung bezüglich lokaler Kontrolle (LC), Gesamtüberleben (OS), Toxizität und prognostischer Faktoren mit Einfluss auf OS und LC.
Ergebnisse
Zwischen Februar 2010 und Dezember 2016 wurden 202 aus 268 Patienten mit nichtresezierbaren Lebermetastasen ausgewählt. Mediane Nachbeobachtungszeit nach SBRT war 33 Monate (Spanne 5–87 Monate). Die 1‑, 3‑ und 5‑Jahres-LC-Raten lagen jeweils bei 92%, 84% und 84%. In der univariaten Analyse waren die Primärhistologie und die früheren ablativen lokalen Therapien signifikant. Das mediane OS lag bei 21 Monaten und die Überlebensraten waren 1, 3 und 5 Jahre nach SBRT jeweils bei 79%, 27% und 15%. In der univariaten Analyse waren Geschlecht, primäre Krankheitshistologie, intra- und extrahepatische Progression signifikante prognostische Faktoren. Diese Analyse bestätigte die Abwesenheit von Spättoxizität >G3.
Schlussfolgerung
Diese Untersuchung bestätigt die Wirksamkeit und die Sicherheit von SBRT für nichtresezierbare Lebermetastasen. Eine Fallauswahl kann die Überlebensrate und die LC verbessern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligometastatic disease is an intermediate stage between absence of metastases and systemic dissemination [1]. According to recent literature, oligometastatic patients are characterized by up to 5 metastases at 1–3 sites, all suitable for ablative treatment [2].
The liver is one of the sites more often affected by metastases from solid malignancies. Colorectal cancer (CRC) is one of the most frequently occurring tumors with solitary or oligometastatic liver disease [3, 4].
In CRC liver metastasis, the introduction of polychemotherapy has increased response rates and median overall survival (OS) to 40–57% and 15–20 months, respectively [5]. Afterwards, surgical resection of liver metastases improved OS, with 1‑ and 5‑year OS rates of 90–95% and 30–60%, respectively, and with a median OS of 40–53 months [6,7,8,9]. The role of surgery of non-CRC liver metastases is controversial: some papers have shown a better prognosis only in a subgroup of neuroendocrine metastases [10,11,12,13]. Adams et al. evaluated outcomes of 1452 patients with limited liver metastases from non-CRC and non-neuroendocrine metastasis [14]. According to this recent literature, only patients with controlled metastatic disease and/or response to systemic therapy should be considered for liver surgery. In these selected patients, surgery may offer a real benefit in terms of survival.
However, only 10–20% of patients were suitable for surgical resection because of technical difficulties, unfavorable tumor factors, or patient comorbidities [7, 8]. In the past decade, minimally invasive loco-regional approaches were introduced as an alternative to surgery, including radiofrequency ablation (RFA), microwave ablation (MWA), trans-catheter arterial chemoembolization (TACE), and selective internal RT (SIRT) [15, 16]. Local thermal approaches, RFA, or MWA can even be a valid option to surgery valid alternative to surgery in selected patients. Five-year survival rates following RFA vary between 17 and 51%.
These minimally invasive approaches were characterized by some limitations: lesions greater than 3 cm in diameter or in the proximity of major blood vessels and main biliary tract or gallbladder. These minimally invasive approaches were characterized by some limitations: lesions greater than 3 cm in diameter or in the proximity of major blood vessels and main biliary tract or gallbladder.
Historically, the role of radiotherapy (RT) was only palliative, due to the low whole-liver tolerance to RT. A high dose of radiotherapy to a large volume of healthy hepatic tissue was a risk for radiation-induced liver disease (RILD). According to the radiobiological model, the liver is a parallel organ, so the risk of RILD is proportional to the mean radiation dose delivered to normal hepatic tissue. Development of stereotactic body radiation therapy (SBRT) allowed delivery of a high dose of radiation in few fractions with maximum sparing of healthy tissues. To date, there are eight prospective trials published regarding SBRT for the treatment of liver metastases [17,18,19,20,21,22,23]. This treatment was well tolerated, with a low risk of RILD, and very effective, with local control rates at 1 year of 70–100%.
Materials and methods
Patient data were retrospectively analyzed after the approval of our Ethical Review Committee.
Study endpoints were to evaluate the efficacy of liver SBRT in terms of local control, overall survival (OS), and radiation treatment-related toxicity. Prognostic factors affecting OS and local control (LC) were investigated.
Inclusion criteria, which were defined in our previously published study, were as follows: liver metastases considered not suitable for surgery, technically or medically inoperable, or because of patient refusal; maximum tumor diameter less than 6 cm; no more than 3 liver lesions; normal liver volume greater than 1000 cm3; no evidence of progressive or untreated gross disease outside of the liver; no prior radiation therapy to the target area; adequate liver function; no concurrent chemotherapy allowed, either within 14 days before SBRT or until the first follow-up evaluation thereafter; no active connective tissue disorders; Karnofsky performance status ≥70; minimum age 18 years; and written informed consent.
Stereotactic body radiation therapy requires highly precise dose planning and delivery. First of all, each patient must be immobilized during the simulation phase. A thermoplastic mask (Klarity®, Newark, OH, USA) with an abdominal compression is used to maximally reduce organ motion related to the respiratory cycle. A contrast-free computed tomography scan and a three-phase contrast-enhanced CT scan are acquired at 3‑mm slice thickness. A 4-dimensional CT (4DCT) is acquired if the lesion is located in the posterior–superior segments, or with a shift greater than 5 mm on the different phases of CT. Multi-modal imaging with contrast-enhanced magnetic resonance imaging (MRI) and/or positron-emission tomography (PET) is used in doubtful or special cases for better target definition. The clinical target volume (CTV) is the lesion visualized on CT images and is equal to the gross tumor volume (GTV). The internal target volume (ITV), for all patients who underwent 4D-CT scan, is defined as the envelope of all GTVs in the different respiratory phases. The planning target volume (PTV) is generated from either the GTV or the ITV by adding an overall isotropic margin of 5 mm (from ITV) or of 7–10 mm in the cranial–caudal axis and 4–6 mm in the anterior–posterior and lateral axes.
Treatment planning SBRT requires a highly conformal dose distribution, with multiple beams using either coplanar or non-coplanar geometries. Stereotactic body radiation therapy for liver metastases is performed with volumetric modulated arc therapy. Before each treatment fraction, patient and liver lesion position are checked with cone-beam CT (CBCT). In selected patients, surgical clips or outcomes of previous treatments are used as fiducial markers.
The prescription dose was 75 Gy in three consecutive daily fractions of 25 Gy each. The dose was chosen in order to overcome the radioresistance of some histologies (such as colorectal cancer) and to potentially increase LC for big lesions. The plan objective was to cover at least 98% of the CTV (ITV) volume with 98% of the prescribed dose (V98% = 98%) and V95% = 95% for the PTV. According to ICRU 91, Dmax < 110%, D2% < 107%, and D98% > 98%.
When full doses were not achievable due to OAR, the total dose was reduced by 10%, 20%, or 30%, with patients receiving 67.5 Gy, 61.89 Gy, or 56.25 Gy, respectively. Regarding constraints for organs at risk, we applied a critical dose–volume model according to the surgical and radiotherapy studies [20, 21, 24, 25]. Conservatively, we required that 700 mL of healthy liver were irradiated with a maximum dose of 15 Gy. Spinal cord, heart, and gastrointestinal organs (stomach, duodenum, and small bowel) must receive less than 18 Gy, 30 Gy, and 21 Gy in three fractions, respectively. For both kidneys and rib, dose constraints were V15 Gy < 35% and D30cm3 < 30 Gy.
We evaluated acute (<6 months) and late toxicity. Common Terminology Criteria for Adverse Events (CTCAE version 4.0) was used to score adverse events. Radiation-induced liver disease is defined by Lawrence’s criteria as a subacute toxicity occurring within 4 months after the end of radiation treatment. The diagnosis of RILD is excluded in the presence of liver disease progression.
In this study, assessment of tumor response was based on European Organization for Research and Treatment of Cancer Response Evaluation Criteria in Solid Tumors (EORTC-RECIST) criteria version 1.1 [26].
Patients were monitored by physical examination at the beginning and the last day of the SBRT, and with basal blood chemistry analysis. After conclusion of SBRT, these examinations were requested 21 days later and then every 3 months. Follow-up was performed every 3 months, which included CT imaging and, in selected cases, MRI. When a PET scan was available pre-treatment, it was also required after 6 months to confirm metabolic response or progression.
Statistical considerations
Actuarial LC and OS curves were generated using the Kaplan–Meier method. All enrolled patients were included in the statistical evaluation. The analysis of LC was defined as the time from the beginning of SBRT to the progression of treated metastases or last follow-up. The influence of patient, disease, and treatment characteristics on OS and LC were evaluated using Cox proportional hazards regression. All analyses were performed using STATA version 1 software and a p-value was considered significant if <0.05.
Results
Between February 2010 and December 2016, we analyzed 202 patients with 268 liver metastases from solid tumors treated with SBRT. Baseline patient and treatment characteristics are listed in Table 1.
Seventy-two patients (35.6%) had synchronous metastatic disease at the time of diagnosis and 44 (21.8%) had metachronous liver metastases with disease-free interval (DFI) ≤12 months. Eighty-six (42.6%) had metachronous liver metastases with extended DFI >12 months. After diagnosis of metastatic disease, 153 (75.7%) patients received one or more different chemotherapy regimens. The majority of patients (64.4%) had undergone a prior local therapy. Number of treated lesions was 1 in 150 patients (74.5%), 2 in 38 patients (18.8%), and 3 in 14 patients (6.9%). Median follow-up time from SBRT was 33 months, with range of 5–87 months.
We observed complete and partial response in 126 (47%) and 29 (10.8%) patients, respectively, and stable disease in 35 (13.1%) patients.
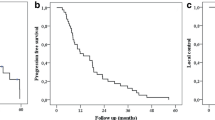
One-, 2‑, 3‑, and 5‑year LC rates were 92%, 87%, 84%, and 84%, respectively (Fig. 1a). Median local control has not been achieved. At univariate analysis, two variables affected local control, as shown in Table 2. Primary histology (Fig. 1b) and previous local ablative therapies (Fig. 1c) were significant for local control (p < 0.03 and p < 0.006, respectively), but the size of metastasis were not related to local relapse. We found that 115 patients (56.9%) had a diagnosis of out-field liver progression and 111 patients (59.4%) had the appearance of extra-hepatic metastases after SBRT.
At the time of analysis, 61 patients (30%) were alive. Median follow-up was 21 months (95% CI 18–24). The OS rates were 79%, 50%, 27%, and 15% at 1, 2, 3, and 5 years after SBRT, respectively. All patients died of out-field progression. Fig. 2 shows the Kaplan–Meyer curves for OS.
At univariate analysis, 4 out of 10 examined variables were significant prognostic factors of survival: sex, primary disease histology, intra-, and extra-hepatic progression, as shown in Fig. 2 and Table 3.
This analysis confirmed the absence of late toxicity >G3 in all patients treated with SBRT in our study. Most frequent acute toxicities were G2 fatigue in 38% of patients and G2 transient transaminase increase in 24% of patients. In all cases, the increased values normalized within the 3–6 months after SBRT. Two patients presented acute skin toxicity at about 2 months after the end of SBRT. One patient experienced G3 skin ulceration. One patient presented G2 skin induration, able to slide skin, unable to pinch skin. Six patients presented G2 nausea and/or vomiting. One case of G2 gastric ulcer was recorded after 2 months of the end of SBRT during esophagus gastroduodenoscopy for epigastric pain. In terms of chronic adverse effects, we recorded two cases of G2 rib fractures after 7 and 11 months of the end of SBRT.
Discussion
This is a report on a large cohort of patients treated with SBRT for unresectable liver metastases, using the ablative high dose of 75 Gy in three fractions.
Considering the outcome of local control, in our analysis, a few factors affected local response rate, but not lesion diameter. Previous studies have demonstrated that the response rate to liver SBRT is correlated to radiation dose. In the study by McCammon et al. [27], 246 liver and lung metastases were treated with SBRT in three fractions. Three-year local control rates were 89.3%, 59.0%, and 8.1% for lesions treated with ≥54 Gy, 36 up to 53.9 Gy, and <36 Gy, respectively. In the phase I–II trial of Rusthoven et al., a dose escalation of 36–60 Gy in three fractions was analyzed for liver metastases with a maximum diameter <6 cm [20]. Forty-seven patients with 63 hepatic metastases were treated, local control at 1 and 2 years was 95% and 92%, respectively, with a median survival of 20.5 months. In this study, dose prescription and lesion diameter affected local control. In a more recent study by McPartlin et al. [28], the relationship between dose and size response was confirmed. In this phase I–II study, large lesions were treated using 33–60 Gy in six fractions. Local control and overall survival at 4 years were 26% and 9%, respectively. So, the authors concluded that dose escalation is needed to improve local control and that patient selection is crucial to choose optimal candidates for liver SBRT. In 2013, the preliminary results of a phase II study on liver SBRT using a higher prescription dose of 75 Gy in three fractions showed the safety and the efficacy of this fractionation, without differences in LC related to lesion size [21]. In 2013, the preliminary results of a phase II study on liver SBRT using a higher prescription dose of 75 Gy in three fractions showed the safety and the efficacy of this fractionation. The relationship between dose and size response was not confirmed [21].
Our experience in a larger cohort of patients with a longer follow-up confirmed the efficacy of dose escalation in the treatment of liver metastases from different primary histologies, with a rate of LC at 3 years of 84%. Hepatic lesions from colorectal cancer are correlated with a lower local control rate of 79% at 3 years, which is, however, comparable to the response rate achieved with other local ablative therapies. These data are according to recent literature. In a report by Ahmed et al., a multigene expression index for tumor radiosensitivity (RSI) was validated. The authors assessed RSI in liver metastases and clinical outcomes after SBRT-based on primary histology. Colorectal adenocarcinoma metastases were determined to be more radioresistant than other histologies such as anal squamous cell cancer, breast, and lung adenocarcinoma [29].
Another factor related to worse local control in our study was prior local liver therapies. One explanation for this could be found in a long disease history and the primary histology of these oligometastatic patients. Surgery, RFA, TACE, and chemotherapy are all directed at reducing the tumor mass. However, in the majority of cases, tumor regrowth and relapse of disease after the end of therapy. However, in the majority of cases, tumor recurs after the end of these local therapies. Although the concept of tumor stem cells has been historically investigated, the demonstration of their existence has only occurred in the last decade. Capacity for self-renewal, production of heterogeneous progeny, and ability to limitlessly divide seem to be some important characteristics of cancer stem cells. CSCs with such characteristics have been reported for many hematological and solid tumors correlated to chemo- and radioresistance [30]. Also in our experience, the worse efficacy of SBRT in heavily locally pretreated lesions could be explained by considering these data.
Evaluating the outcome of overall survival, in our analyses, the relevant prognostic factors were primary disease histology of metastases and progression of disease after radiotherapy. Patients with CRC, breast, and gynecological cancer liver disease had a better OS according to surgical literature. In CRC liver metastases, surgery is widely accepted to have a better prognosis. In non-colorectal non-neuroendocrine liver metastases (NCNNLM), the indication for hepatic surgery is controversial, owing to the low number of cases and the heterogeneity of the primary disease [31]. Prognostic and risk factors associated with NCNNLM for different histologies were analyzed by Takemura et al. [31]. The greatest predictor of survival was primary tumor. The authors reviewed 10 studies with more than 40 patients who underwent surgery for liver metastases from breast cancer. The 3‑ and 5‑year OS rates were 49–68% and 27–53%, respectively, with median survival times of 41–115 months [14, 32,33,34,35,36,37,38,39]. Considering the good results of surgery, an increasing number of experiences about SBRT on breast and gynecological cancer liver metastases have been published [40, 41].
Recently, Andratschke et al. published a pooled analysis as part of the German Society for Radiation Oncology. They evaluated 474 patients with 623 liver oligometastases treated with SBRT. Median overall survival was 24 months. Authors confirmed that overall survival is mainly influenced by histology [42].
The second factor correlated to a worse overall survival was progression of disease after SBRT. This data is also evidenced in a recent paper by Klement et al. [43]. The authors analyzed 388 patients with 500 liver and lung metastases. Median overall survival was 25.4 months in patients with local failure after SBRT versus 30.6 months in patients without progression.
Number of lesions, metastases diameter, and extra-hepatic disease at the time of SBRT are not statistically significant for OS rates. These data suggest that an optimal selection of patients in multidisciplinary team is mandatory.
Conclusion
This study confirms the efficacy and safety of SBRT for unresectable liver metastases. Selection of cases with positive prognostic factors may improve survival and local control of these oligometastatic patients. A multidisciplinary evaluation is mandatory to define the best treatment for selected patients, in the outlook in the perspective of tailored therapeutic strategy.
Further evaluation of biological and genetic tumor profile is needed to select oligometastatic patients with indolent disease who should benefit from local ablative treatment.
References
Weichselbaum RR, Hellman S (2011) Oligometastases revisited. Nat Rev Clinoncol 8:378–382
Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M (2012) Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 17:1100–1117
Yoon SS, Tanabe KK (1999) Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist 4:197–208
Scheele J, Stangl R, Altendorf-Hofmann A (1990) Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br J Surg 77:1241–1246
Bengmark S, Hafstrom L (1969) The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 23:198–202
Cummings LC, Payes JD, Cooper GS (2007) Survival after hepatic resection in metastatic colorectal cancer: A population-based study. Cancer 109:718–726
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S (2006) Survival after hepatic resection for colorectal metastases: A 10-year experience. Ann Surgoncol 13:668–676
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 230:309–318
Leonard GD, Brenner B, Kemeny NE (2005) Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clinoncol 23:2038–2048
Berney T, Mentha G, Roth AD, Morel P (1998) Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg 85:1423–1427
Lindell G, Ohlsson B, Saarela A, Andersson R, Tranberg KG (1998) Liver resection of noncolorectalsecondaries. J Surgoncol 69:66–70
Benevento A, Boni L, Frediani L, Ferrari A, Dionigi R (2000) Result of liver resection as treatment for metastases from noncolorectal cancer. J Surgoncol 74:24–29
van Ruth S, Mutsaerts E, Zoetmulder FA, van Coevorden F (2001) Metastasectomy for liver metastases of non-colorectal primaries. Eur J Surgoncol 27:662–667
Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M et al (2006) Association Française de Chirurgie. Hepatic resection for noncolorectalnonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 244:524–535
Nosher JL, Ahmed I, Patel AN, Gendel V, Murillo PG, Moss R et al (2015) Non-operative therapies for colorectal liver metastases. J Gastrointestoncol 6:224–240
Gillams A et al (2013) Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting. Eur Radiol 2015(25):3438–3454
Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H et al (2006) Phase II study on stereotactic body radiotherapy of colorectal metastases. ActaOncol 45:823–830
van der Pool AE, Méndez Romero A, Wunderink W, Heijmen BJ, Levendag PC, Verhoef C et al (2010) Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg 97:377–382
Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R et al (2009) Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clinoncol 27:1585–1591
Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ et al (2009) Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clinoncol 27:1572–1578
Scorsetti M, Arcangeli S, Tozzi A, Comito T, Alongi F, Navarria P et al (2013) Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiatoncolbiolphys 86:336–342
Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G et al (2010) Dose escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiatoncolbiolphys 78:486–493
Ambrosino G, Polistina F, Costantin G, Francescon P, Guglielmi R, Zanco P et al (2009) Image guided robotic stereotactic radiosurgery for unresectable liver metastases: Preliminary results. Anticancer Res 29:3381–3384
Schefter TE, Kavanagh BD (2011) Radiation therapy for liver metastases. SeminRadiatOncol 21:264–270
Penna C, Nordlinger B (2002) Colorectal metastasis (liver and lung). Surgclin North Am 82:1075–1090 (Review)
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B (2009) Observation of a dose control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiatoncolbiolphys 73:112–118
McPartlin A, Swaminath A, Wang R, Pintilie M, Brierley J, Kim J et al (2017) Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int J Radiatoncolbiolphys 99:388–395
Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue B et al (2016) Radiosensitivity Differences Between Liver Metastases Based on Primary Histology Suggest Implications for Clinical Outcomes After Stereotactic Body Radiation Therapy. Int J Radiatoncolbiolphys 95:1399–1404
Oishi N, Yamashita T, Kaneko S (2014) Molecular biology of liver cancer stem cells. Liver Cancer 3:71–84
Takemura N, Saiura A (2017) Role of surgical resection for non-colorectal non-neuroendocrine liver metastases. World J Hepatol 9:242–251
Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S et al (2006) Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 244:897–907
Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC et al (2012) Hepatectomy for non colorectal non neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Collsurg 214:769–777
Hoffmann K, Bulut S, Tekbas A,Hinz U, Büchler MW, Schemmer P (2015) Is Hepatic Resection for Non-colorectal, Non-neuroendocrine Liver Metastases Justified? Ann Surg Oncol 22(Suppl. 3):1083–1092
Pocard M, Pouillart P, Asselain B, Salmon R (2000) Hepatic resection in metastatic breast cancer: results and prognostic factors. Eurj Surgoncol 26:155–159
Elias D, Maisonnette F, Druet-Cabanac M, Ouellet JF, Guinebretiere JM, Spielmann M et al (2003) An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg 185:158–164
Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, Eichbaum M et al (2010) Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surgoncol 17:1546–1554
Abbott DE, Brouquet A, MittendorfEA,Andreou A, Meric-Bernstam F, Valero V et al (2012) Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery 151:710–716
Sadot E, Lee SY, Sofocleous CT, Solomon SB, Gönen M, Kingham TP et al (2016) Hepatic Resection or Ablation for Isolated Breast Cancer Liver Metastasis: A Case-control Study With Comparison to Medically Treated Patients. Ann Surg 264:147–154
Scorsetti M, Franceschini D, De Rose F, Comito T, Villa E, Iftode C et al (2016) Stereotactic body radiation therapy: A promising chance for oligometastatic breast cancer. Breast 26:11–17
Iftode C, D’Agostino GR, Tozzi A, Comito T, Franzese C, De Rose F et al (2018) Stereotactic Body Radiation Therapy in Oligometastatic Ovarian Cancer: A Promising Therapeutic Approach. Int J Gynecol Cancer 28(8):1507–1513 (Oct)
Andratschke N, Alheid H, Allgäuer M, Becker G, Blanck O, Boda-Heggemann J et al (2018) The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. Bmc Cancer 13;18(1):283 (Mar)
Klement RJ, Abbasi-Senger N, Adebahr S, Alheid H, Allgaeuer M, Becker G et al (2019) The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. Bmc Cancer 26;19(1):173 (Feb)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Clerici, T. Comito, C. Franzese, L. Di Brina, A. Tozzi, C. Iftode, P. Navarria, P. Mancosu, G. Reggiori, S. Tomatis, and M. Scorsetti declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Clerici, E., Comito, T., Franzese, C. et al. Role of stereotactic body radiation therapy in the treatment of liver metastases: clinical results and prognostic factors. Strahlenther Onkol 196, 325–333 (2020). https://doi.org/10.1007/s00066-019-01524-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01524-8