Abstract
Purpose
The aim of this study was to retrospectively study survival and long-term morbidities of children with nasopharyngeal carcinoma (NPC) treated by induction chemotherapy and concurrent chemoradiation (CRT). The total dose of radiation was adapted to the response following neoadjuvant chemotherapy.
Methods
Children with non-metastatic NPC treated in France between 1999 and 2015 were retrospectively included in the study. The strategy combined neoadjuvant platinum-based chemotherapy, followed by adapted CRT to tumor response.
Results
In total, 95 patients (median age 15 years [range, 7–23 years], male-to-female ratio 1.8) with undifferentiated NPC were included; 59% of patients had TNM stage IV. Intensity-modulated radiotherapy (IMRT) was delivered to 57 patients (60%), while the other patients were treated with conformal RT (3D-RT). After a median follow-up of 4.5 years [range, 3.6–5.5 years], 13 relapses and seven deaths had occurred. The 3‑year overall and relapse-free survival (RFS) were 94% [95% CI, 85–97%] and 86% [77–92%], respectively. The locoregional failure rate was 6% [95% CI, 2–14]. Long-term treatment-related sequelae of grade 2+ were reported by 37 (50%) patients; odynophagia was significantly reduced treated by IMRT vs. conventional 3D-RT (7% vs. 55%, p = 0.015). Using a reduction dose of 59.4 Gy, 54 Gy, and 45 Gy, respectively, to the primary, involved, and uninvolved neck nodes, after a favorable tumor response, was not associated with an increased locoregional failure rate.
Conclusions
The survival rates for NPC have been considerably improved by means of multimodal therapy, but long-term locoregional morbidity remains common. Use of IMRT may induce less residual odynophagia. Radiation dose reduction adapted to chemotherapy response does not have a negative impact on outcome. These findings support the use of an RT protocol adapted to the tumor response to neoadjuvant chemotherapy for a long-lasting improvement in the patient’s quality of life.
Zusammenfassung
Zielsetzung
Retrospektive Analyse der Überlebenszeit und der Langzeitmorbidität von Kindern mit Nasopharynxkarzinom (NPC), die mit neoadjuvanter Chemotherapie und zeitgleicher Chemoradiotherapie (CRT) behandelt wurden. Die Strahlendosis wurde an die Reaktion auf die neoadjuvante Chemotherapie angepasst.
Methoden
Kinder mit nichtmetastasiertem NPC, die zwischen 1999 und 2015 in Frankreich behandelt wurden, wurden retrospektiv in die Studie eingeschlossen. Die Behandlungsstrategie bestand aus einer Kombination von neoadjuvanter platinbasierter Chemotherapie, gefolgt von einer an das Ansprechen auf Chemotherapie angepassten Strahlentherapie.
Ergebnisse
Fünfundneunzig Patienten (mittleres Alter 15 Jahre, Spanne7–23 Jahre; Verhältnis Männer/Frauen 1,8) mit NPC vom Typ III konnten in die Studie eingeschlossen werden; 59 % der Patienten hatten ein TNM-Stadium IV. Bei 57 Patienten (60 %) wurde eine intensitätsmodulierte Strahlentherapie (IMRT) verabreicht, während die anderen Patienten mit einer konformen RT (3D-RT) behandelt wurden. Nach einer medianen Nachbeobachtungszeit von 4,5 Jahren (Spanne 3,6–5,5 Jahre) traten 13 Rückfälle und 7 Todesfälle auf. Die 3‑Jahres-Gesamtüberlebensrate und die rezidivfreie Überlebensrate (RÜR) betrugen 94 % (95 %-Konfidenzintervall [KI] 85–97 %) bzw. 86 % (95%-KI 77–92 %). Die regionale Rückfallrate betrug 6 % (95 %-KI 2–14). Langzeitfolgeerscheinungen von Grad 2 oder höher traten bei 37 Patienten (50 %) auf; Odynophagie war bei Patienten, die mit IMRT behandelt wurden, im Vergleich zu herkömmlicher 3D-RT signifikant reduziert (7 % vs. 55 %; p = 0,015). Die Verwendung einer Reduktionsdosis von 59,4 Gy, 54 Gy bzw. 45 Gy für die primären, betroffenen und nichtbetroffenen Halsknoten nach günstiger Tumorantwort war nicht mit einer erhöhten lokoregionalen Ausfallrate verbunden.
Schlussfolgerung
Die NPC-Überlebensraten konnten durch eine multimodale Therapie erheblich verbessert werden; eine lokoregionale Langzeitmorbidität besteht jedoch nach wie vor. Das IMRT-Verfahren kann zu einer Verringerung der Odynophagie führen. Die an das Ansprechen auf Chemotherapie angepasste Dosisreduktion hat keinen negativen Einfluss auf die Überlebensraten. Diese Ergebnisse unterstützen die Verwendung eines RT-Protokolls, das an das Ansprechen auf neoadjuvante Chemotherapie angepasst ist, um die Lebensqualität des Patienten nachhaltig zu verbessern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is rare in children, but nevertheless represents the most common of the very rare pediatric tumors, accounting for about 1% of all pediatric malignancies in France. The incidence worldwide may vary strongly according to geographical differences [1]. The median age at diagnosis in the pediatric population is between 12 and 15 years [2,3,4]. Tumor stage is often locally advanced (>40–55% of TNM stage IV) at diagnosis, although distant metastases are rare (<5–10%; [3, 5,6,7,8,9]).
Sensitivity to radiotherapy (RT) and chemotherapy (ChT) has been established for a long time. Irradiation of the nasopharynx and cervical nodes therefore remains the standard local treatment with total radiation doses between 50 and 70 Gy. In recent series, based on multidisciplinary strategies, 5‑year overall survival (OS) is higher than 75% [3, 5, 10,11,12]. Other factors, such as age at onset and RT doses delivered, do not constitute prognostic factors in most pediatric studies [3, 5, 6, 13].
Distant metastasis is the predominant mode of tumor failure in pediatric NPC and occurs within the first 2 years of follow-up. Neoadjuvant ChT was initially used in children to reduce metastatic tumor spread and RT field volume. Although no prospective study has compared outcomes for pediatric patients receiving RT, with or without ChT, the OS was higher with the RT/ChT combination currently used suggesting that neoadjuvant ChT should be administered systematically in all cases of NPC in childhood and therefore it remains the standard treatment in childhood NPC [3, 5, 7, 14].
Locoregional relapse is also rare, with local control rates of up to 90–95% in the most recent studies [5, 7, 13, 15, 16]. Long-term head and neck sequelae, mainly related to irradiation, are nevertheless commonly observed in NPC in childhood [3, 5, 7, 11, 12]. In order to improve long-term locoregional control and reduce sequelae, several groups have therefore proposed the use of more conformal RT techniques with adaptation of the radiation dose to the response to neoadjuvant ChT, by decreasing the total dose and radiation volume in the case of a favorable response to neoadjuvant ChT [3, 5, 7, 10, 16].
This retrospective multicenter national study was designed to analyze therapeutic aspects and long-term toxicity data with the current systematic use of conformal RT, and to analyze an adapted strategy based on tumor response to neoadjuvant ChT in pediatric patients with NPC.
Patients and methods
This study retrospectively reviewed the medical charts of all patients with NPC treated from 1999 to 2015 in seven large French pediatric and adult oncology centers (Institut Curie, Paris; Gustave Roussy, Villejuif; Centre Oscar Lambret, Lille; Centre Léon Bérard, Lyon; Centre Paul Straus, Strasbourg; Institut Claudius Regaud, Toulouse; Institut Jean Godinot, Reims), satisfying the following inclusion criteria: biopsy-proven NPC (World Health Organization [WHO] type II or III, non-keratinizing and undifferentiated carcinoma), diagnosis before the age of 25 years, nonmetastatic disease, treated with conformal RT (3D-RT) or intensity-modulated radiation therapy (IMRT) in combination with ChT ± maintenance therapy. This study was approved by the Lille IRB (Institutional Review Board) and CCTIRS agreement of the 9.1.2016 (Advisory Committee on Information Processing in Material Research in the Field of Health).
Patients were staged according to the 5th edition of the American Joint Committee of Cancer staging system (2010; [17]). Staging and routine assessments at diagnosis included ear–nose–throat specialists using clinical examination, upper endoscopy, and head and neck magnetic resonance imaging (MRI) with ultrasound of cervical nodal regions. Radiological assessment by head and neck MRI was recommended after two or three courses of ChT [3]. Complete response (CR) to neoadjuvant ChT was defined as no evidence of residual disease; partial response (PR) was defined as a decrease of ≥50% and no evidence of new lesions; stable disease (SD) was defined as minor changes that did not meet criteria for PR or progressive disease (PD); very good PR (VGPR) was defined as no evidence of measurable disease, but persistent asymmetry of the nasopharyngeal tumor region or contrast enhancement or a decrease of ≥90% of the sum of the greatest dimensions of target lesions; and PD was defined as an increase of ≥25% of target lesions or the appearance of new lesions [18]. Favorable response was considered in the case of CR, VGPR and PR, while other cases were considered to be an unfavorable response.
Initially, ChT regimens and dosing schedules were heterogeneous, as they were based on physician decisions and initial tumor response Patients with NPC diagnosed after 2011 were systematically registered after parents/guardians’ consent in a database set up by the French very rare tumor group (FRACTURE group; [19]). Therapeutic guidelines propose neoadjuvant ChT regimens comprising three courses of 5 FU-cisplatin, followed by nasopharyngeal and cervical node RT using doses adapted to the initial response to ChT, with concurrent ChT using cisplatin (optional for patients in VGPR) followed by optional maintenance beta-interferon (IFN-β; Rebif®) therapy for 6 months [10]. One patient was previously included in a trial [15]. Severe (≥grade 2) long-term locoregional toxicities (fibrosis, xerostomia, trismus, odynophagia, xerostomia, ototoxicity, dental caries, endocrine disorders, etc.) were retrospectively graded according to the worst Common Terminology Criteria for Adverse Events v4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/), and compared after IMRT and 3DRT. The Brock grading scale was used for assessment of hearing loss [20].
Radiotherapy guidelines
Optimal immobilization of patients was recommended in a supine position with a thermoplastic mask covering the head to the shoulders. The primary lesion and lymph node involvements were defined at diagnosis by conventional scan with contrast, axial contrast-enhanced MRI with thin slices (<3 mm), and endoscopy examination. Another axial contrast-enhanced MRI with thin slices (<3 mm) was recommended again after two or three cycles of induction ChT, to evaluate the ChT response, in order to allow for RT dose adaptation. It was recommended to combine at least the second MRI with computed tomography (CT) planning.
Briefly, gross tumor volume (GTV) included the extent of disease in the primary tumor (GTV T) and cervical lymph nodes (GTV N), as observed on pre-radiation MRI or CT after neoadjuvant ChT. The clinical target volume (CTV1) included primary tumor region (GTV T and N), the whole nasopharynx, the parapharyngeal lymph nodes, as well as all sites of potential subclinical disease and all visible, and enlarged (>1 cm) cervical lymph nodes, usually with a 0.5-1-cm safety margin. A second clinical target volume (CTV2) included lymph node levels III, IV, and V as well as the supraclavicular regions, even if not involved at diagnosis. Margins from CTV to PTV were between 3 and 5 mm, depending both on the physician’s choice and RT technique. A daily dose of 1.8–2.2 Gy was delivered 5 days/week by conventional fractionation. Details of RT doses according to tumor response to neoadjuvant ChT proposed by the FRACTURE group are reported in Table 1. The total duration of RT was reviewed and considered to be non-optimal in the case of premature RT discontinuation or RT delayed by more than 15% with respect to the initially scheduled dates. The main critical organs to be delineated and to be spared as much as possible were the cochlea, parotid glands, brain stem, spinal cord, pituitary gland, eyes, lens, chiasma, optic nerves, larynx, and brachial plexus.
Statistical methodology
Survivals estimates were calculated using the Kaplan–Meier method [21]. Overall survival was defined as the time interval from the date of biopsy to the date of death from any cause and relapse-free survival (RFS) was defined from the date of biopsy until the date of first relapse (localized and/or metastatic), tumor progression, or death from any cause. Patients with no events were censored on the date of the last follow-up. For univariate analysis, the statistical significance of each variable was first tested by the log-rank test. A stepwise variable selection procedure was applied to the covariates with a p value ≤0.10 in univariate analysis. Variables with p < 0.10 were entered into multivariate analyses and considered significant if p ≤0.05. Prognostic factors for RFS were analyzed using a Cox model. Associations between two variables were analyzed with Fisher’s exact test or the Wilcoxon Mann–Whitney test. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated according to the Wald method. Stata software v13.1 (StataCorp LP, Stata Statistical Software: Release 11, College Station, TX,USA) was used for statistical analysis.
Results
Patient and tumor characteristics
This study included 95 patients with a median age at diagnosis of 15 years (range, 7–23). Clinical and tumor characteristics are detailed in Table 2. Two thirds of patients were treated in a pediatric department (online supplemental Fig. 1). One patient had a family history of nasopharyngeal carcinoma (father and uncle). The diagnosis was based on cavum biopsy for 33 patients (52%), on cervical lymph node biopsy for eight patients (12%), or on both for 23 patients (36%), with missing data on the biopsy site for 31 patients. The main histological type was WHO type III for 93 patients (99%) and the presence of Epstein–Barr virus (EBV) antigen or DNA in tumor tissue (EBER probe) was positive in almost all patients for whom this information was available (43/44 patients). Only three patients (3%) had stage II disease, while the remaining 91 patients (97%) had stage III or IV disease.
Treatment characteristics
In total, 90 patients (95%) received neoadjuvant ChT, but five patients did not receive neoadjuvant ChT for various reasons: stage II disease (two cases) and patients treated in an adult medical oncology department (two cases), physician’s decision (one case; online resource 1). Details of the therapy are listed in Table 3. Neoadjuvant ChT consisted of cisplatin and 5‑fluorouracil for 82% of patients and was cisplatin-based in 99% of cases. Docetaxel was added in 27% of cases (TPF regimen). Overall, 70 of 87 patients (80%) received at least three cycles of neoadjuvant ChT (median 3; range: 1–6).
Tumor response
Response to neoadjuvant ChT based on imaging (CT and/or MRI) was assessed after two cycles in 41 patients (65%), after three cycles in 21 patients (33%), and after four cycles in one patient (2%; missing data for 21 cases). In six patients, evaluation was available but the timing of this evaluation was not specified. Among the 69 patients for whom tumor response was available based on local assessment, 24 patients (35%) achieved CR or VGPR, 36 patients (52%) achieved PR, eight patients (12%) presented SD, and one patient (1%) presented PD of the primary tumor. Overall, the primary response rate—RR = (CR + PR + VGPR) / total number of patients—to neoadjuvant ChT was 87%, and the cervical node RR was 89%.
All patients received external RT (Table 3), consisting of conformal RT in 38 patients (40%) and IMRT for 57 patients (60%). Median radiation dose to the primary tumor was 65 Gy (range: 45–74 Gy) in 25–38 fractions (median: 33) and 60 Gy (range: 45–72 Gy) in 25–37 fractions (median: 30) to involved cervical lymph nodes. Among the 95 patients, based on medical decisions, 14 had a lower dosage and 28 a higher dosage than the recommended guidelines. The distribution of patients according to total dosage to primary is detailed in the online supplemental resource 2. Median doses to the primary tumor was 60.0 Gy (range: 50.0–70.0) in the case of favorable response (CR/VGPR) vs. 64.0 Gy (50.4–70.0) after a PR and 66.6 Gy (59.4–70) in the case of SD or PD (p < 0.01). Overall, 55 patients (59%) received concurrent platinum-based single-agent ChT (with cisplatin in 45 cases, cisplatin/carboplatin combination in four cases, other regimen in one case, and missing data in five cases). Amifostine was added in eight cases.
Maintenance therapy after completion of ChT and ChT/RT was received by 29 patients (31%): 17 patients received IFN-β and 11 patients received conventional ChT (treatment not specified in one case) for a median duration of 5.8 months (range: 0.7–6.6).
Outcome
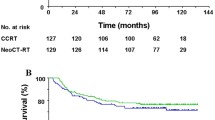
All but one of the patients who presented early locoregional and metastatic progression had been considered to be in remission at the end of initial therapy. After a median follow-up of 4.5 years (range: 0.6–18), 14 patients had experienced relapse or progression at a median follow-up of 8 months (range: 1–102; Table 4), and 7´seven had died from progression despite salvage therapy. The tumor status at the end of follow-up, for the whole cohort, is indicated in Table 4. The 3‑year OS was 94% (95% CI: 85–97%) and RFS was 86% (95% CI: 77–92%); (Fig. 1). The locoregional failure rate was 6% (95% CI: 2–14): two local relapses, one cervical nodal relapse (in RT fields), and one locoregional progression. Among the 13 patients with relapses, only one had favorable response (VGPR), seven had PR and three SD/PD (missing data in three cases). Median total dose to primary for these 13 patients was 66 Gy (range: 54.8–72 Gy).
Prognostic analysis
The only significant prognostic variables for RFS on univariate analysis were median age at onset (<14 vs. ≥14 years), and stage of disease (Table 5). No difference was shown in survival between patients receiving a median dose over or under 60 Gy on the primary tumor. Concurrent ChT did not appear to influence survival, but maintenance therapy with interferon tended to have a favorable impact on outcome. The 3‑year RFS and OS rates among patients treated by IMRT were 89% (77–95%) and 93% (80–98%), respectively, and were not different when compared with conformal RT. In multivariate analysis, only time to diagnosis of >5 months remained prognostic (HR 1.2 [95% CI: 1.01–1.35]; p = 0.03) but not gender, stage, median dose to cervical nodes, or interferon administration.
Long-term morbidities
The incidence of long-term toxicities of any grade was 95% (69/73 patients with data on long-term follow-up), including 37 patients (50%) with ≥grade 2 toxicity. Main head and neck sequelae were neck fibrosis, xerostomia, or caries (online resource 3). The residual odynophagia rate of grade 2+ was significantly higher in the group treated with 3D-RT compared with the group treated with IMRT (55% vs. 7%; p = 0.015, Fisher’s exact test). Hypothyroidism was observed in 64% of cases, and trismus was observed in 43% of cases. Two patients developed a second malignancy: A 13-year-old girl diagnosed with NPC developed squamous cell carcinoma of the tonsils 6 years after treatment of the NPC (including RT with a total dose of 63 Gy), and is currently in remission 5 years after surgery; a 17-year-old girl diagnosed with NPC developed squamous cell carcinoma of the tongue 8 years after treatment of the NPC (including RT with a total dose of 70 Gy), and is currently in remission 7 years after surgery and brachytherapy.
Discussion
This series confirms the very good long-term tumor control and survival in pediatric patients with NPC currently obtained with multimodal strategies comprising ChT and concurrent chemoradiation using a dose reduction of 10% in the case of response following induction ChT. The 3‑year OS in the present cohort was satisfactory at 94% (85–97%). Overall survivals rates with combined strategies are fairly satisfactory (around 80% and sometimes up to 95%, as in the present cohort) with local tumor failure rates of less than 10% [10,11,12, 16]. Most tumor events therefore correspond to early distant metastatic relapses mainly occurring during the first 2 years of follow-up, systematically raising the question of medical therapy in addition to locoregional irradiation. Notably, in this series, despite RT dose reduction (<60 Gy) for patients with a favorable response, RT adaptation did not appear to have any negative impact on outcome [3, 10, 14, 22]. To date, there have been some prospective pediatric NPC studies adapting RT by response to induction ChT. In the study by Buehrlen et al., the radiation dosage to the primary tumor was reduced from 59 to 54 Gy in patients with tumor who showed a complete response after three cycles of ChT by MRI [10]; the approach seemed to be feasible as none of the patients treated with a reduced total dose relapsed. In the other study by Rodriguez-Galindo et al., the radiation dosage to the primary tumors was increased from 61.2 Gy to 71.2 Gy in patients who did not have a CR or PR after three cycles of ChT. Event free survival for all patients was 92% in the first study (after a median follow-up of 30 months) and 85.5% in the latter one. Based on the two prospective NPC-91-GPOH [14] and NPC-2003-GPOH [10] studies, a German group (GPOH) published treatment guidelines [23]. In these studies, the majority of patients received dosages below 60 Gy. Moreover, in the GPHO experience, patients in CR after three cycles of ChT received a lower dose to the primary of 54 Gy.
Pediatric NPC frequently presents as advanced locoregional disease (59% of our patients had stage IVA or IVB disease), but distant metastases are rare at diagnosis (<5–10%; [15]) and are difficult to treat in this setting (5-year DFS 18% for stage IVC [metastatic patients]; [24]). Overall, pediatric NPC patients appear to have a better prognosis despite more advanced disease compared with adults [8, 13, 25]. Treatment regimens currently remain highly heterogeneous throughout the world. In most series, patients receive cisplatin-based neoadjuvant ChT, often in combination with 5‑FU. Heterogeneous RT doses were used, both within studies and between series, with median doses to the cavum ranging from 59 to 72 Gy. Therapy remained heterogeneous in the present study, despite official national pediatric guidelines proposed by the very rare tumor group. These differences can be explained by the absence of strong prospective comparative randomized trials precisely defining the role of concurrent ChT, the possibility to reduce the primary irradiation dosage in the case of a favorable response to neoadjuvant ChT, the role of maintenance therapy, and the best dosage of locoregional RT.
Patients were exclusively treated with IMRT in two series [7, 12]. The German series treated patients with NPC exclusively with 3D-RT [10]. The other seven series, including the present series, report the results of patients treated by both techniques. Optimum total dose to the primary tumor region remains controversial with some studies in favor of high RT doses (>60 Gy; [26,27,28,29]), while others are not [6, 12, 30]. It should be noted that some patients in the series by Orbach et al. and Liu et al. were treated with 2D-RT [3, 31].
In the present cohort, 95% of patients experienced at least one late toxicity, as commonly reported in the literature. In addition, long-term sequelae might be underrepresented as the long-term follow-up was not specifically structured. In this tumor, long-term effects are mainly due to head and neck RT and some appear be correlated to the type of RT. The most frequent toxicities after head and neck irradiation are locoregional sequelae, with xerostomia, neck fibrosis, hypothyroidism, ototoxicity, and dental abnormalities. Cheuk et al., in their retrospective series of 59 patients under the age of 20 treated in the United States before 2004, reported a cumulative incidence of late morbidities of 84% after a 15-year follow-up [30]. Laskar et al. showed that patients treated with IMRT had significantly less acute grade 3 odynophagia, grade 3 mucositis, and grade 3 dermatitis than patients treated with conventional RT. They also showed that the median time to onset of all acute toxicities was significantly longer in patients treated with IMRT compared with those treated with 3D-RT [32]. In our series, significantly fewer patients experienced odynophagia in the IMRT group (7%) compared with the conformal RT group (55%, p < 0.01). In addition, Cheuk et al. reported four cases of secondary malignancies: two cases of basal cell carcinoma of the neck and head region 23 and 27 years after the first diagnosis, one case of parotid muco-epidermoid carcinoma 2 years after NPC treatment, and one case of brain stem tumor 24 years after diagnosis. One patient also presented with multiple carcinomas including colorectal adenocarcinoma, maxillary squamous cell carcinoma, and esophageal adenocarcinoma in a context of a germline P53 mutation [30]. Secondary head and neck cancers are common after treatment of pediatric NPC with an incidence of 0–8.5% [5, 13, 30, 33, 34]. Two patients in the present series developed secondary malignancies in irradiated volumes 6 and 8 years after treatment, respectively. All these findings argue strongly in favor of reduction of head and neck RT doses in children with NPC after a favorable response in an attempt to reduce long-term sequelae.
The long recruitment period (1999 to 2015) associated with the changing management of this disease may have impacted the quality of diagnostic imaging (with the advent of PET scanning and improvement of MRI), and RT techniques have also improved with the development of IMRT. However, our inclusion criteria required patients to be recently treated by conformal RT or IMRT and had therefore systematically undergone CT planning. Intensity-modulated radiotherapy is a time-consuming technique for delineation and treatment, but RT planning and delineation are crucial when margins are small, with a risk of under-coverage of target volumes or a risk of toxicity due to coverage of critical organs. Treatment planning is effectively assisted by contrast-enhanced CT/MRI fusion to delineate targets and critical organs, which cannot be reliably performed on CT only (especially without contrast; [35]). The major advantage of CT/MRI fusion is better delineation of CTV [31]. The potential benefit of IMRT in NPC children compared with other conventional RT techniques is the possibility to substantially reduce long-term toxicity while maintaining excellent tumor control and thereby improving quality of life in the individual child and young adult. No comparative study in pediatric NPC has been published. Nevertheless, recent large retrospective studies have confirmed the benefit of IMRT in terms of local control (+9%) and survival (+14%; [16, 32, 33]). A randomized trial in adults showed better local control (+6 to 11%) and DFS/OS (+5 to 12%) due to better target coverage using IMRT [36,37,38]. The role of proton therapy in pediatric NPC has not been clearly defined. The sharp dose fall-off results in high radiation doses to the tumor with a minimal exit dose ensures improved sparing of normal tissues. However, very few data are available concerning the clinical impact of proton therapy in NPC, and no series of pediatric NPC has been published. Dosimetric comparisons showed similar target coverage to that obtained with IMRT. This technique should therefore be the subject of clinical trials owing to the significantly lower doses to the parotid glands, cochlea, maxillary, and larynx, although it does not appear to have any clinical impact on thyroid function [39,40,41]. Moreover, the potential value of new imaging modalities, such as RT adapted to PET scan changes, may allow for a potential reduction of tumor volume, and metabolic activity also needs to be prospectively assessed throughout treatment.
Despite the large size of this cohort, we acknowledge that the major limitation of this study is represented by is its retrospective nature and the biases associated with this type of methodology, particularly selection and monitoring biases. The main selection bias arises from the fact that patients were recruited by radiotherapists of the French Pediatric Radiotherapy Group (GFRP), which means that all patients in our study come from a referral center and the vast majority (91/95) were derived from the five French RT centers treating the largest number of children in France, which may not be strictly representative of the general population with NPC. In addition, this was a multicenter study, but the overall characteristics of the study population were similar to those described in the literature, which limits the risk of selection bias. In this study, 67% of patients were treated in a pediatric department and 33% were treated in an adult medical oncology department. The majority of young adults (18–25 years) were treated in an adult medical oncology department. However, many studies have shown that adolescents and young adults (AYA) have improved survival when treated for certain diseases in a pediatric department [42], as AYAs are less often included in clinical trials and therefore do not benefit from progress in pediatric oncology or adult medical oncology [43, 44]. Many pediatric oncology departments have set up specific medical and psychosocial care for this population. One study in the United States specifically focused on the survival of AYAs with rare tumors, particularly NPC (25 patients), and did not demonstrate any significant difference in terms of 10-year OS according to the site of management (adult medical oncology vs. pediatric oncology; [45]). However, it seems to be important that these patients are being treated in specific structures used to take care of young patients. Moreover, significant late toxicities require specialized long-term follow-up.
The exact role and type of neoadjuvant ChT have not been clearly determined. In adult NPC, patients treated by induction ChT followed by radiochemotherapy had a better survival than patients treated by immediate radiochemotherapy (3-year failure-free survival: 80% vs. 72%, p = 0.034; [46]). The only available phase 2 comparative prospective randomized study in children with NPC showed that the addition of docetaxel to cisplatin-5-fluorouracil induction therapy did not provide any benefit in terms of local control rate and outcomes in children and adolescents with NPC [15]. In addition, the role of concurrent chemoradiotherapy in NPC remains unclear in children. Since a beneficial effect of concurrent radiochemotherapy has been shown in adults, this principle of therapy has been introduced in prospective trials on NPC in children and adolescents [10, 15]. Meta-analyses showed that the greatest benefit of ChT in adults with NPC was obtained when ChT was delivered concurrently to RT [47, 48] with an absolute 5‑year survival benefit of 6%. Recently, a meta-analysis of 20 randomized trials concluded in favor of systematic usage of ChT to RT with better locoregional and distant tumor control for protocols including induction ChT. Protocols with adjuvant ChT always ranked better than those with concomitant ChT alone [49]. Nevertheless, schedules containing more than one timing of ChT generally resulted in more toxicity than the use of only one timing [49, 50]. Finally, the place of maintenance therapy after RT has never been scientifically defined. Most patients in the various studies, except in the German series [10], did not receive systematic adjuvant therapy. However, treatment comprising neoadjuvant ChT, concurrent RT/ChT followed by 6 months of interferon therapy was associated with a 3-year OS of 97% [10]. In the present series no relapse was observed in patients who received interferon maintenance therapy. No multivariate analysis could be performed because of the small number of events in this series. According to this analysis, the FRACTURE group has updated their national guidelines for NPC in childhood and adolescence to ensure more homogeneous treatment (online resource 4). Overall prognosis remains dismal for patients with initially metastatic tumor or after relapse. For those patients, owing to the specific immune environment of EBV-associated NPC, rational targets for immunotherapy seem promising and need to be developed for pediatric patients [51].
Conclusion
The survival rates for NPC have been considerably improved by means of multimodal therapy, but locoregional morbidity remains common and needs long-term follow-up. Treatment with IMRT may induce less residual odynophagia compared with 3D-RT treatment. Radiation dose reduction adapted to ChT response has no negative impact on outcome. These findings support the use of an RT protocol adapted to the tumor response to neoadjuvant ChT in order to lastingly improve the patient’s quality of life. Authors recommend an adapted protocol with dose reductions after favorable tumor response to induction ChT. For patients with tumor in CR or VGRP, recommended dosages are for PTV-T1 54 Gy, PTV-N1 54 Gy, and PTV N0 45 Gy; for patients with PR, PTV-T2/N2 59.4 Gy, PTV-T1/N1 54 Gy, and PTV-N0 45 Gy; and for patients with poor tumor response (SD/PD), PTV-T2/N2 66.6 Gy, PTV-T1/N1 54 Gy, and PTV-N0 45 Gy.
References
Desandes E, Lacour B, Sommelet D et al (2004) Cancer incidence among adolescents in France. Pediatr Blood Cancer 43(7):742–748
Downing NL, Wolden S, Wong P et al (2009) Comparison of treatment results between adult and juvenile nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 75(4):1064–1070
Orbach D, Brisse H, Helfre S et al (2008) Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: Radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer 50(4):849–853
Dourthe ME, Bolle S, Temam S et al (2018) Childhood Nasopharyngeal Carcinoma: State-of-the-Art, and Questions for the Future. J Pediatr Hematol Oncol 40:85–92
Casanova M, Bisogno G, Gandola L et al (2011) A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian Rare Tumors in Pediatric Age (TREP) project. Cancer 118(10):2718–2725
Yan Z, Xia L, Huang Y et al (2013) Nasopharyngeal carcinoma in children and adolescents in an endemic area: a report of 185 cases. Int J Pediatr Otorhinolaryngol 77(9):1454–1460
Tao CJ, Liu X, Tang LL et al (2013) Long-term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin. J Cancer 32(10):525–532
Wu SG, Liao XL, He ZY et al (2017) Demographic and clinicopathological characteristics of nasopharyngeal carcinoma and survival outcomes according to age at diagnosis: A population-based analysis. Oral Oncol 73:83–87
Gioacchini FM, Tulli M, Kaleci S et al (2017) Therapeutic modalities and oncologic outcomes in the treatment of T1b glottic squamous cell carcinoma: a systematic review. Eur Arch Otorhinolaryngol 274(12):4091–4102
Buehrlen M, Zwaan CM, Granzen B et al (2012) Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer 118(19):4892–4900
Sahai P, Mohanti BK, Sharma A et al (2017) Clinical outcome and morbidity in pediatric patients with nasopharyngeal cancer treated with chemoradiotherapy. Pediatr Blood Cancer 64(2):259–266
Guo Q, Cui X, Lin S et al (2016) Locoregionally advanced nasopharyngeal carcinoma in childhood and adolescence: Analysis of 95 patients treated with combined chemotherapy and intensity-modulated radiotherapy. Head Neck 38(Suppl 1):E665–672
Daoud J, Ghorbal L, Siala W et al (2013) Is there any difference in therapeutic results of nasopharyngeal carcinoma between adults and children? Cancer Radiother 17(8):763–767
Mertens R, Granzen B, Lassay L et al (2005) Treatment of nasopharyngeal carcinoma in children and adolescents: definitive results of a multicenter study (NPC-91-GPOH). Cancer 104(5):1083–1089
Casanova M, Ozyar E, Patte C et al (2016) International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5‑fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol 77(2):289–298
Qiu WZ, Peng XS, Xia HQ et al (2017) A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 143(8):1563–1572
Edge S, Byrd D, Compton C et al (2010) AJCC cancer staging handbook AJCC cancer staging manual, 7th edn. Springer, New York, pp 63–79
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47(1):207–214
Reguerre Y, Lacour B, Andre N et al (2010) Epidemiology and management of rare paediatric tumours within the framework of the French Society for Children Cancer. Bull Cancer 97(9):1041–1045
Brock PR, Bellman SC, Yeomans EC et al (1991) Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol 19(4):295–300
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:467–471
Rodriguez-Galindo C, Wofford M, Castleberry RP et al (2005) Preradiation chemotherapy with methotrexate, cisplatin, 5‑fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer 103(4):850–857
Kontny U, Franzen S, Behrends U et al (2016) Diagnosis and treatment of Nasopharyngeal carcinoma in children and adolescents—recommendations of the GPOH-NPC study group. Klin Padiatr 228(3):105–112
Lee AW, Lin JC, Ng WT (2012) Current management of nasopharyngeal cancer. Semin Radiat Oncol 22(3):233–244
Gioacchini FM, Tulli M, Kaleci S et al (2017) Prognostic aspects in the treatment of juvenile nasopharyngeal carcinoma: a systematic review. Eur Arch Otorhinolaryngol 274(3):1205–1214
Laskar S, Sanghavi V, Muckaden MA et al (2004) Nasopharyngeal carcinoma in children: ten years’ experience at the Tata Memorial Hospital, Mumbai. Int J Radiat Oncol Biol Phys 58(1):189–195
Wolden SL, Steinherz PG, Kraus DH et al (2000) Improved long-term survival with combined modality therapy for pediatric nasopharynx cancer. Int J Radiat Oncol Biol Phys 46(4):859–864
Ozyar E, Selek U, Laskar S et al (2006) Treatment results of 165 pediatric patients with non-metastatic nasopharyngeal carcinoma: a Rare Cancer Network study. Radiother Oncol 81(1):39–46
Guruprasad B, Tanvir P, Rohan B et al (2013) Paediatric nasopharyngeal carcinoma: an 8‑year study from a tertiary care cancer centre in South India. Indian J Otolaryngol Head Neck Surg 65(Suppl 1):131–134
Cheuk DK, Billups CA, Martin MG et al (2011) Prognostic factors and long-term outcomes of childhood nasopharyngeal carcinoma. Cancer 117(1):197–206
Liu T (1999) Issues in the management of nasopharyngeal carcinoma. Crit Rev Oncol Hematol 31(1):55–69
Laskar S, Bahl G, Muckaden M et al (2008) Nasopharyngeal carcinoma in children: comparison of conventional and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 72(3):728–736
Liu W, Tang Y, Gao L et al (2014) Nasopharyngeal carcinoma in children and adolescents—a single institution experience of 158 patients. Radiat Oncol 9:274
Sultan I, Casanova M, Ferrari A et al (2010) Differential features of nasopharyngeal carcinoma in children and adults: a SEER study. Pediatr Blood Cancer 55(2):279–284
Lin H, Huang S, Deng X et al (2014) Comparison of 3D anatomical dose verification and 2D phantom dose verification of IMRT/VMAT treatments for nasopharyngeal carcinoma. Radiat Oncol 9:71
Zhang MX, Li J, Shen GP et al (2015) Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer 51(17):2587–2595
Chen JL, Huang YS, Kuo SH et al (2017) Intensity-modulated radiation therapy achieves better local control compared to three-dimensional conformal radiation therapy for T4-stage nasopharyngeal carcinoma. Oncotarget 8(8):14068–14077
Peng G, Wang T, Yang KY et al (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104(3):286–293
Widesott L, Pierelli A, Fiorino C et al (2008) Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys 72(2):589–596
Lewis GD, Holliday EB, Kocak-Uzel E et al (2016) Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 38(Suppl 1):E1886–1895
Taheri-Kadkhoda Z, Bjork-Eriksson T, Nill S et al (2008) Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol 3:4
Shaw PH, Reed DR, Yeager N et al (2015) Adolescent and young adult (AYA) oncology in the united states: a specialty in its late adolescence. J Pediatr Hematol Oncol 37(3):161–169
Downs-Canner S, Shaw PH (2009) A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol 31(12):927–929
Keegan TH, Ries LA, Barr RD et al (2016) Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 122(7):1009–1016
Cash T, Qayed M, Ward KC et al (2015) Comparison of survival at adult versus pediatric treatment centers for rare pediatric tumors in an adolescent and young adult (AYA) population in the State of Georgia. Pediatr Blood Cancer 62(3):456–462
Sun Y, Li WF, Chen NY et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17(11):1509–1520
Baujat B, Audry H, Bourhis J et al (2006) Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma. Cochrane Database Syst Rev 4:CD4329
Blanchard P, Lee A, Marguet S et al (2015) Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 16(6):645–655
Ribassin-Majed L, Marguet S, Lee AWM et al (2017) What is the best treatment of locally advanced Nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 35(5):498–505
Machtay M, Moughan J, Trotti A et al (2008) Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 26(21):3582–3589
Hong M, Tang K, Qian J et al (2018) Immunotherapy for EBV-Associated Nasopharyngeal Carcinoma. Crit Rev Oncog 23(3–4):219–234
Acknowledgements
The French Fracture database is supported by the “Enfants, cancers et Santé” association who did not take part in any analyses of this study. We thank Dr G. Schleiermacher for the editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Jouin, S. Helfre, S. Bolle, L. Claude, A. Laprie, E. Bogart, C. Vigneron, H. Potet, A. Ducassou, A. Claren, F.G. Riet, M.P. Castex, C. Faure-Conter, B. Fresneau, A.S. Defachelles, and D. Orbach declare no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence (bias) their work.
Caption Electronic Supplementary Material
66_2019_1461_MOESM2_ESM.docx
ONLINE RESOURCE 3: SUPPLEMENTAL TABLE ST2. Long-term head and neck severe morbidities with comparison between patients treated with 3D-RT and those treated with IMRT
66_2019_1461_MOESM4_ESM.doc
ONLINE RESOURCE 4: SUPPLEMENTAL FIGURE SF2. NPC French FRACTURE group proposals adapted to the response to induction chemotherapy
Rights and permissions
About this article
Cite this article
Jouin, A., Helfre, S., Bolle, S. et al. Adapted strategy to tumor response in childhood nasopharyngeal carcinoma: the French experience. Strahlenther Onkol 195, 504–516 (2019). https://doi.org/10.1007/s00066-019-01461-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01461-6