Abstract
Aim
The purpose of this work was to retrospectively evaluate survival and local control rates of triple-negative breast cancer subtypes classified as five marker negative (5NP) and core basal (CB), respectively, after breast-conserving surgery and intraoperative boost radiotherapy with electrons (IOERT) followed by whole breast irradiation.
Methods and materials
A total of 71 patients with triple-negative breast cancer were enrolled, who were treated with lumpectomy, axillary lymph node dissection, and IOERT with 9.6 Gy (median Dmax) followed by normofractionated whole breast irradiation to median total doses of 54 Gy. Chemotherapy was applied in a neoadjuvant (12 %), adjuvant (75 %), or combinational setting (7 %).
Results
After a median follow-up of 97 months (range 4–170 months), 5 in-breast recurrences were detected (7.0 %). For all patients, 8-year actuarial rates for local control, metastases-free survival, disease-specific survival, and overall survival amounted to 89, 75, 80, and 69 %, respectively. All local recurrences occurred in grade 3 (G3) tumors irrespective of their specific immunohistochemical phenotype; thus, the local control rate for grades 1/2 (G1/2) was 100 % for both 5NP and CB, while for G3 it was 88 % for 5NP and 90 % for CB (p = 0.65 and 0.82, respectively, n.s.). For disease-specific survival, only the difference of the best-prognosis group 5-NP/G3 vs. the worst-prognosis cohort CB/G1/2 was statistically significant: 90 % vs. 54 % (p = 0.03).
Conclusion
Boost-IOERT provides acceptable long-term in-breast control in triple negative breast cancer. The best subgroup in terms of disease-specific survival was represented by 5NP in combination with tumor grading G3.
Zusammenfassung
Hintergrund
Ziel der Studie war es, im Rahmen einer retrospektiven Analyse Überlebens- und Lokalkontrollraten bei triple-negativen Mammakarzinomen zu untersuchen. Die Tumoren waren in 5NP(5-Marker-negative)- und CB(core basal)-Subtypen klassifiziert und die Patientinnen hatten nach brusterhaltender Operation und intraoperativem Elektronenboost (IOERT) eine Ganzbrustbestrahlung erhalten.
Material und Methoden
Insgesamt 71 Patientinnen mit triple-negativem Mammakarzinom erhielten während einer Lumpektomie und axillärer Lymphknotendissektion eine IOERT (med Dmax 9,6 Gy ) und danach eine Ganzbrustbestrahlung in konventioneller Fraktionierung (mediane Gesamtdosis 54 Gy). Eine Chemotherapie wurde in neoadjuvanter (12 %), adjuvanter (75 %) oder kombinierter (7 %) Sequenz durchgeführt.
Ergebnisse
Nach einer medianen Follow-up-Phase von 97 Monaten (Bereich 4–170) wurden 5 ipsilaterale In-Brust-Rezidive festgestellt (7%). Die aktuarischen Achtjahresraten aller Patientinnen für lokale Kontrolle bzw. metastasenfreies, krankheitsspezifisches und Gesamtüberleben lagen entsprechend bei 89, 75, 80 und 69 %. Unabhängig vom immunhistochemischen Phänotyp traten alle Lokalrezidive bei Tumoren mit niedrigem Differenzierungsgrad G3 auf [Lokalkontrollen: G1/2 (CB und 5NP) 100 % vs. G3 88 % (5NP) und 90 % (CB), p = 0,65 bzw. 0,82; n.s.]. Bezüglich des krankheitsspezifischen Überlebens zeigte der Vergleich zwischen der Subgruppe mit der besten Prognose 5NP/G3 und der mit der schlechtesten Prognose CB/G1/2 statistische Signifikanz: 90 vs. 54 % (p = 0,03).
Zusammenfassung
Bei konservativ operierten triple-negativen Mammakarzinomen erzielt die IOERT als Boostmodalität vor einer Ganzbrustbestrahlung auch langfristig akzeptable Lokalkontrollraten. Die Kombination eines 5NP-Subtyps mit dem Tumordifferenzierungsgrad G3 zeigt einen signifikanten Vorteil im krankheitsspezifischen Überleben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer represents one of five breast cancer receptor subtypes [1]. Lowery et al. [2] identified 15 retro- and prospective trials which investigated the occurrence of both in-breast and locoregional recurrencesFootnote 1 each seperately depending on their receptor phenotype. A respective meta-analysis of these trials [2] comprised cohorts between 149 and 2985 patients, which ruled out the triple-negative phenotype as the breast cancer subgroup with the worst outcome. If treated within a breast-conserving concept, 5-year rates for locoregional and in-breast recurrences after median follow-up periods of 3–10 years were reported to range widely between 3.2–14 % and 2.8–16 %, respectively, in the single studies.
Considering all evaluable patients in a meta-analysis, luminal subtype tumors (ER/PR+) showed a lower risk of locoregional recurrences than triple-negative tumors [relative risk (RR) 0.38; 95 % confidence interval (CI) 0.23–0.61]. Hence, triple-negative breast cancer has increasingly come into the focus of molecular biological research in order to explore cellular characteristics and identify further profiles which could help to explain these clinical results. Therefore, subtypes of “triple-negative” classified breast cancer were identified either by pattern of gene profiles [3–5] or immunohistochemical staining of cell biomarkers. Subtypes determined by immunohistochemical methods were compared to each other concerning in-breast relapses and survival rates [6–8].
Among others, cytokeratine 5/6 and epidermal growth factor receptor were identified to be biomarkers with high relevance for this kind of subclassification [6, 7], dividing the triple-negative phenotype into two groups: The five-marker negative phenotype (5NP), defined as negative for epidermal growth factor receptor and cytoceratine 5/6, and the core basal (CB) type, which appears positive for epidermal growth factor receptor and/or cytoceratine 5/6 [6]. Corresponding to this classification, a retrospective analysis of 639 patients with triple-negative breast cancer, who were treated in the British Columbia Cancer Agency of Vancouver, was performed to clarify its prognostic value [6] for breast cancer-specific survival. In comparison of 303 5NP-type to 336 CB-type patients, the latter group showed a 10 % lower 10-year breast cancer-specific survival (72 vs. 62 %). This difference increased to 26 % (p = 0.00164) if both groups received anthracycline-based adjuvant chemotherapy. However, a respective evaluation for in-breast and locoregional recurrences1 was not performed in this study.
In recent decades, in-breast relapses could be reduced steadily for numerous reasons, not least by whole breast irradiation after breast-conserving surgery [9] and local dose escalation to the tumor bed as the region with the highest risk for recurrence [10]. Since 1998, we use an intraoperative single-shot delivery of electrons (IOERT) as a standard boost technique, promising to be advantageous in terms of accuracy and, hence, local control, sparing tissues at risk, and shortening the overall treatment time. Clinical data with respect to this approach have been published [11, 12], including a long-term pooled analysis conducted by the European Group of the International Society of Intraoperative Radiotherapy (ISIORT Europe; [13]) with actuarial in-breast recurrence rates of 0.8 % after a 6-year follow-up period [14]. Nevertheless, there is still a lack of information evaluating how IOERT is able to influence local control and survival rates in the high-risk constellation of a triple-negative phenotype, which encouraged us to initiate a retrospective analysis of such classified patients who received boost IOERT at our institution from 1998–2005.
Material and methods
Between 1998 and 2005, 1000 breast cancer patients with clinical stages I–II were treated with breast-conserving surgery, IOERT as anticipated tumor bed boost, and subsequent whole breast irradiation. Of these, 71 patients with triple-negative breast cancer phenotype were retrospectively identified, with a median age of 55 years (range 29–77 years). Tumor characteristics are summarized in Table 1. The surgical procedure consisted of a lumpectomy and dissection of axillary lymph node levels I and II which was preceded by sentinel lymph node biopsy.
In all patients, a final R0 resection status was achieved, with a median resection margin of 4 mm (range 0.5–20 mm). After tumor removal, freedom of margins was first determined by frozen section histology, then the tumor bed was treated by a median IOERT boost dose (Dmax) of 9.6 Gy (range 7–12 Gy) using a dedicated linear accelerator in the operating room. Technical details on the procedure, planning target volume definition, and dose prescription have been previously described [12, 14]. Median tube sizes of 6 cm (range 4–8 cm) and median electron energies of 6 MeV (range 4–18 MeV) were used, corresponding to a median treated tissue volume which was encompassed by 90 % of the prescribed dose of 8 ml (range 2–26 ml). In 6 cases (8.3 %) with close resection margins after final histopathological assessment (invasive or in situ components), a re-excision was performed.
After a median time lapse of 15 weeks (range 3–32 weeks), radiotherapy was continued by whole breast irradiation based on a tangential three-dimensional conformal radiotherapy technique (6 MV photons) with conventional fractionation of 1.6–1.85 Gy (5 fractions/week) up to median total doses of 54 Gy (range 51–57.6 Gy). According to their nodal status, 8 of 71 patients (9.8 %) received additional regional node irradiation encompassing the supra-/infraclavicular fossa alone in 4 patients and combined with the ipsilateral internal mammary chain in an additional 3 patients. In one isolated case, the internal mammary chain was irradiated after scintigraphic detection of a suspicious lymph node adjacent to the primary tumor site in the upper-inner quadrant. Doses to regional nodes were applied in normofractionation with median total doses of 46.2 Gy (range 44.5–52 Gy). A total of 67 patients (94 %) received chemotherapy either in an adjuvant (75 %), neoadjuvant (12 %), or combined setting (7 %), respectively (Table 2). In order to evaluate the impact on survival and local control according to triple-negative subclassifications, four subgroups were defined in combination with tumor grading: 5NP + G1/2, 5NP + G3, CB + G1/2, and CB + G3.
Statistical analysis
Actuarial 8-year rates for local control, metastases-free survival, disease-specific survival, and overall survival were calculated using the Kaplan–Meier method [15] based on the Kaplan–Meier product limit estimator. All data were presented with 95 % confidence intervals (CI) calculated by logarithmic transformation of Greenwood’s variance estimate. Comparisons between subgroups were done with the Gehan–Wilcoxon test since proportional hazard cannot be assumed. P-values less than 0.05 were considered statistically significant and were not adjusted for multiple testing, due to the explorative nature of the study. All calculations were performed with NCSS 8 (Kaysville, UT, USA). For graphical presentation, MedCalc 13.2 (Ostend, Belgium) was used.
Results
After a median follow-up of 97 months (range 20–170 months), 5 in-breast recurrences (7.0 %) in the former index quadrant and no regional axillary relapses were observed, corresponding to an 8-year local control rate of 89 % (95%CI 76–95 %). All in-breast relapses were characterized by a tumor grading G3, independently of their classification into 5NP (n = 3) or CB (n = 2). In all, 17 patients (24 %) developed distant metastases, of whom 15 died of their cancer. In addition, 1 patient died due to a secondary cancer, and a further 4 patients died for unknown reasons. Five patients developed contralateral breast cancer, and another 4 patients secondary (nonbreast-related) malignancies, respectively. The time lapse between IOERT and the first occurrence of progression averaged 77 months (range 19–89 months) for in-breast relapses and 28 months (range 5–132 months) for systemic failure (all median values). At the time of final analysis, 47 patients (66 %) were alive without evidence of disease.
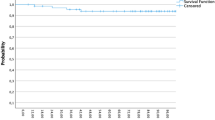
All data are presented as actuarial 8-year rates, expressed as percentages in 95 % CI and respective ranges. For all patients, metastasis-free survival, disease-specific survival, and overall survival values averaged 75 % (range 63–83 %), 80 % (68–87 %), and 69 % (57–78 %), respectively. According to subgroup analyses for 5NP + G1/2, 5NP + G3, CB + G1/2, and CB + G3, actuarial 8-year rates were 83 % (27–97 %), 90 % (71–97 %), 54 % (13–83 %), and 79 % (56–91 %) for disease-specific survival, 67 % (19–90 %), 83 % (64–93 %), 57 % (17–84 %), and 77 % (55–89 %) for metastasis-free survival, and 54 % (13–83 %), 83 % (64–93 %), 54 % (13–83 %), and 65 % (44–80 %) for overall survival, respectively (Fig. 1a–c).
After matching for tumor grading, 8-year rates for disease-specific and metastasis-free survival between triple-negative subgroups revealed the following outcomes by comparing CB to 5NP subtypes: for G1/G2 tumors, disease-specific survival in 5NP types averaged 83 % vs. 54 % for CB (p = 0.28), while for G3 tumors, 5NP was 90 % vs. 79 % for CB (p = 0.31). Results of rates for metastasis-free survival for G1/G2 averaged 67 % for 5NP vs. 57 % CB (p = 0.34) and for G3 tumors 83 % for 5NP vs. 77 % for CB (p = 0.49).
Survival analyses for tumor grading G3 compared to G1/2 in each triple-negative phenoptype could be described as follows: for 5NP G3 vs. G1–2, the disease-specific survival was 90 vs. 83 % (p = 0.8); metastasis-free survival was 83 vs. 67 % (p = 0.68); overall survival was 83 vs. 54 % (p = 0.28); while for CB G3 vs. G1–2, the disease-specific survival was 79 vs. 54 % (p = 0.22); metastasis-free survival was 77 vs. 57 % (p = 0.31); and overall survival was 65 vs. 54 % (p = 0.55; Fig. 1a–c). Only rates of disease-specific survival by comparing the “worst-prognosis” constellation CB G1/2 (54 %) with the “best-prognosis” group 5NP G3 (90 %) reached statistical significance (p = 0.03; Fig. 1a).
If focussing on local control, all recurrences were associated with tumor grading G3 independent of their respective phenotype [5NP/CB plus G1/2 was 100 %, 5NP plus G3 was 88 % (p = 0.65), and CB plus G3 was 90 % (p = 0.82)], but were not statistically significant (Fig. 1d).
Due to the low incidences of in-breast recurrences no serious statistical calculations of univariate and multivariate hazard regression models were possible (Table 3). Of note, all five events were observed in patients with negative axillary nodal status, tumor grading G3, and following IOERT with tube diameters smaller than 6 cm.
Discussion
Up to now, trials considering triple-negative phenotype subanalysis for risk evaluation of subsequent local and locoregional relapse1 following breast-conserving treatment are scarce. Out of a cohort of 2985 breast cancer patients, Voduc et al. [16] provide data of 556 triple-negative breast cancer patients divided into basal-like (n = 295, positive for cytoceratine 5/6 or epidermal growth factor receptor—corresponding to CB in our definition) and non-basal (n = 261, negative for cytoceratine 5/6 and epidermal growth factor receptor—categorized as 5NP in the present study). Of these patients, 246 (134 basal-like and 114 non-basal) were allocated to breast-conserving surgery followed by adjuvant radiotherapy, without further information on dose and technique. After a median follow-up of 12 years, 19 in-breast relapses were described in the basal-like group, compared to 9 events in the non-basal cohort, corresponding to 10-year local recurrence-free survival rates of 86 and 92 %, respectively, and to a crude in-breast failure rate for all patients of 11.4 % (basal-like 14.4 %, 8 % non-basal). Millar et al. [17] analyzed a cohort of 394 invasive breast cancer patients and identified 68 patients with a triple-negative receptor constellation, all of them treated with breast-conserving surgery, followed by whole breast irradiation up to total doses of 45–50 Gy (1.8–2 Gy/fraction) and a tumor bed boost of 16 Gy (2 Gy/fraction). Of these, 52 patients were categorized as “basal-like” (positive for epidermal growth factor receptor and/or cytoceratine 5/6) and 16 patients as “unclassified” (negative for epidermal growth factor receptor and cytoceratine 5/6) subtypes. In-breast recurrences occurred more frequently (n = 5) in the basal-like group compared to the unclassified one (n = 1), corresponding to 5 and 10 years in-breast failure rates of 9.6 and 6.3 %, respectively. After a median follow-up of 84 months, the crude in-breast recurrence rate of all 68 patients averaged 9 % (n = 6/68). Of all 394 patients, triple-negative subtypes [hazard ratio (HR) 2.4, p = 0.016] and of these, the “unclassified” ones (HR 3.4, p = 0.042) developed significantly more breast cancer-specific deaths. The heterogeneity of TNBC phenotypes and their different implication on survival has been described previously [6, 8] but also with controversial results [18].
Our own study population could be interpreted as a high-risk subselection of patients with a triple-negative phenotype, of which 80, 55, and 41 % were diagnosed with tumor grading G3, KI 67 % ≥ 20 %, and CB subtype, respectively. These histopathological parameters stand for both poor prognosis in cancer-specific survival and local failure probability [6, 17, 19]. In contrast to previous observations [6, 8], we detected no statistically relevant influence of CB subtype if compared to 5NP classification either in terms of survival or local control. All recurrences were associated with tumor grading G3, which was ruled out as the strongest histopathological feature in developing local failure as described in a recent pooled analysis of the ISIORT Europe [14], and in patients who were treated with IOERT tube sizes < 6 cm. Due to very small number of in-breast events, no clear statistical and, thus, reliable parameters for clinical decision-making could be identified. Despite these restrictions, it could be hypothesized that especially when treating high-grade tumors, a boost volume has to be designed generously, e.g., by choosing sufficient IOERT tube diameters. Apart from the obvious precision in terms of topographic accuracy, it has been assumed that an intraoperatively administered tumor bed boost is not only capable of reducing the risk of recurrences by activation of biologic pathways [20–22] but also seems to have—if not preventive—at least a time-prolonging effect until the first occurrence of in-breast relapses.
In the present cohort, the observed median time lapse of 77 months between IOERT and an in-breast event clearly differs from a previously described peak within the first 3 years after treatment [7, 23]. Such a “blockade” in cellular kinetics, resulting in a delay of first in-breast relapses longer than 2 years, has been associated with a significant effect on fewer cumulative incidences of distant metastases [24] and could therefore result in a survival benefit. This biologic effect could be also more relevant in “high-risk” triple-negative subtypes due to their known tendency for worse local control and, hence, possibly also survival [25–27].
Why patients with tumor grading G3 did better in survival analyses than those with G1/2 cannot be answered in detail. One could hypothesize that G3 tumors represent a subgroup with a possibly better clinical response to systemic treatment, corresponding to the experience from clinical trials investigating tumor response rates after primary systemic treatment. In these trials, a triple-negative phenotype and tumor grading G3 ruled out as characteristics with a high probability for histologically proven pathological complete response, henceforth, also possibly contributing to improved overall survival [28].
Limitations to the article are its retrospective character and the small number of patients, which could distort statistical accuracy.
Conclusion
IOERT as boost modality during breast-preserved operated patients with triple-negative breast cancer, provides acceptable local control after long-term follow-up and prolongs the time interval to the first occurrence of in-breast recurrences. Triple-negative breast cancer characterized as 5NP together with tumor grading G3 was identified to be the best subgroup in terms of disease-specific survival.
Notes
In-breast and regional recurrences together
References
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Lowery AJ, Kell MR, Glynn RW et al (2012) Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 133:831–841
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767
Perou CM (2010) Molecular stratification of triple-negative breast cancers. Oncologist 15:39–48
Peddi PF, Ellis MJ, Ma C (2012) Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer 2012:217185. doi:10.1155/2012/217185
Cheang MC, Voduc D, Bajdik C et al (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14:1368–1376
Cho EY, Chang MH, Choi YL et al (2011) Potential candidate biomarkers for heterogeneity in triple-negative breast cancer (TNBC). Cancer Chemother Pharmacol 68:753–761
Uhm JE, Park YH, Yi SY et al (2009) Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer 124:1457–1462
Sedlmayer F, Sautter-Bihl ML, Budach W et al (2013) DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 189:825–833
Bartelink H, Maingon P, Poortmans P et al (2015) Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 16:47–56
Reitsamer R, Sedlmayer F, Kopp M et al (2006) The Salzburg concept of intraoperative radiotherapy for breast cancer: results and considerations. Int J Cancer 118:2882–2887
Sedlmayer F, Fastner G, Merz F et al (2007) IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: results of an ISIORT pooled analysis. Strahlenther Onkol 183:32–34
Krengli M, Calvo FA, Sedlmayer F et al (2013) Clinical and technical characteristics of intraoperative radiotherapy. Analysis of the ISIORT-Europe database. Strahlenther Onkol 189:729–737
Fastner G, Sedlmayer F, Merz F et al (2013) IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol 108:279–286
Kaplan EL, Meier P (1958) Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 53:457–481
Voduc KD, Cheang MC, Tyldesley S et al (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28:1684–1691
Millar EK, Graham PH, O’Toole SA et al (2009) Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 27:4701–4708
Elsawaf Z, Sinn HP, Rom J et al (2013) Biological subtypes of triple-negative breast cancer are associated with distinct morphological changes and clinical behaviour. Breast 22:986–992
Luporsi E, Andre F, Spyratos F et al (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132:895–915
Vaidya JS, Baldassarre G, Massarut S (2009) Beneficial effects of intraoperative radiotherapy on tumor microenvironment could improve outcomes (Int J Radiat Oncol Biol Phys 2008;72:1575–1581). Int J Radiat Oncol Biol Phys 74:976
Belletti B, Vaidya JS, D’Andrea S et al (2008) Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 14:1325–1332
Herskind C, Griebel J, Kraus-Tiefenbacher U et al (2008) Sphere of equivalence—a novel target volume concept for intraoperative radiotherapy using low-energy X rays. Int J Radiat Oncol Biol Phys 72:1575–1581
Pogoda K, Niwinska A, Murawska M et al (2013) Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol 30:388
Montagna E, Bagnardi V, Rotmensz N et al (2012) Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol 23:324–331
Nguyen PL, Taghian AG, Katz MS et al (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence afte breast-conserving therapy. J Clin Oncol 26:2373–2378
Moran MS (2015) Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol 16:e113–e122
Zaky SS, Lund M, May KA et al (2011) The negative effect of triple-negative breast cancer on outcome after breast-conserving therapy. Ann Surg Oncol 18:2858–2865
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Fastner, C. Hauser-Kronberger, A. Moder, R. Reitsamer, F. Zehentmayr, P. Kopp, C. Fussl, T. Fischer, H. Deutschmann, and F. Sedlmayer state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Rights and permissions
About this article
Cite this article
Fastner, G., Hauser-Kronberger, C., Moder, A. et al. Survival and local control rates of triple-negative breast cancer patients treated with boost-IOERT during breast-conserving surgery. Strahlenther Onkol 192, 1–7 (2016). https://doi.org/10.1007/s00066-015-0895-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0895-2
Keywords
- Triple-negative breast neoplasms
- Intraoperative radiotherapy
- Breast conserving surgery
- Electrons
- Recurrence