Abstract
Background and Purpose
Loeys-Dietz syndrome (LDS) is a connective tissue disorder characterized by arterial aneurysms and dissections. This study sought to assess and describe the arterial changes of the cervical arterial vasculature of such patients, with an emphasis on the carotid bifurcation.
Material and Methods
A retrospective review of patients with a known diagnosis of LDS was carried out. The maximum diameters of the external carotid artery (ECA) and internal carotid artery (ICA) origins, common carotid artery (CCA) terminus, maximum transverse and craniocaudal dimensions of the carotid bulb, and bifurcation angle were measured. The presence of a chalice sign was defined as a carotid bifurcation angle of ≥80°. A semi-quantified analysis of vertebral artery tortuosity was completed as well. All measurements were compared to a cohort of age-matched controls.
Results
A total of 21 patients with LDS were included. Compared to normal controls, the presence of a chalice sign had 61.9% sensitivity and 100.0% specificity for LDS if present bilaterally; the sensitivity and specificity of a unilateral chalice sign were 66.7% and 82.3%, respectively. Patients with LDS also had significantly higher rates of a bilateral chalice sign compared to patients with vascular Ehlers-Danlos syndrome (vEDS) (61.9% versus 0%, P <0.0001) and patients with Marfan syndrome (61.9% versus 14.3%, P = 0.001).
Conclusion
Patients with LDS have characteristic findings of the cervical arterial vasculature that enables them to be distinguished from normal controls as well as patients with connective tissue diseases, such as Marfan syndrome and vEDS; most notably including marked widening of the carotid bifurcation angle in what is proposed to be named the chalice sign.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loeys-Dietz syndrome (LDS) is an autosomal dominant inherited connective tissue disorder (CDS) characterized by a triad of arterial disease, cleft palate or a bifid uvula, and hypertelorism [1,2,3,4,5,6]. Although a genetic disorder, family history is a poor screening tool as 75% of cases arise from de novo genetic mutations [7]. Both arterial and skeletal abnormalities have been described in LDS patients, including aortic aneurysms and dissections, widespread arterial tortuosity, scoliosis, and pectus excavatum [8,9,10,11,12]. Arterial changes are likely progressive, as pseudoaneurysms and dissections tend to be seen in older patients [13]. The progressive aortic and peripheral arterial aneurysmal disease amongst LDS patients leads to early morbidity and mortality and the mean age of death is 26 years [14].
Prior descriptions of the head and neck arterial vasculature have focused on the vascular tortuosity and/or ectasia and the incidence of arterial aneurysms [14]. The prevalence of cervical vascular tortuosity has been reported to be 84–100% of affected patients [13, 14]. Such findings have prognostic significance amongst LDS patients as the severity of vertebral artery tortuosity portends adverse cardiovascular outcome [15]. More recently, it has been noticed that LDS patients tend to have a very capacious carotid bulb and a relatively wide angle of bifurcation of the carotid artery when compared to the general population. Changes related to the carotid bifurcation have gone relatively unreported to date. The purpose of this study was to perform a systematic and quantified analysis of the carotid bulb and bifurcation in LDS patients, with a particular emphasis on the angle of the bifurcation. Secondarily, this study assessed the severity of vertebral artery tortuosity amongst such patients.
Methods
Patient Selection and Review
Institutional review board approval was obtained for the purposes of this study. A retrospective review was completed of all patients with a confirmed diagnosis of LDS who underwent computed tomography angiography (CTA) or magnetic resonance angiography (MRA) imaging of the carotid arterial vasculature at this institution between 31 January 2008 and 4 March 2019. Diagnostic criteria for LDS included a confirmed mutation in one of the known LDS genes in the setting of aortic aneurysms or dissections; in some cases, a significant family history was taken into account. The major diagnostic criteria for vascular Ehlers-Danlos syndrome (vEDS) included the following: family history of vEDS with a variant in the COL3A1 gene, arterial rupture at young age, spontaneous sigmoid colon perforation, uterine rupture during the 3rd trimester of pregnancy, or carotid-cavernous sinus fistula formation without trauma [16]. The diagnosis of Marfan syndrome was based on the criteria laid out in the revised Ghent nosology [17].
Pediatric patients (≤18 years old) were excluded in order to account for the progressive nature of the syndrome. Patients were also excluded if motion or other artifacts significantly affected the quality of the examiantions. The electronic medical record (EMR) was used to review for a history of hypertension, dyslipidemia, diabetes mellitus, tobacco use, stroke or coronary artery disease amongst the included cohort.
The same number of age-matched control patients were selected via a review of the EMR. Control patients had no history of any connective tissue disease and no acute findings or anatomic variants on imaging. To account for the heterogeneity of imaging modality in the LDS cohort (CTA versus MRA), the same proportion (6/21; 28.5%) of computed tomography (CT) to magnetic resonance imaging (MRI) images was used in both the LDS and control cohorts.
Imaging Protocol
Nearly all CTA imaging was performed on a 128-slice multidetector scanner (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany). Slice thickness was 0.75 mm, and tube voltage and tube current were 120 kV and 415 mAs, respectively. Matrix for scans was 512 × 512; pitch = 0.6.
The majority of MRA imaging was performed with contrast (CE-MRA), on a 3T magnet (GE Healthcare, Waukesha, WI, USA). Imaging was performed in the coronal plane following a bolus of contrast medium (TE = 1.6, TR = 4.2, FOV = 26, matrix = 384 × 224, 70 slices, slice thickness = 1.2 mm, total acquisition time = 34 s).
Sensitivity Analysis
In addition to studying the association between the chalice sign and LDS using a set of age-matched controls, a sensitivity analysis was also performed to determine if the chalice sign was a finding specific to LDS or if it was associated with other vascular connective tissue diseases. To do this, the prevalence of the chalice sign was compared among patients with vEDS and Marfan syndrome. The vEDS and Marfan syndrome cohorts were chosen from patients with confirmed diagnoses that were sequentially imaged with CTA or MRA over the same time period. The vEDS and Marfan syndrome patients were not age and sex-matched due to the lack of sufficient patients.
Imaging Review and Interpretation
All images from both patient cohorts were reviewed by two neuroradiologists independently. Multiple measurements were obtained of the carotid bifurcation, including the maximum diameters of the ECA and ICA origins, maximum diameter of the CCA terminus, maximum transverse and craniocaudal dimensions of the bulb, and carotid bifurcation angle based on a line drawn along the medial aspects of the ECA and ICA (Fig. 1). The presence of a chalice sign was defined as a carotid bifurcation angle of ≥80°. Interobserver agreement for the chalice sign was calculated. Cases of disagreement were resolved with a consensus read between the two reviewers.
A carotid artery bifurcation in a 26-year-old age-matched control patient. The maximum diameters of the external carotid artery (ECA) origin (A), internal carotid artery (ICA) origin (B), and common carotid artery (CCA) terminus (C), maximum right-left (D) and craniocaudal (E) dimensions of the carotid bifurcation, and angle between the ICA and ECA origins (F) were measured
Vertebral artery tortuosity was assessed on 3D reconstructions of CTA or MRA images by dividing the intraluminal length of a vertebral artery by the sum of its straight course lengths: 1) a straight line drawn from the vertebral artery origin to the C1 transverse foramen and 2) a straight line from the transverse foramen to the arterial terminus (Fig. 2). Severity of vertebral artery tortuosity was based on the ratio of intraluminal length to straight line length: <1.15 = none, 1.15–1.40 = mild, 1.40–1.75 = moderate, and ≥1.75 = severe.
Vertebral arteries of a 26-year-old age-matched control patient. Semiquantification of vertebral artery tortuosity was obtained by comparing the true arterial length (depicted by intraluminal black lines in the right vertebral artery) to summed distance of lines drawn between 1) the vertebral artery origin and the artery as it passes through the C1 transverse foramen and 2) the artery at the C1 transverse foramen and the basilar artery origin (depicted by white lines along the left vertebral artery)
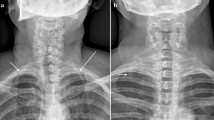
A 52-year-old male with LDS. Both the right (a) and left (b) carotid arteries have markedly increased bifurcation angles, as well as increased craniocaudal and transverse dimensions of the bifurcations. There is significant tortuosity of both vertebral arteries (c) as well as focal ectasia of the proximal right vertebral artery (curved arrow). Incidentally noted is fenestration of the proximal basilar artery (straight arrow)
Statistical Analysis
All statistical analyses were performed using JMP 13.0 (www.jmp.com, Cary, NC, USA). Comparison of continuous variables was performed using a paired t‑test and comparison of categorical variables was performed using a χ2-test. With respect to arterial measurements, comparisons were performed on a per-artery basis (i.e. right carotid compared to right carotid, left carotid compared to left carotid). In addition, the sensitivity and specificity of various imaging findings were calculated in identification of LDS patients. Interobserver agreement was calculated using the kappa statistic.
Results
Patient Characteristics
A total of 21 LDS patients were included in the final cohort (average age 43.5 ± 15.2 years) and 8/21 (38.1%) were female. Visualization of the vertebral arteries was of insufficient quality for evaluation in 6 LDS patients; the quality of carotid vascular imaging was sufficient for evaluation in all patients. The same number of age-matched controls was included. Of 24 patients with vEDS, 3 were excluded (2 pediatric patients, 1 with significant motion artifact degradation of images). Comorbidities are shown in Table 1 and 8/21 (38.1%) had hypertension, 5/21 (23.8%) had a history of tobacco use, 5/21 (23.8%) had dyslipidemia, and 1/21 (4.8%) had a prior stroke. No patients had a history of diabetes or coronary artery disease.
Carotid Arteries
Right and left carotid artery measurements are given in Table 2. Regarding the right carotid artery, the carotid bifurcation angle was significantly larger in LDS patients than in control patients (86.4 ± 44.7° versus 41.7 ± 26.6°, respectively; p = 0.0002). The maximum transverse bulb dimension of LDS patients was larger than controls (16.6 ± 4.5 mm versus 11.6 ± 2.7 mm, respectively; p < 0.0001), as were the maximum craniocaudal dimension of the bifurcation (9.2 ± 3.7 mm versus 6.3 ± 2.3 mm, respectively; p = 0.0021), the CCA terminus diameter (7.4 ± 1.3 versus 6.7 ± 0.9 mm, respectively; p = 0.0254), the ICA origin diameter (8.5 ± 1.8 mm versus 7.0 ± 1.4 mm, respectively; p = 0.0024), and the ECA origin diameter (5.2 ± 1.1 mm versus 4.3 ± 1.1 mm, respectively; p = 0.01).
Results of the left carotid artery were largely similar to the right. The carotid bifurcation angle was significantly larger in LDS (91.0 ± 40.3° versus 38.5 ± 19.5° in control patients; p < 0.0001). The maximum transverse bifurcation dimension of LDS patients was larger than controls (16.5 ± 5.0 mm versus 11.1 ± 2.3 mm, respectively; p < 0.0001), as was the maximum craniocaudal dimension of the bifurcation (8.4 ± 3.0 mm versus 6.5 ± 1.5 mm, respectively; p = 0.0059), the ICA origin diameter (8.3 ± 2.0 mm versus 6.6 ± 1.2 mm, respectively; p = 0.0011), and the ECA origin diameter (5.3 ± 1.5 mm versus 4.2 ± 0.9 mm, respectively; p = 0.0046). The diameter of the CCA terminus was not significantly different among the cohorts (7.5 ± 1.7 mm in LDS versus 6.7 ± 1.3 mm in controls; p = 0.507).
In total 14 LDS patients and 2 controls had a chalice sign of the right carotid bifurcation (P < 0.0001), 13 LDS patients and 1 control had a chalice sign of the left carotid bifurcation (P < 0.0001) and 13 LDS patients and no controls had bilateral chalice sign (P < 0.0001) (Fig. 3). The sensitivity of the chalice sign was 61.9% if present bilaterally. A bilateral chalice sign had 100.0% specificity. The presence of a unilateral chalice sign had a sensitivity 66.7% and specificity of 82.3%.
Vertebral Arteries
The average intraluminal length of the right vertebral artery was 248.6 ± 50.9 mm, compared to 199.8 ± 19.3 mm in controls. On the left, the average intraluminal vertebral artery length was 251.9 ± 33.8 mm versus 206 ± 55.7 mm in controls. In comparison, the average straight course lengths of the right and left vertebral arteries were 173.1 ± 14.8 mm (versus 167.3 ± 12.0 mm in controls) and 179.3 ± 22.3 mm (versus 178.1 ± 17.0 mm in controls), respectively. Of both vertebral arteries of LDS patients 2/30 (6.7%) had no tortuosity, 15/30 (50.0%) had mild tortuosity, 8/30 (26.7%) had moderate tortuosity, and 5/30 (16.7%) had severe tortuosity. In control patients 11/30 of the vertebral arteries had no tortuosity (36.7%) and the tortuosity of the remaining control patients was mild in 18/30 (60.0%) and moderate in 1/30 (3.3%). The LDS patients had significantly greater tortuosity of the left vertebral artery compared to controls (p = 0.0047). No significance difference was seen between the degree of vertebral artery tortuosity on the right side (p = 0.1334).
There was an association between the presence of a bilateral chalice sign and vertebral artery tortuosity. Among patients with a bilateral chalice sign, the tortuosity index of the right vertebral artery was 1.5 ± 0.3 compared to 1.2 ± 0.1 for those without a bilateral chalice sign (p = 0.01). Among patients with a bilateral chalice sign, the tortuosity index of the left vertebral artery was 1.5 ± 0.3 compared to 1.2 ± 0.1 for those without (p = 0.006). Of the patients with a bilateral chalice sign 100% had some degree of right vertebral artery tortuosity compared to 65.3% of those without bilateral chalice signs (p = 0.02). All patients with bilateral chalice signs had some degree of left vertebral artery tortuosity compared to 67.3% without bilateral chalice signs (P = 0.03) (Fig. 4).
Sensitivity Analysis
A total of 21 vEDS patients and 21 Marfan syndrome patients were included in the sensitivity analysis. The mean age of the vEDS patients was 39.9 ± 16.6 years (P = 0.44 compared to LDS) and tean age of the Marfan syndrome patients was 50.4 ± 14.0 years (P = 0.15 compared to LDS). There were 8 males in the vEDS group (P = 0.21) and 9 males in the Marfan syndrome group (P = 0.35). When comparing LDS patients to vEDS patients, 14 LDS patients and 1 vEDS patients had a chalice sign of the right carotid bifurcation (P <0.0001), 13 LDS patients and 3 Marfan patients had a chalice sign of the left carotid bifurcation (P = 0.002), 13 LDS patients and no vEDS patients had a chalice sign of the bilateral carotid bifurcations (P <0.0001). When compared to vEDS patients, the sensitivity of the chalice sign was 61.9% if present bilaterally. A bilateral chalice sign had 100.0% specificity. The presence of a unilateral chalice sign had a sensitivity 66.7% and specificity of 81.0%.
When comparing LDS patients to Marfan syndrome patients, 14 LDS patients and 4 Marfan syndrome patients had a chalice sign of the right carotid bifurcation (P = 0.002). 13 LDS patients and 3 Marfan patients had a Chalice sign of the left carotid bifurcation (P = 0.06), 13 LDS patients and 3 Marfan syndrome patients had a chalice sign of the bilateral carotid bifurcations (P = 0.001). When compared to Marfan syndrome patients, the sensitivity of the chalice sign was 61.9% if present bilaterally. A bilateral chalice sign had 85.7% specificity. The presence of a unilateral chalice sign had a sensitivity 66.7% and specificity of 61.9%.
Interobserver Agreement
Interobserver agreement for the presence of the chalice sign was excellent with k = 1.00.
Discussion
This study set out to assess and describe the cervical arterial vasculature of patients with LDS. The results indicated that LDS patients have a markedly enlarged bifurcation angle, resembling the broad curvature of a goblet in what is proposed to be referred to as the chalice sign. The presence of the chalice sign is specific for LDS, particularly when bilateral. This was true even when comparing the presence of the chalice sign among patients with LDS to those with Marfan syndrome or vEDS. The chalice sign was very reproducible as well with a high level of interobserver agreement. In addition to the chalice sign, LDS patients have characteristic findings of the carotid bifurcations, including larger dimensions of the ECA and ICA origins and carotid bifurcations compared to age-matched controls.
Age-related changes to the carotid vasculature are expected findings in all patients. A longitudinal study by Ngo et al. showed that the bifurcation angle increased with age over a 10-year period amongst 114 patients, being 33.2° on average at baseline, and 34.9° on average at follow-up [18]. The CCA diameter was also found to increase in size over time [18]. A review of 300 subjects by Jeon et al. similarly found that the CCA and ICA diameters and bifurcation angle increased with age; the angle increased from 34.4° in patients 21–30 years old to 48.5° in patients >70 years old [19]. Kamenskiy et al. found that the bifurcation angle, bulb diameter, and tortuosity of the carotid arteries increased with age, positing that such findings could be secondary to degradation of the arterial intramural elastin [20]. Nevertheless, the changes related to LDS appear to outpace those of normal aging; nearly all of the measured dimensions of the carotid bifurcation were greater among LDS than age-matched controls.
The vascular changes observed in LDS patients are hypothesized to be related to fragmentation of elastic fibers, accumulation of amorphous matrix within the media, and excessive arterial wall collagen [21,22,23]. Because these histological characteristics are observed in young children, they are thought to be due to a defect in elastogenesis rather than elastin destruction [21]. Such changes lead to markedly thin and weak arterial walls that are prone to dissections and aneurysm formation [24]. Presumably, the same processes contribute to the increased carotid bifurcation angle, vascular ectasia, and tortuosity observed amongst LDS patients in the current study.
It is interesting that it was found that the Chalice sign, especially when bilateral, was specific to LDS when compared to controls with Marfan syndrome or vEDS especially given the fact that LDS is commonly reported to have similar clinical features to Marfan syndrome and EDS. Marfan syndrome is known to result from a mutation in the extracellular matrix protein fibrillin 1 [25]. This protein serves as the structural component of calcium-binding microfibrils, which provide structural support in both elastic and non-elastic connective tissues. The vEDS is caused by mutations in the COL3A1 gene which is responsible for making type III collagen, the dominant collagen protein of blood vessels [26]. The LDS is known to result from one of five mutations involving the TGF-beta receptor gene, SMAD 3 gene or the TGF beta gene. It is hypothesized that because LDS is due to mutations involving protein receptors and signaling molecules, its affect on vascular structures may go beyond impairments in single proteins (as Marfan syndrome and vEDS) and thus result in a more extreme phenotype of vascular tortuosity and disease.
Ultimately, the end result of the arterial changes seen in LDS is significant morbidity and mortality. Loeys et al. reported a mean age of death of 26.0 years (range 0.5–47.0 years), usually due to aortic dissection (89% of either thoracic or abdominal aorta) and less commonly due to intracranial hemorrhage (7%) [27]. The same study found that the mean age of an LDS patient’s first vascular surgery was 19.8 years, and the mean age of a first vascular dissection was 26.7 years [27]. The average age of the current study cohort was older than the previously reported mean age of death (43.5 years old). It’s possible that increased awareness of LDS since its relatively recent discovered in 2005 has led to better prevention of early mortality; however, it is also possible the current cohort represented an older representation of LDS patients that had undergone chronic arterial changes not seen in a more typical LDS population. Nevertheless, it is noteworthy that the oldest member of the cohort was only 67 years old.
The reason that greater vertebral tortuosity was observed in LDS patients on the left side but not the right is likely related to normal anatomic differences in the lengths and courses of the arteries. Nevertheless, vertebral artery tortuosity is a known feature of LDS. Combined right and left data demonstrated either moderate or severe tortuosity in just under half of the measured vessels in LDS patients (43.3%), whereas the same degree of tortuosity was observed in only 1/30 (3.3%) of the normal control vertebral arteries.
No study is devoid of limitations. The current study is limited by heterogeneity of modalities by which patients were imaged; most were imaged with MRA, while the minority was imaged with CTA. Attempts were made to negate such inconsistencies by matching the proportion of CTA to MRA imaging within the control cohort. Also, as stated above, the average age of included LDS patients was 43.5 years, above the typical life expectancy of 26 years old. Consequently, the results may have been somewhat skewed by an atypical subset of patients that has undergone arterial changes of a longer period of time than usual.
Conclusion
Patients with LDS undergo characteristic changes of the cervical arterial vasculature, including marked widening of the carotid bifurcation angle in what is proposed to be referred to as the chalice sign. The presence of a bilateral chalice sign is highly specific for LDS. Additionally, LDS patients have significantly larger diameters of the ICA and ECA origins and larger carotid bifurcation dimensions, compatible with the arterial ectasia and tortuosity that are known hallmarks of the syndrome.
References
Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–81.
Kane BS, Shamsa K. Preventing a catastrophe: increasing awareness of Loeys-Dietz syndrome. Tex Heart Inst J. 2019;46:41–3.
Hara H, Takeda N, Fujiwara T, Yagi H, Maemura S, Kanaya T, et al. Activation of TGF‑β signaling in an aortic aneurysm in a patient with Loeys-Dietz syndrome caused by a novel loss-of-function variant of TGFBR1. Hum Genome Var. 2019;6:6.
MacCarrick G, Black JH 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–87.
Van Hemelrijk C, Renard M, Loeys B. The Loeys-Dietz syndrome: an update for the clinician. Curr Opin Cardiol. 2010;25:546–51.
Patel ND, Alejo D, Crawford T, Hibino N, Dietz HC, Cameron DE, Vricella LA. Aortic root replacement for children with Loeys-Dietz syndrome. Ann Thorac Surg. 2017;103:1513–8.
Beaulieu RJ, Lue J, Ehlert BA, Grimm JC, Hicks CW, Black JH 3rd. Surgical management of peripheral vascular manifestations of Loeys-Dietz syndrome. Ann Vasc Surg. 2017;38:10–6.
Jawaid Y, Aqtash O, Mansoor K, Ajmeri AN, Fofie F, Amro A, Dial L. Loeys-Dietz syndrome complicated by right coronary artery pseudoaneurysm. Case Rep Cardiol. 2018;2018:8014820.
Aftab M, Cikach FS, Zhu Y, Idrees JJ, Rigelsky CM, Kalahasti V, Roselli EE, Svensson LG. Loeys-Dietz syndrome: Intermediate-term outcomes of medically and surgically managed patients. J Thorac Cardiovasc Surg. 2019;157:439–50.e5.
MacFarlane EG, Parker SJ, Shin JY, Kang BE, Ziegler SG, Creamer TJ, et al. Lineage-specific events underlie aortic root aneurysm pathogenesis in Loeys-Dietz syndrome. J Clin Invest. 2019;129:659–75.
Wang S, Kernodle A, Hicks CW, Black JH 3rd. Endovascular repair of tortuous recurrent femoral-popliteal aneurysm in a patient with Loeys-Dietz syndrome. J Vasc Surg Cases Innov Tech. 2018;4:156–9.
Kirby DJ, Dietz HC, Sponseller PD. Spondylolisthesis is common, early, and severe in Loeys-Dietz syndrome. J Pediatr Orthop. 2018;38:e455–61.
Rodrigues VJ, Elsayed S, Loeys BL, Dietz HC, Yousem DM. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol. 2009;30:1614–9.
Loughborough WW, Minhas KS, Rodrigues JCL, Lyen SM, Burt HE, Manghat NE, et al. Cardiovascular manifestations and complications of Loeys-Dietz syndrome: CT and MR imaging findings. Radiographics. 2018;38:275-86.
Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–96.
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26.
Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–85.
Ngo MT, Kwak HS, Ho Chung G, Koh EJ. Longitudinal study of carotid artery bifurcation geometry using magnetic resonance angiography. Vascular. 2019;27:312-7.
Jeon SJ, Kwak HS, Chung GH. Widening and rotation of carotid artery with age: geometric approach. J Stroke Cerebrovasc Dis. 2018;27:865–70.
Kamenskiy AV, Pipinos II, Carson JS, MacTaggart JN, Baxter BT. Age and disease-related geometric and structural remodeling of the carotid artery. J Vasc Surg. 2015;62:1521–8.
Loeys BL, Dietz HC. Loeys-Dietz Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A, editors. GeneReviews. Seattle: University of Washington; 1993.
Maleszewski JJ, Miller DV, Lu J, Dietz HC, Halushka MK. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome (LDS). Am J Surg Pathol. 2009;33:194–201.
Johnson PT, Chen JK, Loeys BL, Dietz HC, Fishman EK. Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol. 2007;189:W29–35.
Rahme RJ, Adel JG, Bendok BR, Bebawy JF, Gupta DK, Batjer HH. Association of intracranial aneurysm and Loeys-Dietz syndrome: case illustration, management, and literature review. Neurosurgery. 2011;69:E488–92. discussion E492–3.
Takeda N, Komuro I. Genetic basis of hereditary thoracic aortic aneurysms and dissections. J Cardiol. 2019;74:136–43.
Kuivaniemi H, Tromp G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene. 2019;707:151–71.
Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
J.C. Benson and W. Brinjikji declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Benson, J.C., Brinjikji, W. The Chalice Sign. Clin Neuroradiol 30, 713–720 (2020). https://doi.org/10.1007/s00062-019-00838-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00838-5