Abstract
Background and Purpose
Cangrelor is a P2Y12 inhibitor that presents the advantage of having a short half-life. Its use may be helpful in the management of antiplatelet therapy for patients with intracranial aneurysms treated by stent-assisted coiling or flow-diverter stents. The purpose of this study was to report early experiences in using cangrelor for such indications.
Material and Methods
From October 2017 to November 2018, 7 consecutive patients (5 females, 2 males, mean age = 56 years) were managed with cangrelor as antiplatelet therapy, combined with aspirin, for stent-assisted coiling embolization and flow-diverter embolization of challenging intracranial aneurysms. Anti-aggregation protocols, including cangrelor, were systematically recorded. Treatment-related complications (minor/major hemorrhagic complications, ischemic complications) as well as clinical and angiographic outcomes (evaluated at 8.7 ± 4.2 and 8.75 ± 10 months, respectively) were retrospectively analyzed.
Results
Of the aneurysms 71.4% (5 out of 7) were ruptured and treated in the acute phase. In one case cangrelor was used as an alternative to clopidogrel in an asymptomatic hemorrhagic complication after stent-assisted coiling for better control of a possible worsening of the intracranial bleeding. Of the patients, 1 (14%) with a complex ruptured MCA aneurysm treated with a flow-diverter stent experienced a severe intracranial hemorrhage, which occurred after switching the cangrelor to ticagrelor and eventually led to death. No hemorrhagic complications under cangrelor were recorded for the six remaining patients. No mRS worsening was observed at discharge, except for the patient who died and six out of the seven patients had a mRS ≤2 at follow-up.
Conclusion
Cangrelor is a new antiplatelet therapy with a P2Y12 inhibiting effect, with a rapid onset and offset of action, owing to its short half-life. This cases series presents a pilot experience with promising results in terms of antiplatelet management for challenging intracranial aneurysms treated by stent assisted coiling or flow-diverter stents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
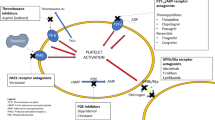
Antiplatelet therapy is a key medication for stent-assisted coiling or flow-diversion treatment of intracranial aneurysms [1, 2]. During the first months after stent deployment, in order to avoid (or a least reduce) the risk of thromboembolic complications, an antiplatelet therapy is required. Derived from the cardiologists’ practice for the management of stents in coronary diseases [3], interventional neuroradiologists usually use dual antiplatelet therapy (aspirin plus a P2Y12 receptor inhibitor, e.g. clopidogrel, prasugrel or ticagrelor) during the first weeks/months after stenting (with no consensus between the different teams around the world), and then switch to a single antiplatelet therapy (for a total of at least 1 year in this institution). The drawback of this strategy is that most of the antiplatelet agents used in the field of interventional neuroradiology have a slow onset of action (2–8 h for clopidogrel, for instance) [4], thus requiring a loading dose before the endovascular treatment. Additionally, the long duration of platelet inhibition induced by P2Y12 inhibitors [4] is challenging for the management of hemorrhagic complications or unplanned high bleeding risk procedures, including the ventricular drainage setting. Moreover, platelet inhibition induced by some antiplatelet agents, such as ticagrelor, is difficult to neutralize, which may lead to dramatic complications in cases of hemorrhage [5].
Cangrelor is a P2Y12 inhibitor, which received Food and Drug Administration (FDA) approval for percutaneous coronary interventions (PCI) in June 2015 [6]. Cangrelor is an active drug, which does not require activation by metabolic conversion. This mechanism is different from other antiplatelet medications such as clopidogrel, which is a prodrug that requires conversion to be activated. Interestingly, cangrelor is the only P2Y12 inhibitor that is available in i.v. form.
The pharmacokinetics of cangrelor are characterized by a rapid onset and offset of action with a clearance of 50 l/h and a half-life of 3–5 min [7]. Moreover, the effect of cangrelor ceases rapidly after the administration has been stopped and normal platelet function is thereafter restored within 1 h [7]. The very short half-life of cangrelor may be interesting in the field of interventional neuroradiology, especially for the management of intracranial hemorrhages complicating stent-assisted coiling or flow diversion. The need for an emergent ventricular drainage in patients recently treated with an intracranial stent may be another situation in which cangrelor may be a valuable medication.
This article presents early experiences in using cangrelor for the management of antiplatelet therapy in the acute phase of stent-assisted coiling or flow-diverter treatment.
Material and Methods
Study Design
This study is a retrospective observational case series.
Patient Population
From October 2017 to November 2018, all adult (≥18 years) patients treated at this institution with cangrelor as an antiplatelet regimen for stent-assisted coiling or flow diversion for an intracranial aneurysm were systematically reviewed. Patient demographics and aneurysm characteristics (e.g. ruptured/unruptured status, aneurysm shape, location, shape, largest diameter and aneurysm neck size) were systematically assessed.
Procedures
In all patients, details about the endovascular procedure were collected from the medical chart. Details about the antiplatelet regimen before and after the procedure as well as for the management in the intensive care unit (ICU) were also systematically recorded.
Cangrelor Administration Protocol
All patients had complete blood assays, including platelet and red blood cell counts as well as coagulation work-up before cangrelor infusion. No platelet aggregometry testing was performed before cangrelor administration, since no patient was preloaded by antiplatelet therapy before stenting, to avoid any bleeding from the aneurysm. Cangrelor was used in all cases with a dose derived from the cardiologists’ experience: 30 μg/kg i.v. bolus followed immediately by 4 μg/kg per minute i.v. infusion [5].
Safety
Procedure-related complications were systematically recorded. These complications were divided into major complications (death, symptomatic intracranial hemorrhage, acute ischemic stroke leading to disability, groin puncture complications requiring surgical repair and blood transfusion) and minor complications (transient ischemic attack, acute ischemic stroke that did not lead to disability, asymptomatic/paucisymptomatic intracranial hemorrhage, minor puncture site complication). Any external or internal bleeding that occurred during the hospital stay or after discharge was systematically recorded.
Angiographic and Clinical Outcomes
Immediate angiographic outcome was evaluated on DSA in working projection at the end of the procedure, and was graded according to the Roy-Raymond [8] grading scale for aneurysms treated by stent-assisted coiling and with the O’Kelly-Marotta [9] and the Çekirge-Saatci [10] scales for those treated by flow-diverter stents. Stent occlusion and in-stent thrombosis were systematically assessed on angiographic control. In 4 out of the 6 surviving patients (66.7%), a short-term (3–6 months) MR angiography was performed. Only 2/6 patients (33%) had a 1-year DSA since several patients were treated recently, less than 1 year ago. The average angiographic follow-up time interval was 8.75 ± 10 months. On MRA follow-up, aneurysm occlusion was graded according to the Roy-Raymond score [8] in patients treated by stent-assisted coiling. In the patients treated by FDS who underwent the 1‑year DSA follow-up, aneurysm sac occlusion was graded on the O’Kelly-Marotta [9] and the Çekirge-Saatci [10] grading scales.
Ethical Statement
The use of cangrelor was off-label in this study. All cases were complex ones, with no alternative to intracranial stenting. The choice to treat these patients under cangrelor was made through a multidisciplinary meeting with neurosurgeons and anesthesiologists. Patient families were informed before each treatment of the strategy that was chosen and gave verbal consent.
The need for patient informed consent for retrospective analyses of records and imaging data was waived by the institutional review board (IRB). This work conforms to the World Medical Association Declaration of Helsinki.
Results
Patient Demographics/Aneurysm Characteristics
Patient demographics, aneurysm characteristics and details of the endovascular procedures are summarized in Table 1.
A total of seven consecutive adult patients undergoing endovascular treatment for an intracranial aneurysm were included from October 2017 to November 2018 in a single center (5 females and 2 males, mean age: 56 ± 10.4 years). Of the patients five presented with a ruptured aneurysm (including two ruptured blister-like aneurysms), not eligible for regular coiling or clipping, treated at the acute phase with a FDS (silk stent; n = 1 ,Balt, Montmorency, France, Pipeline Embolization Device, PED; n = 2, eV3/Medtronic, Irvine, CA, USA and P64; n = 2, Phenox, Bochum, Germany). In one patient (patient # 1), 2 FDS (Silk) were used in a telescopic fashion and one of the patients treated by FDS had a compressive large partially thrombosed aneurysm at the distal aspect of the basilar artery. In none of the aneurysms treated by FDS additional coils were used. Indeed, intrasaccular coiling was deemed too dangerous in the two blister-like aneurysms cases, and for the remaining fusiform or partially thrombosed aneurysms, coiling seemed inappropriate. Of the patients two were treated using non-flow diverter stents (Baby Leo, Balt): in the first case, cangrelor was used in an asymptomatic hemorrhage complicating a stent-assisted coiling of an unruptured aneurysm (the patient bled under ticagrelor; which was switched to cangrelor to reduce the risk of bleeding worsening); in the second case, cangrelor was required for a bail-out stenting in a ruptured pericallosal aneurysm treated firstly by balloon-assisted coiling. In the latter case, a stent was deployed due to coil loop protrusion.
Of the aneurysms 2 (28.6%) were located on the MCA bifurcation/trifurcation. Of these 2 patients 1 (patient # 1) was treated by a flow diverter stent because no other option (either endovascular or surgical) was deemed feasible; the aneurysm involved both the M1 segment and the M2 branches of a MCA trifurcation. According to the literature [11, 12] and to local experience, FDS was deemed to be the most suitable option in this challenging case. Of the aneurysms two were located on the distal internal carotid artery (ICA) (28.6%), one on the A1 anterior cerebral artery (ACA) (14.3%), one on the pericallosal artery and a last one on the basilar artery (14.3%). The average maximum diameter of the aneurysms was 9.0 ± 8.1 mm (range: 1.9–23 mm); average neck diameter was 5.8 ± 5.3 mm (range: 0.5–13 mm).
Stenting Procedures/Antiplatelet Management
All the patients were under general anesthesia via a femoral approach during treatment. All stentings were performed with a tri-axial system using a 6F long sheath, an intermediate supple catheter (5F or 6F) and a microcatheter. Of the patients five were treated with a flow diverter stent (for a ruptured MCA trifurcation aneurysm, for an unruptured compressive basilar artery aneurysm, for a ruptured serpentine ICA aneurysm and for two blister aneurysms); two patients were treated with a low profile braided stent for an unruptured MCA aneurysm and for a ruptured pericallosal aneurysm. For the five patients treated in the acute phase, no preprocedural antiplatelet loading was performed (Fig. 1). The patients received the IV bolus of cangrelor within the 10 min before the stent positioning and an IV bolus of 250 mg aspirin 30 min before the stent deployment. Then, cangrelor infusion was pursued with an electric pump for 12 to 48 h, before switching to ticagrelor 90 mg twice a day.
50-year-old female patient admitted for a World Federation of Neurological Surgeons (WFNS) II subarachnoid hemorrhage. a CT-scan, axial slice showed thin subarachnoid hemorrhage graded Fisher II, with no vascular cause found on primary angiogram (b). Control angiogram 8 days later, selective left internal carotid artery (ICA) injection (c and d, black arrow) and 3D rotational angiography; volume-rendering reconstruction (e) showing a blister-like aneurysm of the left A1 segment. Periprocedural unsubtracted angiograms (f and g): deployment of a flow diverter stent (Pipeline Flex 2.5 × 10 mm, Medtronic) in left A1. h Control flat panel volume CT angiography with 20% contrast medium dilution performed in the angiosuite at the end of the procedure showing optimal coverage of aneurysmal neck and satisfactory wall apposition of the stent. Note the contrast media stagnation in the aneurysms (arrow)
All patients systematically had a postoperative CT scan to avoid early hemorrhagic complication. In case of clinical worsening during the following hours after the procedure, a brain MRI, including at least diffusion-weighted and T2*-weighted sequences, was performed to depict any ischemic or hemorrhagic complication.
Antiplatelet Therapy Bridging
In three patients, cangrelor was used in a bridging fashion. In one patient (patient # 5) the treatment was switched from ticagrelor to cangrelor at day 21 for ventricular drainage removal. In one patient (patient # 3), the cangrelor was used as an alternative to ticagrelor for better control of a potential worsening of an intracranial hemorrhage in the aftermath of a stent-assisted coiling. The patient experienced a spontaneous and asymptomatic intraparenchymal hematoma following stent-assisted coiling of an unruptured MCA aneurysm depicted at day 1 on CT scan. The patient was treated by aspirin (75 mg/day) and ticagrelor (90 mg x2/day) for bleeding. The aspirin was pursued at the same dose and the ticagrelor stopped. Cangrelor was started 36 h after the bleeding event for 24 h. Then, ticagrelor was resumed, and the cangrelor stopped 3 h after the first dose of ticagrelor. The patient did not experience growth of the hematoma or rebleeding and was discharged free of symptoms.
Finally, the last patient (patient # 2) required ventricular drainage due to the occurrence of a hydrocephalus 20 days after the treatment of a large basilar artery aneurysm. The hydrocephalus was revealed by major headache, and consciousness disorder. Dual antiplatelet therapy was modified preoperatively as follows: aspirin (75 mg/day) was continued, ticagrelor (90 mg twice a day) was stopped; cangrelor (dose: 0.75 μg/kg/min) was introduced 36 h after ticagrelor discontinuation. This time interval was chosen because it corresponds approximately to 5 times the half-life of cangrelor. Additionally, the P2Y12 effect was regularly monitored by aggregometry tests. The ventricular drainage was then positioned 2 h after having stopped the cangrelor. Afterwards, the cangrelor was resumed 12 h after setting the ventricular drainage. Finally, the cangrelor was stopped and the ticagrelor reintroduced at day 2.
Safety
Only one (14%) major procedure-related complication was recorded (patient # 1), which consisted of an acute intracranial hemorrhage 12 h after the endovascular procedure with FDS in an MCA aneurysm, while the patient was under ticagrelor, after switching from cangrelor to ticagrelor. According to the MRI findings, this complication corresponded to a hemorrhagic transformation of an acute ischemic infarct, which eventually led to death due to rapid growth of the hematoma. A minor (i.e. asymptomatic) intracranial bleeding occurred (patient # 3). In the five remaining patients, no bleeding (either intracranial or from the groin puncture) was recorded. Interestingly, the two patients (patients # 1 and 3) who experienced intracranial bleeding were both under ticagrelor and one patient (patient # 1) presented with a bleeding worsening after having switched to cangrelor. No in-stent thrombosis or stent occlusion was recorded.
Clinical and Angiographic Outcomes
At the end of the procedure, among the patients treated by stent-assisted coiling, one had a Roy-Raymond [8] grade A occlusion, while the other one had a grade C. In patients treated by FDS, all patients had a grade 4A on the Çekirge-Saatci [10] grading scale and a grade A or B on the O’Kelly-Marotta [9] scale on immediate post-procedural DSA (Table 1). The average clinical follow-up was 8.7 ± 4.2 months (range 4–13) and one patient (patient # 1) died 6 days after the procedure from an intracranial hemorrhage. At discharge, 5 patients (71.4%) were mRS 0–2 and 2 patients (28.6%) were mRS >2. At the last clinical follow-up, all surviving patients (6/6) has a mRS ≤2. Angiographic follow-up was available in 4/6 of the surviving patients (66.7%). A Roy-Raymond [8] grade B occlusion was seen in the patient treated by stent-assisted coiling who had MR angiography follow-up (patient # 6), two patients treated with FDS had a 1-year DSA, which showed grade 3 and grade 4A results on the Çekirge-Saatci [10] grading scale, respectively (Table 1). The remaining patients did not have angiographic follow-up since they were treated very recently.
Discussion
This short case series underlines the feasibility of using cangrelor for the management of challenging cases of stent-assisted coiling or flow-diverter stent embolization.
Safety
In this series, no cases of stent occlusion or in-stent thrombosis were recorded. Only one case of severe hemorrhage occurred in the short case series, in a highly challenging ruptured MCA aneurysm. Interestingly, the hemorrhage occurred 12 h after the treatment under ticagrelor, after switching from cangrelor to ticagrelor. In the remaining cases, cangrelor enabled successful and safe treatment of ruptured aneurysms treated with flow diverter stents, hemorrhagic complications in an unruptured aneurysm treated by stent assisted coiling (switch from ticagrelor to cangrelor to avoid increasing of the hematoma), and a case of hydrocephalus following flow diversion which required ventricular drainage in emergency.
It is noteworthy that all the hemorrhages recorded in this series (n = 2, with 1 asymptomatic hemorrhage) occurred under ticagrelor and not under cangrelor. In these cases, ticagrelor was switched for cangrelor for better control of the risk of worsening of the hematoma. In one case, the hematoma increased in size, leading to a fatal outcome; in the other one it remained stable and asymptomatic. Thus, no definitive conclusion can be drawn for the use of cangrelor as a bridging antiplatelet therapy for the management of intracranial bleeding.
The rationale for using ticagrelor in ruptured aneurysms is that the resistance to ticagrelor is very low, compared to clopidogrel. It appeared to be too dangerous to give the patients a loading dose for platelet aggregometry testing in ruptured aneurysms, owing to the significant early rebleeding risk. That is the reason why ticagrelor was chosen instead of clopidogrel, in order to reduce the risk of resistance, and thus the risk of thromboembolic complications. It is acknowledged that the bleeding risk may be slightly higher with ticagrelor compared to clopidogrel; however, there are some data comparing both antiplatelet therapies for intracranial stenting, showing no significantly higher bleeding risks with ticagrelor [2].
Rationale for Using Cangrelor in Interventional Neuroradiology
Cangrelor is an emerging medication used in the cardiology field for PCI [13,14,15]. It has been shown that cangrelor reduces the occurrence of periprocedural thromboembolic complications after PCI compared to clopidogrel, without increase of the periprocedural severe/moderate bleeding risk [16]. So far, only one case series has been published on the safety and effectiveness of cangrelor for intracranial/cervical stenting [17]. In this article, the authors reported their preliminary experience with cangrelor in 8 patients. Most of the patients of this series (7/8; 87.5%) had stenting for intracranial (1/7; 14.3%) or cervical (6/7; 85.7%) arterial occlusion in the setting of acute ischemic stroke in most cases (5/7; 74.4%) and only 1 patient was treated for an intracranial aneurysm with a flow diverter stent.
Recent advances in the design of FDSs, like coating or surface modification with phosphorylcholine, may help to treat patients with ruptured intracranial aneurysms with FDS under a single antiplatelet therapy. Animal studies [18] as well as first case series in humans [19] using these devices are promising; however, strong evidence on the safety and effectiveness of such devices with only one antiplatelet therapy is still lacking. Additionally, even though complete aneurysm occlusion is rarely obtained at the end of the procedure in flow diversion, data from the literature [20] as well as local experiences [21] show that dual antiplatelet therapy is still a reasonable option in patients treated with FDSs in the acute phase; the flow diversion, by redirecting the blood flow in the parent artery, probably reduces the stress exerted on the aneurysm wall and thus prevents rebleeding in most cases.
Cangrelor Dose
It has been recently shown in the literature that an adequate platelet inhibition could be obtained with lower cangrelor doses (even <0.5 μg/kg/min) than the standard one for PCI (bolus of 30 μg/kg, then infusion of 4 μg/kg/min). Aguilar-Salinas et al. [17] proposed in a recent case series of intracranial/cervical stenting (mainly in the setting of acute ischemic stroke) the use of half of the standard dose for PCI (15 μg/kg) bolus, followed by a 2.0 μg/kg/min i.v. infusion of cangrelor for a minimum of 2 h; however, in this series the full dose of the protocol used by the cardiologists was used (bolus of 30 μg/kg, then infusion of 4 μg/kg/min) because it was thought that the risk a ischemic complication/stent occlusion was high in the patients treated by braided stents, most of them being flow diverter stents (71.4%) which are known to be highly thrombogenic. Some data are also available in the cardiology literature on the safety of cangrelor overdose in PCI. There is not a statistically significant higher bleeding risk in patients who received cangrelor overdose as compared to the standard dose for PCI [22]; however, it should be kept in mind that risk of intracranial bleeding, which may lead to devastating consequences, is much higher for intracranial stenting than for PCI.
Advantages of Cangrelor
In addition to its “on-off” activity, another major advantage of cangrelor is that its efficacy does not depend on its metabolism. Contrary to clopidogrel, the efficacy of cangrelor is not impacted by genetic variations. Moreover, no dose adjustment is required for patients with renal or hepatic impairment. In these cases, in contrast with the recommendations for cardiac percutaneous interventions [16], it was decided to continue the cangrelor for 48h before switching for ticagrelor since there was a high estimated bleeding risk (most of the patients of this cases series had ruptured aneurysms presenting a potential risk of rebleeding, who may also secondarily require a ventricular drainage).
In bridging strategy (for the placement of a ventricular drainage for instance), a dose of 0.75 μg/kg/min was chosen based on the results of both phase 2 studies evaluating the dose-response curve of cangrelor on healthy subject and on randomized controlled trials focused on coronary artery bypass grafting surgery [23]. According to these data, a dose of 0.75 μg/kg/min is sufficient to ensure a platelet aggregation inhibition while not significantly increasing the bleeding risk.
Cangrelor Drawbacks
There are two main drawbacks for the use of cangrelor. First, it requires careful management, preferentially in an intensive care unit, to guarantee a continuous i.v. infusion. Thus, clearly defined protocols should be used to avoid any discontinuity in platelet inhibition due to the short half-life of cangrelor. Second, cangrelor is an expensive medication (350 euros/vial) [24]. Thus, this medication should be used with parsimony and cannot be reasonably administered over a long time period.
Limitations of the Study
The major limitation of the case series is its retrospective fashion and the small number of patients included. Additionally, there was no control group with regular antiplatelet therapy in the study; however, the goal of this study was to present early experiences in the use of cangrelor, to share it with the interventional neuroradiology community and to provide new insights for emergency stent-assisted coiling treatment and flow-diverter embolization.
Conclusion
This case series shows the feasibility of using cangrelor, combined with aspirin, as an antiplatelet therapy in stent-assisted embolization or flow-diverter therapy for acutely ruptured aneurysms. This medication has a rapid onset and offset of action, owing to its short half-life, which could be useful in challenging cases where high hemorrhagic and thrombotic risks coexist. It may also be a useful option for patients requiring dual antiplatelet therapy while facing unplanned ventricular drainage; however, comparison with a standard antiplatelet regimen in a larger prospective patient cohort is warranted.
Abbreviations
- ACA:
-
Anterior cerebral artery
- DSA:
-
Digital subtraction angiography
- FDS:
-
Flow diverter stent
- ICA:
-
Internal carotid artery
- MCA:
-
Middle cerebral artery
- mRS:
-
Modified Rankin Scale
- PCI:
-
Percutaneous coronary intervention
References
Fiorella D, Thiabolt L, Albuquerque FC, Deshmukh VR, McDougall CG, Rasmussen PA. Antiplatelet therapy in neuroendovascular therapeutics. Neurosurg Clin N Am. 2005;16:517–40.
Narata AP, Amelot A, Bibi R, Herbreteau D, Angoulvant D, Gruel Y, Janot K. Dual antiplatelet therapy combining aspirin and ticagrelor for intracranial stenting procedures: A retrospective single center study of 154 consecutive patients with unruptured aneurysms. Neurosurgery. 2019;84:77-83.
Webster TD, Vaishnava P, Eagle KA. Perioperative management of dual anti-platelet therapy. Hosp Pract. 2016;44:237–41.
Song JW, Soh S, Shim JK. Dual antiplatelet therapy and non-cardiac surgery: Evolving issues and anesthetic implications. Korean J Anesthesiol. 2017;70:13–21.
Godier A, Taylor G, Gaussem P. Inefficacy of platelet transfusion to reverse ticagrelor. N Engl J Med. 2015;372:196–7.
Norgard NB. Cangrelor: A novel P2Y12 receptor antagonist. Expert Opin Investig Drugs. 2009;18:1219–30.
Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: A direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50:27–35.
Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004.
O’kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16:133–7.
Cekirge HS, Saatci I. A new aneurysm occlusion classification after the impact of flow modification. AJNR Am J Neuroradiol. 2016;37:19–24.
Iosif C, Mounayer C, Yavuz K, Saleme S, Geyik S, Cekirge HS, Saatci I. Middle cerebral artery bifurcation aneurysms treated by extrasaccular flow diverters: Midterm angiographic evolution and clinical outcome. AJNR Am J Neuroradiol. 2017;38:310–6.
Michelozzi C, Darcourt J, Guenego A, Januel AC, Tall P, Gawlitza M, Bonneville F, Cognard C. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: Complications, aneurysm sac occlusion, reabsorption, recurrence, and jailed branch modification at follow-up. J Neurosurg. 2018 Dec 21:1-12. doi: 10.3171/2018.
Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, Iwaoka RS, Stuckey TD, Gogia HS, Gruberg L, French WJ, White HD, Harrington RA; CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet. 2013;382:1981–92.
Alexopoulos D, Pappas C, Sfantou D, Lekakis J. Cangrelor in percutaneous coronary intervention: Current status and perspectives. J Cardiovasc Pharmacol Ther. 2018;23:13–22.
Keating GM. Cangrelor: A review in percutaneous coronary intervention. Drugs. 2015;75:1425–34.
Abtan J, Steg PG, Stone GW, Mahaffey KW, Gibson CM, Hamm CW, Price MJ, Abnousi F, Prats J, Deliargyris EN, White HD, Harrington RA, Bhatt DL; CHAMPION PHOENIX Investigators. Efficacy and safety of cangrelor in preventing periprocedural complications in patients with stable angina and acute coronary syndromes undergoing percutaneous coronary intervention: The CHAMPION PHOENIX trial. JACC Cardiovasc Interv. 2016;9:1905–13.
Aguilar-Salinas P, Agnoletto GJ, Brasiliense LBC, Santos R, Granja MF, Gonsales D, Aghaebrahim A, Sauvageau E, Hanel RA. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: Preliminary experience in a single-center pilot study. J Neurointerv Surg. 2019;11:347-51.
Marosfoi M, Clarencon F, Langan ET, King RM, Brooks OW, Tamura T, Wainwright JM, Gounis MJ, Vedantham S, Puri AS. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. 2018;10:406–11.
Manning NW, Cheung A, Phillips TJ, Wenderoth JD. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: Multicentre experience. J Neurointerv Surg. 2019;11:694-8.
Zhu D, Yan Y, Zhao P, Duan G, Zhao R, Liu J, Huang Q. Safety and efficacy of flow diverter treatment for blood blister-like aneurysm: A systematic review and meta-analysis. World Neurosurg. 2018;118:e79–86.
Capocci R, Shotar E, Di Maria F, Rolla-Bigliani C, Al Raaisi A, André A, Mahtout J, Boch AL, Degos V, Sourour N, Clarençon F. Delayed treatment (≥5 days) by flow diversion of ruptured blister-like cerebral aneurysms: Case series of 8 consecutive patients. Clin Neuroradiol. 2019 Jan 25. doi: 10.1007/s00062-019-00758-4. [Epub ahead of print]
Angiolillo DJ, Bhatt DL, Steg PG, Stone GW, White HD, Gibson CM, Hamm CW, Price MJ, Prats J, Liu T, Mahaffey KW, Harrington RA. Impact of cangrelor overdosing on bleeding complications in patients undergoing percutaneous coronary intervention: Insights from the CHAMPION trials. J Thromb Thrombolysis. 2015;40:317–22.
Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, Voeltz MD, Chandna H, Ramaiah C, Brtko M, Cannon L, Dyke C, Liu T, Montalescot G, Manoukian SV, Prats J, Topol EJ; BRIDGE Investigators. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: A randomized controlled trial. JAMA. 2012;307:265–74.
Sibbing D, Kastrati A, Berger PB. Pre-treatment with P2Y12 inhibitors in ACS patients: Who, when, why, and which agent? Eur Heart J. 2016;37:1284–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Abdennour, M. Drir, K. Premat, E. Shotar, G. Taylor, A. Godier, J. Mathout, S. Lenck, R. Bernard, A. Carpentier and V. Degos declare that they have no competing interests. F. Clarençon reports conflict of interest with Medtronic, Guerbet, Balt Extrusion (payment for readings), Codman Neurovascular (core lab). N. Sourour is consultant for Medtronic, Balt Extrusion, Microvention, Stock/Stock Options: Medina. The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical standards
Patient families were informed before each treatment of the strategy that was chosen and gave verbal consent. The need for patient informed consent for retrospective analyses of records and imaging data was waived by the IRB. This work conforms to the World Medical Association Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Abdennour, L., Sourour, N., Drir, M. et al. Preliminary Experience with Cangrelor for Endovascular Treatment of Challenging Intracranial Aneurysms. Clin Neuroradiol 30, 453–461 (2020). https://doi.org/10.1007/s00062-019-00811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00811-2