Abstract
Purpose
Although high-resolution 3D-imaging has markedly improved the imaging of the pediatric pineal gland, the prevalences of typical and atypical cysts as well as in vivo volumes are unknown.
The purpose of this study was to compare the frequency of typical and atypical cysts using high-resolution 3D-sequence true fast imaging with steady state precession (trueFISP) and standard sequences and to directly measure the pineal volume in a large pediatric population.
Methods
In 54 consecutively examined children (age 0–17 years, mean age 5.4 ± 5.6 years, 44% female, 56% male) the prevalence of typical and atypical cysts (thickened rim, trabeculations, asymmetry) was determined using trueFISP (isotropic, 0.8 mm) and standard sequences, 1.5-T, T1-weighted spin echo (T1-SE), T2-weighted turbo spin echo (T2-TSE) and fluid attenuated inversion recovery (FLAIR). Indistinct findings were noted separately. Volumetry was based on the trueFISP datasets. Solid and cystic compartments were approached separately. The pineal volume was correlated to gender and age.
Results
The detected frequency of pineal cysts was higher in trueFISP (57.4%) than in standard sequences (T1-SE 7.4%, T2-TSE 14.8%, and FLAIR 13.0%). In trueFISP 66.3% of the detected cysts were classified as atypical (standard sequences 0%). Indistinct findings were lowest in trueFISP. The mean pineal volume was 94.3 ± 159.1 mm³ and no gender related differences were found. Age and volume showed a moderate correlation (r = 0.382) which was remarkably higher in completely solid glands (r = 0.659).
Conclusions
TrueFISP imaging improves the detection of pineal cysts in children. A typical cysts are frequently detected as an incidental finding. Volumetric analysis of the pediatric pineal gland is feasible and reveals enormous variation. Whereas gender effects are negligible, the pineal volume in children is dependant on age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The radiological evaluation of the pineal gland in children can be problematical if the gland is large or contains complex cysts. Besides neoplasms, physiological variation also has to be considered. However, the interindividual variation of the pineal gland in childhood is not exactly known.
For the definition of normal pineal morphology and morphometry, traditionally autopsy studies have been used [1–4]. However, up to now adequate autoptical information does not exist for children. Computed tomography (CT, [5]) and magnetic resonance imaging (MRI) studies [6–11] have characterized the pineal gland mainly in adults whereas only sparse information is available for the pediatric pineal gland.
Most of the cross-sectional imaging studies suffer from inadequately high slice thickness (4–10 mm) for a structure with a mean diameter of 6–10 mm in adults. The substantial impact of the partial volume effect on pineal size measurements has been explicitly documented by Schmitz et al. [5].
To overcome the partial volume effect high-resolution 3D-MRI sequences (1 mm isotropic voxel size or less) have recently been used to characterize the pineal gland in adults in more detail [8,10–12]. Sun et al. [11] defined the normal pineal size for healthy young Chinese adults (20–30 years). Nolte et al. described atypical pineal cysts (thick rim, trabeculation, not round/ovoid) as a frequent finding of high-resolution 3D-MRI in adults [8]. Obviously, adult values cannot be directly transferred to children. In children however, exact data on the volume and morphology of the pineal gland is still lacking.
This study utilized the potentials of high-resolution 3D sequences with true fast imaging with steady state precession (trueFISP) for imaging of the pediatric pineal gland. To demonstrate the superiority of trueFISP imaging, conventional MRI sequences using T1-weighted spin echo (T1-SE), T2-weighted turbo spin echo (T2-TSE) and fluid attenuated inversion recovery (FLAIR) were compared to those obtained with trueFISP. In a second step the volume and the spectrum of the microstructure (extent and morphology of cystic changes) of the pineal gland in children is presented for the first time. The question whether the high interindividual variation of pineal volume and microstructure found in adults [8,12,13], which is unique for an organ of the central nervous system) can also be found in children is also addressed.
Finally, it is unknown which morphological component (cystic or solid) determines the variability. Consequently, the 3D data were used to understand which morphological parts of the pineal gland contribute in detail to the high variability. As the underlying causes for the variability are not well understood, the relationship of gender, age and age-related factors with the volume and microstructure of the pineal gland was investigated.
Materials and Methods
Population and Magnetic Resonance Imaging Protocol
The institutional review board (IRB) gave a waiver for approval for this study. A total of 54 consecutive patients enrolled for cranial MRI between June 2007 and November 2009 were included in this retrospective study. Exclusion criteria were suspected pineal mass lesions as well as distortions of the pineal region from adjacent pathologies, intracranial mass lesions or artifacts prohibiting the evaluation of the pineal gland in any of the sequences.
The MRI scans were performed using two 1.5-T MR scanners (Siemens Sonata and Avanto, Siemens, Erlangen, Germany) and the MR sequences for all patients comprised transversal T1-SE (TR/TE 434/11), T2-TSE (TR/TE 3900/101) and FLAIR (TR/TI/TE 9000/2500/89; 5 mm slice thickness, 20% gap, field of view 205 ´ 230 mm, matrix 448 ´ 304 ), as well as trueFISP (TR/TE 7.1/3.5, field of view 178 ´ 220 mm, matrix 212 ´ 275, slice thickness 800 µm). The body height, weight and the diagnosis of patients were extracted from the medical records.
Evaluation
The datasets were anonymized for the analysis. All images were evaluated digitally using OsiriX software (www.osirix-viewer.com). Pineal cysts were categorized as atypical if they were not round to ovoid in shape or displayed atypical microstructural details (trabeculations and thickened rim more than 2 mm in size). The frequency of typical and atypical cysts was determined using trueFISP and standard sequences (1.5-T, T1-SE, T2-TSE, FLAIR). If it was not clear if the pineal gland contained cysts or not the rating was classified as indistinct. The volume of the pineal gland (PGV) was measured by manually defining the pineal borders on transversal reconstructed trueFISP images (800 µm isotropic voxel size). If cysts were detected in trueFISP the cyst volume was measured as previously described [8,9]. Pineal parenchyma volume (PPV) was defined as PGV−cyst volume. For further statistical analysis the PPV was analyzed separately in solid glands (PPVsolid) and cystic glands (PPVcystic).
To investigate the intrarater variability the volume assessment was repeated with a time gap of 3 weeks. To determine the interrater variability 10 datasets were assessed by a second reader unaware of the results of the first evaluation. If other pathological changes of the pineal gland were present they were additionally noted. The head circumference was measured on transversal T1-weighted images at the level of the basal ganglia.
Statistics
For statistical analysis SPSS 18.0 (Illinois, USA) was used. For the intrarater and interrater variability of the volume measurements the Pearson correlation coefficient (r) was determined. ANOVA analysis was used to detect disease-related differences concerning PGV, PPV, cyst volume and the number of cysts. As the hydrocephalus group constituted the largest subgroup it was tested separately against the non-hydrocephalus group for the same parameters using Student’s t-test. The Student’s t-test was also used to detect gender differences of PGV, PPV, and cyst volume. The Pearson correlation coefficient was computed for the relation of age, weight, height and head circumference to PPV, PPVsolid and cyst volume. For all tests a significance level of 0.05 was used. Descriptive values are given as mean ± standard deviation if not otherwise specified.
Results
Population
The study population comprised 54 individuals (5.4 ± 5.6 years, range 0–17 years, median 2.0 years, 44.4% females and 55.6% males). Diagnoses included hydrocephalus (55.6%), congenital malformation (13.0%), hemorrhage (7.4%), other abnormalities (11.1%) and exclusion of abnormalities (13.0%). Using ANOVA analysis no disease-related differences of PGV (p = 0.586), PPV (p = 0.594), cyst volume (p = 0.255) and the number of cysts (p = 0.452) were found.
There were no differences between the largest subgroup, hydrocephalus and the non-hydrocephalus subgroups regarding PGV (p = 0.721), PPV (p = 0.692), cyst volume (p = 0.671) and the number of cysts (p = 0.738).
Reliability of Volume Measurements
Concerning the intrarater variability, the mean of the first assessment was 94.02 ± 159.00 mm3 and of the second assessment 94.59 ± 159.21 mm3. The Pearson correlation coefficient was 1.00 (p < 0.05) and the mean difference between both readings was 2.63 ± 0.85 mm3.
Concerning the interrater variability there were no significant differences between the two readers (mean for reader 1: 164.42 ± 254.46 mm3, mean for reader 2: 163.93 ± 274.65 mm3, mean difference 16.58 mm3, p = 0.948). The correlation coefficient between both readers was 0.998 (p < 0.05).
Comparison of Imaging Techniques
The trueFISP imaging technique was superior in delineating pineal microstructure (Fig. 1). Figure 2 illustrates the spectrum of pineal volumes and cysts in trueFISP. The frequency of pineal cysts was highest in trueFISP (57.5%) in comparison to FLAIR (13.0%), T2-TSE (14.8%) and T1-SE (7.4%). The percentage of indistinct findings was lowest in trueFISP (1.9%) compared to FLAIR and T2-TSE (both 18.5%) and T1-SE (24.1%).
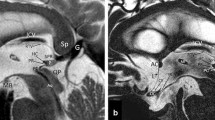
Microstructural details could only be distinguished in trueFISP with a frequency of atypical components among all cystic glands of 64.5% (thick rim 29.0%, trabeculation 48.4%, not round/ovoid: 38.8%). More than one cyst was detectable only in trueFISP (n = 5). In six cases the shape of a small pineal cyst was fusiform (Fig. 3a,b) and in two cases the inner cyst surface was cleft (Fig. 3c,d).
Spectrum of pineal findings. Magnified view of the pineal gland in trueFISP. The smallest pineal gland (0.25 mm3,a) was found in a 2-year-old boy with Chiari malformation, the largest solid gland (95 mm3,b) in a 13-year-old girl with severe cephalgia andc shows the largest cystic gland with a cyst volume of 422 mm3 in a 14-year-old boy with a prolongated seizure
Pineal cysts with extraordinary morphology. Magnified view of the pineal gland in trueFISP. Ina andb the pineal cyst of a 2-year-old macrocephalic boy shows a fusiform shape reminiscent of the pineal diverticulum present during the fetal period [23],c andd show an extremely cleft, multicystic gland in a 7-year-old girl with hydrocephalus
Pineal Volume and Gender
In both females and males PGV, PPV and cyst volume display high interindividual variation (Table 1). The median PGV, PPV and cyst volume were slightly higher in females than in males. Due to more extreme values and/or outliers the corresponding mean values were higher in males, although not statistically significant (Table 1). The values for PPVsolid were higher in females (mean and median) although not statistically significant.
Pineal Volume and Age
Table 2 shows the range for the different age groups (PGV, PPV, cyst volume, PPVsolid, and percentage of cystic glands).
The correlation between age and PGV/PPV (all glands/cystic glands/solid glands)/cyst volume is shown in Table 3. The analysis shows a correlation of moderate strength between age and the volume of the pineal gland as a whole (correlation coefficient 0.382, p = 0.004). A similar highly significant correlation of moderate strength was found for age and the cyst volume.
A separate analysis of solid and cystic glands show that PPVsolidwas more strongly positively correlated with age (r = 0.659, p = 0.001) than PPVcystic (r = 0.419, p = 0.019, not shown in Table 3).
To detect the influence of age-related factors on PGV the relation of body weight, body height and head circumference on the PGV was analyzed (Table 3). The PGV correlated better with body weight than with body height and head circumference. Cyst volumes revealed an interestingly high correlation with body weight which was stronger than with height, head circumference and age.
The correlation with age, height and head circumference was stronger for solid than for cystic glands and consequently likewise stronger for solid than for solid and cystic glands together (not shown in Table 3).
Taken together, the data show an increase of pineal volume with age (especially strong if cystic glands are excluded) and age-related body parameters.
Interindividual Variation for Quantification of Solid and Cystic Components
The mean and median values of the PGV, PPV, cyst volume and PPVsolid are shown in Table 2. As expected the interindividual variability of the PGV was high (SD 169% of the mean PGV, 94.3 ± 159.1 mm3). In solid glands the variability was substantial (SD 102% of the mean PPVsolid, 26.5 ± 27.2 mm3) but lower than in cystic glands (SD 150% of the mean PGVcystic, 144.6 ± 195 mm3, 134%, not shown in Table 2). Cysts, if present, showed an extreme variation of 210.8% of the mean (40.6 ± 85.6 mm3).
Discussion
The pineal gland is an endocrine gland located in the center of the brain. It secretes the hormone melatonin which regulates the day-night cycle (circadian rhythm) and is considered the most important transmitter in chronobiology. In 2007 disruption of the day-night cycle (chronodisruption) was classified as a probable human carcinogen (class 2A) by the International Agency for Research for Cancer (IARC).
In the last decades cross-sectional imaging methods (CT and MRI) have dramatically improved the visualization of the pineal gland but only sparse data still exist concerning the in vivo microstructure and volume of the pineal gland.
Adequate autopsy studies are not available for children. In adults reference values have been reported for pineal weight [3,12,14] as well as approximated volumes derived from the diameters (factor (depending on geometric model) ´ length ´ width ´ height). Because of shrinking artifacts and dehydration during the fixation process histologic measurements of the pineal microstructure and cysts cannot be directly transferred to in vivo MRI.
A number of studies have addressed the morphology of the pineal gland in conventional MRI sequences (T1-SE, T2-TSE; [7,15–19]). The pineal gland is usually isointense to grey matter in T1-weighted and T2-weighted imaging. The appearance of cysts is dependent on the protein content and therefore varies from isointense to hyperintense compared to cerebrospinal fluid (CSF) in T1-weighted imaging [15,18,19]. Because the pineal gland lacks a blood-brain barrier the pineal gland and the cyst wall usually show at least a partial enhancement [20]. In trueFISP the solid pineal gland is isointense to brain whereas cysts show CSF-like signal intensity [8,9].
This study uses high-resolution 3D MRI (trueFISP) for the imaging of the pediatric pineal gland for the first time. In this study the superiority of trueFISP in comparison to conventional imaging was documented using standard parameters. The degree of certainty regarding pineal cysts (lower limit of diameter 2 mm) was substantially better in trueFISP. The prevalence of pineal cysts was by far higher in trueFISP than in conventional sequences (57.5% compared to 7.4–14.8%) and the prevalence in children is higher than the prevalence in adults reported by Nolte et al. [8] in trueFISP (35%).
Atypical features of cysts (thick rim, trabeculation, not round/ovoid) were only detectaequivocal pineal findings as pineal contrastble in trueFISP (64.5% of all cysts). A study by Pastel et al. found atypical features in 60% of pineal cysts in children in a very small population (n = 5, [21]). The study by Nolte et al. in adults found a lower percentage of atypical features (41%, [8]). The question whether there is a decrease of cysts and of atypical features with age remains to be elucidated by follow-up studies.
Prior MRI studies based on conventional MRI tended to recommend a clinical follow-up of typical pineal cysts [22]. The relevance of atypical features in children however, has not been discussed. The high prevalence of atypical features in this study as well as in the study in adults by Nolte et al. [8] does not justify follow-up examinations based on atypical features only.
Concerning the origin of pineal cysts three theories are discussed [3,4,8,23–26]:
-
Embryological remnant of the pineal diverticulum: the pineal gland is a result of the fusion and proliferation of the walls of the pineal diverticulum. If the fusion is incomplete a cystic cavity remains which is lined by cells that may differentiate to glial or ependymal cells.
-
Residual from chronic ischemic injury: this theory is based on histological findings of glial-lined cysts containing a central glial plaque.
-
Residual from degeneration or necrosis of pinealocytes: in cases supporting this theory the central cavity does not contain glial or ependymal cells.
The results of this study add microstructural information to this discussion as six cases with a fusiform shape of the pineal cyst were found the morphological appearance of which resembles the fetal pineal cavity as presented by Cooper [23]. A cleft-like appearance was present in two cases resembling a central necrosis. It is tempting to interpret these finding as pineal diverticulum and central necrosis but as long as histological proof is missing these interpretations are speculative.
Besides microstructural analysis a direct volumetric analysis of the pediatric pineal gland was performed for the first time. This proved that volumetric assessment of the pediatric pineal gland is feasible and reliable as documented by high intrarater and interrater reliability. Consequently, the method is an appropriate tool for the clinical imaging of the pineal gland in children (especially for follow-up examinations).
The volumetric analysis is of special importance for the clinical follow-up of equivocal pineal findings as pineal contrast enhancement is of limited value (the pineal gland is one of the circumventricular organs that lack a blood-brain barrier.) The volumetric results reported can help radiologists to decide whether equivocal findings are within or outside the range of a clinical population.
A limitation of the study is that it is not based on a healthy population. For this reason ANOVA testing and additional t-tests were performed for the largest subgroup, the hydrocephalus patients and no indications of a confounding effect were found.
Putting aside this point, up to now there has been no true volumetric data available for the pineal gland in children and for obvious reasons it will be extremely difficult to gather MRI information of healthy young children in the future. Prior studies were also based on patients and not on healthy volunteers [7].
Concerning the range of the pineal volume, only sparse knowledge exists in adults and even less in children. The results of most of the respective studies can only be interpreted with caution. Most of the hitherto performed MRI studies used two-dimensional acquisition with an inadequately high slice thickness (4 mm or more) that allowed only an approximation of the pineal volume and did not allow sufficient differentiation of parenchymal and cystic components [7,27–31]. Such estimated volumes derived from measurements(length, width and height) in 2D imaging correlate only weakly with the true volumetric values [11]. The provisional nature of these studies is mirrored by the fact that the prevalence of pineal cysts in these studies (0.14–4.3%) is much lower than those reported after autopsy (33–40%, [2,12]). However, adequate MRI protocols are extremely important for the imaging of the pediatric brain [32] and especially of midbrain architecture [33].
The alternative to two-dimensional measurements is the direct volumetric assessment with 3D sequences (using a voxel size of 1 mm or less). State-of-the art isotropic 3D sequences, such as constructive interference with steady state (CISS) or magnetization prepared rapid gradient echo (MPRAGE) are the current standards for the volume assessment of other small regions of the central nervous system, such as the hippocampus [34] or the trigeminal nerve [35]. For the pineal gland such sequences have so far been applied only by Sun et al. [11] in a Chinese population in adults and not in children.
Pineal volumes in this study ranged from 0.3 mm3 to 1440.60 mm3 with a mean volume of 94.3 ± 159.1 mm3. In an MRI study of pediatric patients using 5 mm slice thickness, Sumida et al. [7] found a mean length of 6.1 ± 1.2 mm, a height of 3.7 ± 0.8 mm and a width of 4.8 ± 1.1 mm for the age group of 2–20 years (n = 223) with a calculated mean volume (0.5×length×height×width) of 56.6 ± 27.6 mm3. These calculated volumes are discrepant to the results of this study. It is suspected that differences in the population partial volume effects as well as suboptimal contrast of the chosen sequence (T1 spin echo) possibly resulted in a systematic underestimation of the pineal volume.
Summarizing the issue of the pineal reference range, this study for the first time reports directly measured volumes in a pediatric population. It is assumed that this approach gives the most exact volume measurement of the pineal gland reported so far in children. Especially in the context of the increasing number of incidental findings in human neuroimaging studies [36,37] the establishment of adequate reference values is important.
Morphologically, the trueFISP sequence facilitated a clear differentiation of solid and cystic parts of the pineal gland. An attempt was therefore made to answer the question whether it is the solid parenchyma and/or the cystic compartments which greatly vary. In this study a substantial but smaller part of the overall variation of the PGV is secondary to the solid compartments. The major part of the variation can be traced back to the cystic compartments. Nevertheless, the high variability of the solid glands is still unique for an organ of the central nervous system.
The etiology of the high interindividual variability of the pineal volume is not clear and no relation of the pineal volume to the underlying disease was found.
Analyzing only the solid glands a strong significant correlation with age (r = 0.659) could be found. In other words in solid glands about 43% (0.6592) of the variance can be explained by the influence of age but means that the major part of the variation is still not explained. These results are similar to the study by Sumida et al. [7] who found significantly smaller estimated diameters in children younger than 2 years than in children older than 2 years.
The analysis of age-related body parameters in this study showed similar correlations for body weight, height and head circumference. In the literature there are, however no studies in children regarding this question. In adults, an autoptic study by Golan et al. found no significant differences between pineal volume and body height or body weight in different age groups [13]. In contrast to these findings, the autoptic study by Rodin and Overall claimed a direct correlation of both pineal size and weight to age [14]. In a recent MRI study of healthy Chinese individuals between 20 and 30 years Sun et al. [11] used a T1-weighted 3D sequence and reported moderate correlations between pineal volume (after eliminating cysts larger than 2 mm) and body height (r = 0.322), body weight (r = 0.306) and head circumference (r = 0.306). In the pediatric population in this study the correlation of these body parameters to PPVsolid was approximately twofold higher. Apart from differences regarding the population the use of a strongly T2-weighted sequence (trueFISP) in this protocol might have played a role: strongly T2-weighted sequences achieved better contrast of the pineal gland against the surrounding cerebrospinal fluid. The superiority is reflected in a study by Nolte et al. [8] which reported a prevalence of pineal cysts detected in trueFISP imaging (35%) which equals that of autopsy results [2,12]. It is assumed that the superior contrast facilitates a more exact delineation of the PG and consequently results in more exact volume measurements.
The influence of gender was minimal in the study reported here and no significant differences could be detected regarding PGV, PPV, cyst volume and PPVsolid/cystic. Due to outliers in the male group the mean values of PGV, PPV, cyst volume and PPVcystic were higher in males but medians were higher in females whereas PPVsolid values were almost equal with a p-value of 0.553. This negligible influence of gender is in accordance with inconsistent findings in other (adult) studies: While Tapp and Huxley [4] found heavier glands in females than in males up to the age of 60 years, Sun et al. [11] reported significantly larger pineal volumes in younger healthy men than in women if glands with cysts of more than 2 mm were eliminated.
An interesting connection of the increasing PPV with age could be the fact that the pineal parenchyma volume seems to be correlated with increased melatonin secretion. Nolte et al. found a correlation between pineal parenchyma volume and melatonin secretion over 24 h, maximum melatonin levels and the slope of the melatonin secretion over 24 h in 15 healthy males [12]. The finding of this study that PPV increases with age in children could correspond to the developing endocrine function of the pineal gland during the first years of life [38]. Concerning this point it would be interesting to analyze if the melatonin levels in small children parallel the growth of the developing gland. However, as intraindividual studies in small children are not justifiable an animal model would be necessary to further investigate this issue.
Conclusions
Atypical cysts are a frequent finding in high-resolution imaging of the pediatric pineal gland and are not a sign of malignancy. Pineal volumetry is feasible using trueFISP imaging. The presented pineal volumes can be used as a comparison for the classification of equivocal MRI findings. It has been shown that the high interindividual variability of the pineal volume is mainly related to the cystic component and to a lesser degree to the solid component. Whereas gender effects on the PGV are negligible, pineal growth in children is dependant on age.
References
Hasegawa A, Mori W. Morphometry of the human pineal gland: relationship to the adrenal cortex. Acta Pathol Jpn. 1980;30(3):407–10.
Tapp E. The human pineal gland in malignancy. J Neural Transm. 1980;48(2):119–29.
Tapp E, Huxley M. The weight and degree of calcification of the pineal gland. J Pathol. 1971;105(1):31–9.
Tapp E, Huxley M. The histological appearance of the human pineal gland from puberty to old age. J Pathol. 1972;108(2):137–44.
Schmitz SA, Platzek I, Kunz D, Mahlberg R, Wolf KJ, Heidenreich JO. Computed tomography of the human pineal gland for study of the sleep-wake rhythm: reproducibility of a semi-quantitative approach. Acta Radiol. 2006;47(8):865–71.
Sener RN. The pineal gland: a comparative MR imaging study in children and adults with respect to normal anatomical variations and pineal cysts. Pediatr Radiol. 1995;25(4):245–8.
Sumida M, Barkovich AJ, Newton TH. Development of the pineal gland: measurement with MR. AJNR Am J Neuroradiol. 1996;17(2):233–6.
Nolte I, Brockmann MA, Gerigk L, Groden C, Scharf J. TrueFISP imaging of the pineal gland: more cysts and more abnormalities. Clin Neurol Neurosurg. 2010;112(3):204–8. doi:s0303-8467(09)00307-2 [pii] 10.1016/j.clineuro.2009.11.010.
Nölte I, Lütkhoff AT, Stuck BA, Lemmer B, Schredl M, Findeisen P, et al. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging. 2009;30(3):499–505. doi:10.1002/jmri.21872.
Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH, et al. High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol. 2007;28(9):1706–9.
Sun B, Wang D, Tang Y, Fan L, Lin X, Yu T, et al. The pineal volume: a three-dimensional volumetric study in healthy young adults using 3.0 T MR data. Int J Dev Neurosci. 2009;27(7):655–60. doi:s0736-5748(09)00117-8 [pii] 10.1016/j.ijdevneu.2009.08.002.
Hasegawa A, Ohtsubo K, Mori W. Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res. 1987;409(2):343–9.
Golan J, Torres K, Staskiewicz GJ, Opielak G, Maciejewski R. Morphometric parameters of the human pineal gland in relation to age, body weight and height. Folia Morphol (Warsz). 2002;61(2):111–3.
Rodin AE, Overall J. Statistical relationships of weight of the human pineal to age and malignancy. Cancer. 1967;20(8):1203–14.
Lee DH, Norman D, Newton TH. MR imaging of pineal cysts. J Comput Assist Tomogr. 1987;11(4):586–90.
Mandera M, Marcol W, Bierzynska-Macyszyn G, Kluczewska E. Pineal cysts in childhood. Childs Nerv Syst. 2003;19(10–11):750–5.
Schmidt F, Penka B, Trauner M, Reinsperger L, Ranner G, Ebner F, et al. Lack of pineal growth during childhood. J Clin Endocrinol Metab. 1995;80(4):1221–5.
Mamourian AC, Towfighi J. Pineal cysts: MR imaging. AJNR Am J Neuroradiol. 1986;7(6):1081–6.
Golzarian J, Baleriaux D, Bank WO, Matos C, Flament-Durand J. Pineal cyst: normal or pathological? Neuroradiology. 1993;35(4):251–3.
Inoue Y, Saiwai S, Miyamoto T, Katsuyama J. Enhanced high-resolution sagittal MRI of normal pineal glands. J Comput Assist Tomogr. 1994;18(2):182–6.
Pastel DA, Mamourian AC, Duhaime AC. Internal structure in pineal cysts on high-resolution magnetic resonance imaging: not a sign of malignancy. J Neurosurg Pediatr. 2009;4(1):81–4. doi:10.3171/2008.5.17681 [pii] 10.3171/2008.5.17681.
Barboriak DP, Lee L, Provenzale JM. Serial MR imaging of pineal cysts: implications for natural history and follow-up. AJR Am J Roentgenol. 2001;176(3):737–43.
Cooper E. The human pineal gland and pineal cysts. J Anatom. 1932;67:28–46.
Engel U, Gottschalk S, Niehaus L, Lehmann R, May C, Vogel S et al. Cystic lesions of the pineal region—MRI and pathology. Neuroradiology. 2000;42(6):399–402.
Hajdu SI, Porro RS, Lieberman PH, Foote FW Jr. Degeneration of the pineal gland of patients with cancer. Cancer. 1972;29(3):706–9.
Megyeri L. Cystic changes in the pineal body. Frankf Z Pathol. 1960;70:699–704.
Caldas JG, Doyon D, Lederman H, Carlier R. Magnetic resonance study of the pineal region. Normal pineal gland and simple cysts. Arq Neuropsiquiatr. 1998;56(2):237–44.
Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282(1):36–9.
Lum GB, Williams JP, Machen BC, Akkaraju V. Benign cystic pineal lesions by magnetic resonance imaging. J Comput Tomogr. 1987;11(3):228–35.
Mamourian A, Towfighi J. MR of pineal cysts. AJNR Am J Neuroradiol. 1994;15(9):1796–7.
Petitcolin V, Garcier JM, Mohammedi R, Ravel A, Mofid R, Viallet JF, et al. Prevalence and morphology of pineal cysts discovered at pituitary MRI: review of 1844 examinations. J Radiol. 2002;83(2 Pt 1):141–5.
Warmuth-Metz M, Bison B, Leykamm S. Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol. 2009;19(4):263–73. doi:1007/s00062-009-9029-5.
Hattingen E, Blasel S, Nichtweiss M, Zanella FE, Weidauer S. MR imaging of midbrain pathologies. Clin Neuroradiol. 2010;20(2):81–97. doi:1007/s00062-010-0009-6.
Briellmann RS, Syngeniotis A, Jackson GD. Comparison of hippocampal volumetry at 1.5 T and at 3 T. Epilepsia. 2001;42(8):1021–4. doi:epi02201 [pii].
Horinek D, Brezova V, Nimsky C, Belsan T, Martinkovic L, Masopust V, et al. The MRI volumetry of the posterior fossa and its substructures in trigeminal neuralgia: a validated study. Acta Neurochir (Wien). 2009;151(6):669–75.
Hentschel F, von Kummer R. Response of the German Society of Neuroradiology to the guideline: “Ethically Appropriate Reaction to Incidental Imaging Findings in Brain Research”, suggested by Thomas Heinemann, Institut fur Wissenschaft und Ethik, and Christian Hoppe, Klinik fur Epileptologie, Universitat Bonn, Germany, on January 9, 2009. Klin Neuroradiol. 2009;19(2):108–10.
Heinemann T, Hoppe C, Weber B, Elger CE. Ethically appropriate handling of incidental findings in human neuroimaging research: letter to the guest editorial of Frank Hentschel and Rudiger von Kummer [2]. Klin Neuroradiol. 2009;19(3):242–3; author reply 4.
Waldhauser F, Kovacs J, Reiter E. Age-related changes in melatonin levels in humans and its potential consequences for sleep disorders. Exp Gerontol. 1998;33(7–8):759–72.
Conflict of Interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bumb, J., Brockmann, M., Groden, C. et al. TrueFISP of the Pediatric Pineal Gland. Clin Neuroradiol 22, 69–77 (2012). https://doi.org/10.1007/s00062-011-0110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-011-0110-5