Abstract
Background
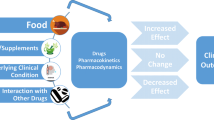
The new oral anticoagulants (NOAC) dabigatran etexilate, rivaroxaban, and apixaban show similar efficacy for stroke prevention in patients with atrial fibrillation (AF) as the vitamin K antagonist warfarin. Absorption of NOACs is dependent on the intestinal P-glycoprotein (P-gp) system and P-gp activity is modulated by a variety of drugs and food components.
Objective
The aim of this review is to give an overview of P-gp-associated drug–drug and drug–food interactions with NOACs in AF patients.
Methods
A literature search was carried out by screening MEDLINE for the terms dabigatran, rivaroxaban, apixaban, P-glycoprotein, and atrial fibrillation from 1998 to 2013. Randomized clinical trials, longitudinal studies, case series, and case reports were included.
Results
Concomitant medication with proton pump inhibitors, amiodarone, clarithromycin, and verapamil increased bioavailability whereas rifampicin decreased the bioavailability of dabigatran. Coadministration of erythromycin, clarithromycin, fluconazole, ketoconazole, and ritonavir increased rivaroxaban plasma concentrations. No data were found on apixaban and P-gp-modulating drugs or on NOACs and food components modulating P-gp. The clinical relevance of interactions between NOACs and P-gp-modulating drugs or food components is largely unknown as bleeding complications under NOACs and P-gp-inhibiting drugs are mainly reported from patients with concomitant renal failure.
Conclusion
There is an urgent need to investigate the role of P-gp-modulating substances as potential sources of drug–drug and drug–food interactions. A thorough analysis of the data accumulated in the three large NOAC trials regarding the role of P-gp-modulating drugs in bleeding and embolic events is desirable. Pharmacological studies should investigate the influence of P-gp-modulating drugs and food on NOAC plasma concentrations and coagulation parameters. When prescribing NOACs, patients should be informed about the potential interactions with drugs and herbal drugs. Patients who develop bleeding or embolic events under treatment with NOACs should be investigated for comedications as well as for over-the-counter drugs and dietary habits. In post-marketing surveillance of NOACs, the association with drug or food intake with complications, bleeding, and embolic events should be registered.
Zusammenfassung
Hintergrund
Die neuen oralen Antikoagulanzien (NOAC) Dabigatranetexilat, Rivaroxaban und Apixaban haben eine ähnliche Wirksamkeit zur Schlaganfallprävention bei Vorhofflimmern (AF) wie der Vitamin-K-Antagonist Warfarin. Die NOAC-Absorption ist abhängig vom P-gp (P-Glykoprotein)-System im Darm, das durch eine Vielzahl von Medikamenten und Nahrungsmittelkomponenten moduliert wird.
Ziel
Ziel der Übersichtsarbeit ist es, einen Überblick über die P-gp-assoziierten Arznei- und Nahrungsmittelwechselwirkungen mit NOAC bei AF-Patienten zu geben.
Methoden
Eine Literaturrecherche erfolgte durch systematisches Screenen in MEDLINE nach Veröffentlichungen zwischen 1998 und 2013 mit den Stichworten Dabigatran, Rivaroxaban, Apixaban, P-Glykoprotein und Vorhofflimmern. Randomisierte klinische Studien, Längsschnittstudien, Fallserien und Kasuistiken wurden eingeschlossen.
Ergebnisse
Die Bioverfügbarkeit von Dabigatran wird durch eine Begleitmedikation mit Protonenpumpenhemmern, Amiodaron, Clarithromycin und Verapamil erhöht, und durch Rifampicin verringert. Die gleichzeitige Gabe von Erythromycin, Clarithromycin, Fluconazol, Ketoconazol und Ritonavir erhöht die Plasmakonzentration von Rivaroxaban. Es wurden weder Daten zu Apixaban und P-gp-modulierenden Medikamenten noch zum Einfluss von P-gp-Modulation durch Nahrungsmittel auf die Bioverfügbarkeit von NOAC gefunden. Die klinische Relevanz der Wechselwirkungen zwischen NOAC und P-gp-modulierenden Medikamenten oder Nahrungsmitteln ist weitgehend unbekannt, da Blutungskomplikationen unter NOAC und einer Komedikation mit P-gp-hemmenden Medikamenten hauptsächlich bei niereninsuffizienten Patienten beschrieben wurden.
Schlussfolgerungen
Die Rolle von P-gp-modulierenden Substanzen als potenzielle Mediatoren von Arzneimittel- und Nahrungsmittelwechselwirkungen zu untersuchen, ist dringend notwendig. Eine gründliche Analyse der in den 3 großen NOAC-Studien gesammelten Daten über die Rolle von P-gp-modulierenden Medikamenten in Hinblick auf Blutungen und ischämische Ereignisse ist wünschenswert. Pharmakologische Studien sollten den Einfluss P-gp-modulierender Substanzen auf die Plasmakonzentration von NOAC und die Gerinnungsparameter untersuchen. Bei der Verordnung von NOAC sollten Patienten auf potenzielle Interaktionen mit Medikamenten und Kräutern aufmerksam gemacht werden. Patienten, die unter NOAC Blutungen oder embolische Ereignisse erleiden, sollten nicht nur über ihre Komedikation, sondern auch nach der Einnahme nichtverschreibungspflichtiger Substanzen sowie nach ihren Ernährungsgewohnheiten befragt werden. Post-Marketing-Analysen von NOAC sollten Assoziationen zwischen Medikamenten- bzw. Nahrungsmittelaufnahme und Komplikationen, wie Blutungen oder Embolien, registrieren und untersuchen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
New oral anticoagulants (NOAC), also termed direct oral anticoagulants (DOAC), such as the thrombin inhibitor dabigatran etexilate or the factor Xa inhibitors rivaroxaban and apixaban show similar efficacy for stroke prevention in patients with atrial fibrillation as the vitamin K antagonist (VKA) warfarin [1, 2, 3]. One of the advantages of the NOACs, compared with VKAs, is said to be the lack of the necessity for laboratory monitoring and its lower rate of drug–drug and drug–food interactions; however, NOAC absorption is dependent on the intestinal P-glycoprotein system [4, 5, 6]. The aim of this review is to give an overview of P-glycoprotein-associated drug–drug and drug–food interactions with special reference to the data on the NOACs dabigatran, rivaroxaban, and apixaban in patients with atrial fibrillation.
Search strategy and selection criteria
A literature search was carried out by systematically screening MEDLINE for publications with the keywords dabigatran, rivaroxaban, apixaban, P-glycoprotein, and atrial fibrillation from 1998 to 2013. Reference lists and older references generated from initial papers were also considered. Randomized clinical trials, longitudinal studies, case series, and case reports were included.
Function of P-glycoprotein
P-glycoprotein 1 (P-gp) also known as multidrug-resistance protein 1 (MDR1), ATP-binding cassette sub-family B member 1 (ABCB1), or cluster of differentiation 243 (CD243) is a glycoprotein that in humans is encoded by the ABCB1 gene. It is a well-characterized ABC transporter that transports a wide variety of substrates across extracellular and intracellular membranes [7] and is a 160-kDa ATP-dependent drug efflux pump for xenobiotic compounds with broad substrate specificity. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. P-gp is expressed in the intestinal epithelium, hepatocytes, renal proximal tubular cells, adrenal glands, and capillary endothelial cells comprising the blood–brain and blood–testis barrier [8]. P-gp also functions as a transporter in the blood–brain barrier and is expressed in many cell types in the brain including the choroid plexus, astrocytes, microglia, and capillary endothelium. The most important physiological effect associated with P-gp expression appears to be in the luminal surface of capillary endothelial cells where the protein prevents the passage of drugs and toxins across the capillary membrane into the brain [9].
Substrates of P-gp
Substrates of P-gp comprise anticancer drugs, human immunodeficiency virus (HIV) protease inhibitors, H2-receptor antagonists, anti-gout agents, antidiarrheal agents, antiemetics, calcium channel blockers, cardiac glycosides, immunosuppressive agents, glucocorticoids, pesticides, anthelmintics, antibiotics, antidepressants, antithrombotic drugs, NOACs, and diagnostic dyes (Tab. 1, [10, 11, 12, 13, 14]).
Modulators of P-gp activity
The activity of P-gp is modulated by several drugs, herbs, and food components. Generally, P-gp inhibitors increase the serum concentration of a substrate, whereas P-gp inducers decrease the serum concentration of a substrate. As shown in Tab. 1, several drugs are substrates as well as modulators of P-gp activity [14, 15].
Studies on pharmacokinetics and pharmacodynamics of drugs affecting P-gp are impeded by the fact that there is considerable overlap in drug specificity for P-gp and CYP3A. Furthermore, many of the data are derived from in vitro studies or experiments with P-gp knockout mice, thus the clinical relevance of the drug–drug interaction in humans is not completely clarified [15]. Several drugs have been identified as strong P-gp inhibitors as listed in Tab. 1 [14].
Food components, dietary supplements, and herbal drugs, such as phenolic acids and analogues, flavonoids, tannins, stilbenes, curcuminoids, coumarins, lignans, and quinones affect the P-gp system (Tab. 2, [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31]) These food compounds are frequently associated with beneficial health effects and are components of many over-the-counter drugs. Similarly with drug–drug interactions, many of these P-gp modulating effects have only been investigated in vitro or in animal models and the clinical relevance in humans is widely unknown.
Additionally, P-gp displays considerable genetic heterogeneity. To date, more than 1,000 single-nucleotide polymorphisms for P-gp have been identified and the impact of these polymorphisms on drug metabolism is under investigation [32].
P-gp and the blood–brain barrier
Drugs modulating P-gp do not only influence the serum concentration of a drug but also the entry of substrates into several organs. That increased drug delivery to the brain by a P-gp inhibiting drug can be clinically relevant has been shown for the first time in humans for the antidiarrheal drug loperamide, which does not produce central nervous system effects at normal doses. After administration of quinidine, a known P-gp inhibitor, respiratory depression occurred that was not explained by increased plasma loperamide concentrations [33]. However, not all substances identified as a substrate of intestinal P-gp are also a substrate of brain P-gp, as has been shown with methadone [34]. Moreover, there are indications of differences between mice and humans in substrate specificity of P-gp, leading to differential P-gp-mediated transport efficiency [35].
Clinical consequences of P-gp modulation
Modulation of P-gp activity in order to improve the delivery of drugs to the target organs is of clinical interest in oncology and neurology, such as in epilepsy [36]. However, P-gp modulation can also become relevant as an unwanted side effect due to drug–drug or food–drug interactions [15].
Several drugs used in patients with cardiovascular disorders including atrial fibrillation are either substrates or modulators of P-gp activity (Tab. 1). A survey in hospitalized patients with atrial fibrillation showed that one or more P-gp-modulating drugs were prescribed to 42 % of the patients [37].
P-gp and oral anticoagulant drugs
The NOACs dabigatran etexilate, rivaroxaban, and apixaban are P-gp substrates, whereas the pharmacokinetics and pharmacodynamics of vitamin K antagonists do not seem to be affected by the P-gp system [38]. The clinical impact of drug–drug interactions between NOACs and P-gp-modulating substances is at present uncertain, mainly due to the lack of data. Most studies investigating drug–drug interactions of NOACs with P-gp-modulating drugs are from laboratories of the NOAC manufacturers [4, 5, 6, 39, 40, 41] and only few pharmacological studies from independent sources [11, 12]. No data about the relevance of food–drug interactions between NOACs and P-gp-modulating food components, herbal drugs, or dietary supplements have been published to date.
Bleeding and embolic complications that occurred during the three large clinical trials investigating NOACs in atrial fibrillation patients have not been analyzed with respect to comedications with P-gp-modulating drugs [1, 2, 3]. There are several case reports of bleeding complications, mainly under the NOAC dabigatran in patients with renal failure, which listed comedications of P-gp-inhibiting drugs (Tab. 2, [42]).
Dabigatran etexilate and P-gp
Dabigatran etexilate is an oral reversible direct thrombin inhibitor that is rapidly absorbed and converted to its active form, dabigatran. Dabigatran has been shown to be a potent, competitive, and reversible inhibitor of thrombin, inhibiting both activity and generation of thrombin. The precursor drug dabigatran etexilate, but not dabigatran, is a P-gp substrate and the bioavailability of dabigatran may be altered by P-gp inhibitors or inducers.
Three studies performed by the manufacturer of dabigatran investigated the effect of P-gp-modulating drugs on the bioavailability of dabigatran: Concomitant medication with proton pump inhibitors, amiodarone and verapamil increased the bioavailability of dabigatran [40]. A further study confirmed that verapamil increased dabigatran bioavailability [4]. A decrease in the bioavailability of dabigatran was found with concomitant intake of the P-gp inducer rifampicin [39]. In all these studies the effects of the changes in dabigatran bioavailability on the coagulation system were not investigated. A further independent study found that the P-gp inhibitor clarithromycin increased dabigatran bioavailability from 6.5 % to 10.1 % which resulted in a proportional prolongation of coagulation as measured by the ecarin clotting time [12]. As dabigatran is mainly excreted by the kidneys, concomitant renal insufficiency might further increase the bioavailability of dabigatran when given together with P-gp inhibitors. This assumption is substantiated by the frequent renal impairment in patients with bleeding complications and P-gp-inhibiting drugs [42].
No data are available about a potential influence of P-gp on dabigatran entry into the brain. Hypothetically, changes in the brain penetration of dabigatran by drugs affecting P-gp may not be expected as it is assumed that only the precursor drug dabigatran etexilate and not dabigatran is a P-gp substrate.
Rivaroxaban and P-gp
Rivaroxaban is an oral direct factor Xa inhibitor. Regarding the effects of drugs affecting P-gp on the pharmacokinetics of rivaroxaban, the manufacturer performed a study testing various P-gp-inhibiting drugs in healthy volunteers. In that study coadministration of erythromycin, clarithromycin, and fluconazole led to a 34–54 % increase in rivaroxaban plasma concentrations, whereas coadministration of ketoconazole and ritonavir led to an increase of 153–158 % [41]. The influence of these increases on blood coagulation has not been investigated.
The influence of P-gp on rivaroxaban entry into the brain has been investigated by two studies: An animal experiment performed by the manufacturer in wild-type and P-gp double-knockout mice demonstrated a slight increase in brain concentrations in P-gp double-knockout mice and decreased excretion into the gastrointestinal tract compared with wild-type mice [5]. Another independent in vitro and in vivo study using knockout mice showed that rivaroxaban is a shared substrate of P-gp and a further efflux transporter, breast cancer resistance protein (BCRP), and that these transporter proteins function synergistically. They appear to be particularly relevant for limiting rivaroxaban central nervous system entry [11]. Whether P-gp inhibitors increase the risk for cerebral bleeding by affecting the blood–brain barrier in rivaroxaban-treated patients has to be established. So far, there has only been one case reported with cerebral bleeding occurring under P-gp-inhibiting therapy [43].
Apixaban and P-gp
Apixaban is an oral direct factor Xa inhibitor. Few data are available about the influence of P-gp on apixaban. Apixaban has been reported as a substrate for P-gp [44]. Animal experiments in rats found a low brain concentration of apixaban suggesting that apixaban has limited penetration through the blood–brain barrier. Again, the efflux transporters P-gp and BCRP are constituents of the blood–brain barrier and are assumed to prevent or reduce drug entry [6].
Conclusion
This review shows that knowledge regarding the relevance of P-gp-modulating substances for drug–drug and drug–food interactions with NOACs is very limited. However, NOACs are already widely recommended and approved in many countries for stroke prevention in patients with atrial fibrillation or primary or secondary prevention of venous thromboembolism [45]. In view of the limited knowledge about drug–drug and drug–food interactions between NOACs and P-gp-modulating substances, there is an urgent need to investigate the role of P-gp-modulating substances as potential sources for drug–drug and drug–food interactions. We suggest a thorough analysis of the data accumulated in the large NOAC trials regarding the role of P-gp-modulating drugs in bleeding and embolic events, especially cerebral bleeding. Furthermore, pharmacological studies should be carried out to investigate the influence of P-gp-modulating drugs and food on NOAC plasma concentrations and coagulation parameters. When prescribing NOACs, patients should be informed about the potential interactions with drugs and herbal drugs. Patients who develop bleeding or embolic events under treatment with NOACs should be investigated with respect to comedications as well as over-the-counter drugs and dietary habits. In post-marketing surveillance of NOACs, the association with drugs or food intake with complications, bleeding, and embolic events should be registered.
References
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Patel MR, Mahaffey KW, Garg J, Pan G et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Granger CB, Alexander JH, McMurray JJ, Lopes RD et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 65:981–992
Härtter S, Sennewald R, Nehmiz G, Reilly P (2013) Oral bioavailability of dabigatran etexilate (Pradaxa(®)) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol 75:1053–1062
Gnoth MJ, Buetehorn U, Muenster U, Schwarz T et al (2011) In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther 338:372–380
Wang L, He K, Maxwell B, Grossmann SJ et al (2011) Tissue distribution and elimination of [14C]apixaban in rats. Drug Metab Dispos 39:256–264
Aller SG, Yu J, Ward A, Weng Y et al (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722
Leslie EM, Deeley RG, Cole SP (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204:216–237
Shen S, Zhang W (2010) ABC transporters and drug efflux at the blood-brain barrier. Rev Neurosci 21:29–53
Schinkel AH, Jonker JW (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29
Gong IY, Mansell SE, Kim RB (2013) Absence of both MDR1 (ABCB1) and breast cancer resistance protein (ABCG2) transporters significantly alters rivaroxaban disposition and central nervous system entry. Basic Clin Pharmacol Toxicol 112:164–170
Delavenne X, Ollier E, Basset T, Bertoletti L et al (2013) A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran: application with clarithromycin. Br J Clin Pharmacol 78:107–113
Taubert D, Beckerath N von, Grimberg G, Lazar A et al (2006) Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther 80:486–501
Wessler JD, Grip LT, Mendell J, Giugliano RP (2013) The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol 61:2495–2502
Marchetti S, Mazzanti R, Beijnen JH, Schellens JH (2007) Concise review: clinical relevance of drug drug and herb drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein). Oncologist 12:927–941
Okura T, Ibe M, Umegaki K et al (2010) Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol Pharm Bull 33:255–259
Jin MJ, Han HK (2010) Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J Food Sci 75:H93–H96
El-Readi MZ, Hamdan D, Farrag N et al (2010) Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol 626:139–145
Ahmed IS, Hassan MA, Kondo T (2013) Effect of lyophilized grapefruit juice on P-glycoprotein-mediated drug transport in-vitro and in-vivo. Drug Dev Ind Pharm (Epub ahead of print)
Fan L, Tao GY, Wang G, Chen Y et al (2009) Effects of Ginkgo biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann Pharmacother 43:944–949
Kumar KK, Priyanka L, Gnananath K et al (2014) Pharmacokinetic drug interactions between apigenin, rutin and paclitaxel mediated by P-glycoprotein in rats. Eur J Drug Metab Pharmacokinet (Epub ahead of print)
Angelini A, Di Ilio C, Castellani ML et al (2010) Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin-resistant sarcoma cells (MES-SA/DX-5): implications for natural sedatives as chemosensitizing agents in cancer therapy. J Biol Regul Homeost Agents 24:197–205
Chula S, Hang L, Yinying B et al (2012) The effects of notoginsenoside R1 on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol 142:136–143
Colombo D, Lunardon L, Bellia G (2014) Cyclosporine and herbal supplement interactions. J Toxicol (Epub 2014 Jan 12)
Bogacz A, Mikołajczak PŁ, Mikołajczak PŁ, Rakowska-Mrozikiewicz B et al (2014) The influence of soybean extract on the expression level of selected drug transporters, transcription factors and cytochrome P450 genes encoding phase I drug-metabolizing enzymes. Ginekol Pol 85:348–353
Zhai XJ, Shi F, Chen F, Lu YN (2013) Capsaicin pretreatment increased the bioavailability of cyclosporin in rats: involvement of P-glycoprotein and CYP 3A inhibition. Food Chem Toxicol 62:323–328
Si M, Zhao J, Li X et al (2013) Reversion effects of curcumin on multidrug resistance of MNNG/HOS human osteosarcoma cells in vitro and in vivo through regulation of P-glycoprotein. Chin Med J 126:4116–4123
Chieli E, Romiti N, Rodeiro I, Garrido G (2010) In vitro modulation of ABCB1/P-glycoprotein expression by polyphenols from Mangifera indica. Chem Biol Interact 186:287–294
Gao LN, Zhang Y, Cui YL, Yan K (2014) Evaluation of genipin on human cytochrome P450 isoenzymes and P-glycoprotein in vitro. Fitoterapia 98:130–136
Yu CP, Hsieh YW, Lin SP et al (2014) Potential modulation on P-glycoprotein and CYP3A by soymilk and miso: in vivo and ex-vivo studies. Food Chem 149:25–30
Schiffman SS, Rother KI (2013) Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev 16:399–451
Ieiri I (2012) Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab Pharmacokinet 27:85–105
Sadeque AJ, Wandel C, He H, Shah S et al (2000) Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther 68:231–237
Kharasch ED, Hoffer C, Whittington D (2004) The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol 57:600–610
Baltes S, Gastens AM, Fedrowitz M, Potschka H et al (2007) Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology 52:333–346
Zhang C, Kwan P, Zuo Z, Kwan P et al (2012) The transport of antiepileptic drugs by P-glycoprotein. Adv Drug Deliv Rev 64:930–942
Jungbauer L, Dobias C, Stöllberger C, Weidinger F (2010) The frequency of prescription of P-glycoprotein affecting drugs in atrial fibrillation. J Thromb Haemost 8:2069–2070
Wadelius M, Sörlin K, Wallerman O, Karlsson J et al (2004) Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. Pharmacogenomics J 4:40–48
Härtter S, Koenen-Bergmann M, Sharma A, Nehmiz G et al (2012) Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol 74:490–500
Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA et al (2011) Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 9:2168–2175
Mueck W, Kubitza D, Becka M (2013) Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 76:455–466
Pfeilschifter W, Luger S, Brunkhorst R et al (2013) The gap between trial data and clinical practice—an analysis of case reports on bleeding complications occurring under dabigatran and rivaroxaban anticoagulation. Cerebrovasc Dis 36:115–119
Stöllberger C, Zuntner G, Bastovansky A, Finsterer J (2013) Cerebral haemorrhage under rivaroxaban. Int J Cardiol 167:e179–e181
Zhang D, He K, Herbst JJ, Kolb J et al (2013) Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos 41:827–835
Beyer-Westendorf J, Ageno W (2014) Benefit-risk profile of non-vitamin K antagonist oral anticoagulants in the management of venous thromboembolism. Thromb Haemost 113 (Epub ahead of print)
Compliance with ethical guidelines
Conflict of interest. C. Stöllberger and J. Finsterer state that they have no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stöllberger, C., Finsterer, J. Relevance of P-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz 40 (Suppl 2), 140–145 (2015). https://doi.org/10.1007/s00059-014-4188-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-014-4188-9