Abstract
Background
There is strong evidence suggesting an association between the peroxisome-activated receptor γ (PPARγ) gene and multimetabolic disorders. The association of PPARγ genetic variants with essential hypertension (EH) has not yet been investigated. The aim of this study was to investigate the association between the PPARγ gene (C681G and intron CT) and EH, examining the polymorphism and haplotype in a Han Chinese population.
Methods
Participants were recruited within the framework of the PMMJS cohort population survey in an urban community of Jiangsu Province, China. Two single-nucleotide polymorphisms (SNPs) previously reported to be associated with multimetabolic disorders and the reasonable coverage of the PPARγ gene region were analyzed with TaqMan SNP genotyping assays.
Results
C681G and intron CT were significantly associated with an increased risk of EH both in the codominant model and the dominant model after adjusting for potential nongenetic risk factors. Analysis of the haplotype association revealed that the risk of EH was significantly increased among individuals carrying the GC (odds ratio, 95 % CI: 1.60, 1.21–2.11), CT (1.45, 1.03–2.11), and GT haplotypes (1.95, 1.17–3.23) compared with those carrying the CC haplotype.
Conclusion
The polymorphisms of C681G and intron CT were significantly associated with the risk of EH, and the GC, CT, and GT haplotypes established by C681G and intron CT are likely to be genetic markers of EH in the Han Chinese population.
Zusammenfassung
Hintergrund
Es gibt deutliche Hinweise auf einen Zusammenhang zwischen dem PPARγ-Gen („peroxisome activated receptor γ“) und multimetabolischen Störungen. Der Zusammenhang genetischer PPARγ-Varianten mit essenzieller Hypertonie ist bisher nicht untersucht worden. Ziel der vorliegenden Studie war es, den Zusammenhang zwischen dem PPARγ-Gen (C681G und Intron-CT) und essenzieller Hypertonie unter Berücksichtigung von Polymorphismus und Haplotyp in einer Population von Han-Chinesen zu untersuchen.
Methoden
Die Teilnehmer wurden im Rahmen der Populationserhebung anhand der Kohorte zur“Prevention of metabolic syndrome (MS) and Multi-metabolic Disorders in Jiangsu Province of China Study” (PMMJS) in der Stadtbevölkerung der Provinz Jiangsu, China, rekrutiert. Zwei Einzelnukleotidpolymorphismen (SNPs), die laut früheren Arbeiten mit multimetabolischen Störungen einhergehen, und – soweit sinnvoll – die PPARγ-Gen-Region wurden mit TaqMan-SNP-Genotyping-Assays analysiert.
Ergebnisse
Sowohl C681G als auch Intron-CT gingen in signifikanter Weise mit einem erhöhten Risiko für essenzielle Hypertonie im kodominanten Modell und im dominanten Modell nach Berücksichtigung des potenziellen nichtgenetischen Risikofaktors einher. Die Haplotypassoziationsanalyse ergab, dass das Risiko für eine essenzielle Hypertonie bei Trägern des Haplotyps G-C (Odds Ratio: 1,60; 95 %-KI: 1,21–2,11), C-T (1,45; 1,03–2,11) und G-T (1,95; 1,17–3,23) gegenüber Trägern des Haplotyps C-C signifikant erhöht war.
Schlussfolgerungen
Die Polymorphismen von C681G und Intron-CT waren signifikant mit dem Risiko einer essenziellen Hypertonie assoziiert, und die Haplotypen G-C, C-T und G-T, die sich aus C681G und Intron-CT ableiteten, stellten wahrscheinlich die genetischen Marker der essenziellen Hypertonie in einer Population von Han-Chinesen dar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Essential hypertension (EH) has been attributed to both genetic determinants and environmental factors. Despite its high prevalence, the pathogenesis of hypertension is not clearly understood. Peroxisome-activated receptor γ (PPARγ), a ligand-activated transcription factor belonging to the nuclear receptor superfamily, was shown to be widely present in endothelial cells and vascular smooth muscle cells, where its activation exerts antiinflammatory and antiproliferative effects [1]. Studies have shown that PPARγ activator (TZDs) treatment was associated with improvement of hypertension, i.e., prevention of vascular remodeling, improvement of endothelial function and carotid intima media thickness, downregulation of inflammatory markers, as well as reduction of the rates of microalbuminuria [2, 3, 4]. Owing to these beneficial effects of PPARγ on blood pressure regulation, experts proposed that the use of PPARγ activators maybe an additional approach to cardiovascular protection associated with antihypertensive treatment [5].
Previous research showed that basic variations in intron sequences might change the structure and function of exon-coding protein molecules by leading to different splicing sites [6]. rs10865710 (C681G) and rs4684847 (intron CT) have been located in one haplotype block with a strong pairwise linkage disequilibrium in the Asian Biotechnology Information SNP database (JPT and CHB) of the International HapMap Project. C681G belongs to the A/B function area of PPARγ located upstream of exon A2 of PPARγ3. Several studies have shown that the polymorphism of C681G could participate in the regulation of lipid metabolism, in obesity, and in proangiotensin of vascular smooth muscle cells [2, 7, 8]. Intron CT located in the intron 7 area of PPARγ was reported to be significantly associated with body weight and blood pressure [9]. Therefore, we speculated that the polymorphism of C681G and intron CT may play an important role in the development of EH. In this study we aimed to investigate the association between the PPARγ gene (C681G and intron CT) and EH focusing on its polymorphism and haplotype in a Han Chinese population.
Methods
Subjects
All subjects were recruited from The Prevention of Metabolic Syndrome (MS) and Multi-metabolic Disorders in Jiangsu Province of China Study (PMMJS), which is an ongoing prospective cohort study in the Jiangsu Province of China. Details on the design of this study have been described elsewhere [10]. For this study, we excluded participants with diabetes, cardiovascular disease (CVD), a body mass index less than 18.5 kg/m2, and secondary hypertension. Finally, we randomly sampled 820 subjects (270 males, 550 females) after fully taking age and geographical region into account. All subjects were of Han Chinese origin and to our knowledge no kinship existed among the subjects. Informed consent was obtained from all participants and the study was approved by the ethics committee of Soochow University. The blood samples for PPARs polymorphism detection, the clinical and biochemical parameters, as well as the demographic and environmental risk factors in this study were all derived from PMMJS. These polymorphisms have been described in detail previously [11]. Above all, the subjects selected were similar to those who were not selected in terms of the extracted indicators.
Blood pressure (BP) was measured using a standard mercury sphygmomanometer with subjects in the sitting position after resting for at least 15 min. BP was re-measured by trained staff, taking three measurements, the average of which was recorded as the subjects’ BP. Hypertensive subjects were defined as having a systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg, or as taking antihypertensive therapy [12]. Normotensive (NT) subjects were those with both SBP and DBP below 140 and 90 mmHg, respectively.
Covariate measurement
Information on demographics, main disease history, family history of hypertension, obesity, smoking status and intensity, alcohol consumption, and use of medications was collected via a structured questionnaire. Anthropometric measurements, including body weight, height, and waist circumference (WC), were performed by trained staff. Blood samples were obtained after a minimum 8-h fast to measure fasting plasma glucose (FPG), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels, all of which were assessed enzymatically on an automatic biochemistry analyzer (Hitachi Inc., Tokyo, Japan) using commercial reagents. All analyses were performed in the same laboratory.
Genotyping
According to the manufacturer’s instructions, genomic DNA from participants was extracted from EDTA-treated whole blood, using a DNA Blood Mini Kit (Qiagen, Hilden, Germany). The two SNPs were detected using fluorescent TaqMan probes (TaKaRa, Dalian, China). Restriction enzyme Taq1 (containing 10 × Taq1 basal buffer and 0.1 BSA) was used to identify and cut a specific sequence, after which PCR was performed with the following primers: forward 5’-ACA ATC ACT CCT TAA ATA TGG TGG-3’ and reverse 5’-AAG TAG GGA CAG ACA GGA CCA GTA-3’. A 25-μl reaction mixture was amplified by PCR, including 20 ng DNA, 0.05 μl Ex Taq DNA polymerase, 1 μl 10 × buffer, 0.8 μl dTNP, 0.1 μl forward primers, and 0.1 μl reverse primers. PCR conditions were as follows: initial denaturation for 3 min and 95 °C, denaturation for 10 s and 95 °C, annealing for 30 s and 63 °C, extension for 30 s and 72 °C, 40 cycles. ABI Prism 7000 software and an allelic discrimination procedure were used for genotyping of the two aforementioned SNPs. The probe sequence for the TaqMan fluorescence probe analysis is presented in Tab. 1. A 25-μl reaction mixture including 1.25 μl SNP Genotyping Assays (20 ×), 12.5 μl Genotyping Master Mix (2 ×), and 20 ng DNA was used, with the following conditions: initial denaturation for 10 min and 95 °C, denaturation for 15 s and 92 °C, annealing and extension for 90 s and 60 °C, 50 cycles.
Statistical analysis
Baseline continuous variables of participants were calculated as means with standard deviation or median with interquartile range according to their distribution, and the categorical variables were presented as percentage. The characteristics of the participants were compared according to EH status using the chi-square test for categorical variables and the t test for continuous variables. A value of p < 0.05, using two-sided tests, was considered statistically significant. The analyses of deviations from the Hardy–Weinberg equilibrium, estimation of linkage disequilibrium between polymorphisms, association of SNP polymorphisms and haplotypes with EH, as well as the odds ratio (OR) of EH and corresponding 95 % confidence interval (CI) were all performed using SNPstats software, available at http://bioinfo.iconcologia.net/SNPstats. Multiple ORs were further adjusted for the potential nongenetic risk factors of gender, age, smoking, and alcohol consumption, FPG, BMI, HDL-C, and family history of hypertension.
Results
Demographic and clinical features of participants
Of the 820 research participants (270 males, 550 females), 269 met the EH criteria; the average age of the participants was 50.05 ± 9.41 years. Age, HDL-C, FPG, BMI, WC, and family history of hypertension were significantly different between the NT and EH groups. Compared with NT subjects, EH subjects had a higher age, FPG, BMI and WC, but lower HDL-C. No statistically significant difference in gender, total cholesterol (TC), TG, current smoking, and alcohol consumption was observed between the NT and EH groups (Tab. 2).
C681G and intron CT
The overall minor allele frequency of C681G and intron CT was 33.0 % and 21.0 %, respectively. The allele frequency distribution of C681G and intron CT in the NT subjects varied from that in the EH subjects (p < 0.001 and 0.030, respectively). No significant deviation from the Hardy–Weinberg equilibrium was detected for the study polymorphisms (p = 0.87 and 0.11 for C681G and intron CT, respectively; Tab. 3).
Association of C681G and intron CT with EH, SBP, and DBP
After adjusting for gender, age, smoking and alcohol consumption, FPG, BMI, HDL-C, and family history of hypertension for C681G, the CG and GG and CG + GG genotype carriers had a significantly increased risk of EH compared to the CC genotype carriers, both in the codominant and dominant models. The adjusted ORs of EH were 1.57 (95 % CI 1.14 2.15), 2.24 (95 % CI 1.38–3.64), and 1.68 (95 % CI 1.24–2.28), respectively, and these carriers also had a significantly increased risk of DBP ≥ 90 and SBP ≥ 140 (ORs and 95 % CI omitted). For intron CT, the risk of EH was significantly increased for CT and CT + TT genotype carriers compared to CC genotype carriers in the codominant and dominant models; the adjusted ORs were 1.56 (95 % CI 1.11–2.18) and 1.46 (95 % CI 1.03–2.09), respectively. The CT and CT + TT genotype carriers were also more likely to have a higher risk of DBP ≥ 90 and SBP ≥ 140, respectively (ORs and 95 % CI omitted). Interestingly, the analysis of the association between C681G and intron CT consistently showed that all the minor allele carriers had a significantly increased risk of DBP ≥ 90, SBP ≥ 140, and EH in the dominant model (Tab. 4).
Association of haplotypes with EH, SBP, and DBP
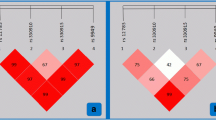
Association analyses revealed that the risk of EH was significantly increased among individuals carrying the haplotypes GC (adjusted OR 1.60 and 95 % CI 1.21–2.11), CT (adjusted OR 1.45 and 95 % CI 1.03–2.04), and GT (adjusted OR 1.95 and 95 % CI 1.17–3.23), compared with those carrying the CC haplotype; the risk of SBP ≥ 140 and DBP ≥ 90 was also significantly increased among individuals carrying the GC, GT and GC, and CT haplotypes, compared with those carrying the most common haplotype CC; the adjusted ORs were 1.48 (95 % CI 1.10–1.99), 1.91 (95 % CI 1.13–3.24), 1.59 (95 % CI 1.15–2.20), and 1.55 (95 % CI 1.05–2.28), respectively (Tab. 5, Fig. 1).
Discussion
Our study was the first to investigate the association between the C681G and intron CT locus of the human PPARγ gene and EH using a haplotype-based analysis in a Han Chinese population. The major novel findings in the present study were that the single-locus association analysis consistently revealed that the polymorphism of C681G and intron CT had an increased risk of EH, both in the dominant and codominant models. Moreover, the haplotype analyses revealed that the GC, CT, and GT haplotypes derived from the C681G and intron CT locus had a hazardous effect on EH. These findings support our hypothesis that the polymorphism of C681G and intron CT plays an important role in the development of EH, and that the GC, CT, and GT haplotypes might be genetic markers of EH in the Han Chinese population.
In the present study, the polymorphism of C681G had an increased risk of EH both in the dominant and codominant models. This was in agreement with the allele frequency distribution between the EH and NT groups, which was statistically significant (p < 0.001). There is little evidence on the association between the C681G polymorphism and EH in population-based studies. Previous studies found an association between the C681G polymorphism and a higher BMI and higher LDL-C and TC levels as well as higher plasma apolipoprotein B and LDL-C levels in a French population [8, 13]. These findings suggest that the C681G polymorphism may affect the incorporation between the signal transduction of the PPARγ promoter region and the transcription activator factor 5 (STAT5) [13]. Of note, STAT5 can regulate immune inflammation, the proliferation, differentiation, and apoptosis of cells, the signal transduction of insulin, the angiotensin in vascular smooth muscle cells, and the transcriptional activator of the sodium grain gene [14].
To date, only one other study has reported the relationship between intron CT and blood pressure: a community-based cohort study conducted in a Washington County, Maryland, population on the association between five SNPs and cardiovascular morbidity and mortality [9]. The results of the study indicated that there was a statistically significant age-adjusted association between intron CT and blood pressure, but the statistically significant association disappeared after correction for multiple factors. The results of our study indicated that the intron CT polymorphism was significantly related to EH, both in the codominant and dominant models, as the participants carrying the intron CT, CT + TT, and CT genotypes were significantly more likely to have a higher risk of EH than individuals carrying the CC genotype even after adjusting for potential nongenetic risk factors.
The most outstanding finding in the single-locus association analysis was the consistent associations of C681G and intron CT with an increasing risk of SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, and EH even after multiple adjustments were made in the dominant model. Additionally, both C681G and intron CT were located in one haplotype block and had very high linkage disequilibrium. Considering this, we established haplotypes CC, GC, CT, and GT based on C681G and intron CT. Our results showed that the risk of EH was significantly increased among individuals carrying the GC, CT, and GT haplotypes compared with those carrying the CC haplotype after multivariable adjustment. Currently, although there is no evidence of the effect of C681G and intron CT on EH, the hazardous haplotypes GC, CT, and GT all harbored the EH-raising alleles of the C681G-G allele and/or the intron CT-T allele, respectively. Moreover, the functions of these haplotypes were consistent with the effect of these variant genotypes among the same subgroup. Furthermore, the GT haplotype that contains both of the EH-raising alleles had the highest OR compared to the GC and CT haplotypes (OR, 1.95 vs. 1.45, 1.60), which contain only one EH-raising allele. These results suggest that the hazardous effect of GC, CT, and GT was probably driven by the C681G-G allele and the intron CT-T allele. To our knowledge, C681G and intron CT are located on PPAR γ. Animal studies indicated that PPARγ activators (TZDs) have a protective role in improving hypertension. The protective role may be inferred by subcellular mechanisms such as mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and ROS-generating enzymes [15]. Evidence from population studies has shown that the TZDs could reduce blood pressure levels in diabetic hypertensive patients [16]. Interestingly, a promising finding in the haplotype association analysis was that the GC, CT, and GT haplotypes exhibited a statistically significant increased risk of EH, and a statistically significant increased risk of SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. These consistent findings suggest that our results were not due to chance. Instead, haplotypes GC, CT, and GT were most likely genetic markers of EH in the Han Chinese population.
PPARs represent an attractive target for reducing global risk in hypertensive patients. Both in animal models and population-based studies, the TZDs played a role in protecting from cardiovascular disease and in reducing blood pressure [15, 16, 17]. Previous studies pointed out that the PPARγ SNPs located in the intron area might influence the expressed region’s transcription rate and time. This could lead to different shear stress in the process of transcription and translation of the PPARγ gene and subsequently can change the structure of the protein and the carriers’ genetic susceptibility to EH [6]. Although it the exact function of the C681G and intron CT polymorphisms remains unclear at present, the available evidence showed that PPARγ agonists may play a protective role in blood pressure [18], stimulate the secretion of C diuresis sodium peptide in endothelial cells [19], downregulate the expression of angiotensin receptor 1 (AT1), and inhibit DNA synthesis by Ang ll stimulation in vascular smooth muscle cell (VSMC). Furthermore, it was shown that PPARγ agonists may inhibit the expression of cell cycle protein [20], increase the release of NO [21], lower the activity of the renin angiotensin system and sympathetic nervous system [22], and limit the inflammatory response [1]. However, how and in which way the C681G and intron CT polymorphisms contribute to the occurrence of hypertension remains unclear, and the exact function of their polymorphisms on hypertension needs to be explored in future studies.
There were several advantages of the present study. First, our study found for the first time that the risk of EH was significantly increased, not only in the single-locus association analysis but in the haplotype association analysis. Second, our regression analysis with a relatively large sample size permits adjustment for the effects of potential nongenetic risk factors of EH and for the incorporation of the end points of SBP ≥ 140 mmHg or DBP ≥ 90 mmHg in evaluating the risk of EH. Third, the haplotype effect is the combined effect of both SNPs that define the haplotype; a specific multilocus haplotype, rather than a single-locus allele, is a more significant determinant of association [23].
There were also limitations in the present study. First, in the single-locus analysis of intron CT, we found that the hazardous effect appeared to be significant in the heterozygote but not in the homozygote subjects. It is possible that the variant allele may have a strong dominant effect so that there was a small difference between the effects of the variant homozygotes and heterozygotes. Alternatively, because of the relatively small number of variant homozygotes observed, the effect of the variant homozygotes might more likely be subject to selection bias or other unfavorable genotypes than that of the heterozygotes [24]. Nevertheless, this speculation needs further support from additional research and functional studies.
Second, both C681G and intron CT were not in a sufficiently strong pairwise linkage disequilibrium owing to their D′ values being below 0.5, but they were located in one haplotype block and had a strong pairwise linkage disequilibrium in the JPT and CHB database. Moreover, the D′ value might more likely be subject to crowd structure, population growth, mutation rate, and natural selection. Such analyses would be interesting and add more value to the present study.
Third, there was a disequilibrium in the gender distribution between participants with and without EH and we could not exclude participants with a family history of hypertension because of the design of the study. To date, gender and a family history of hypertension were well-known risk factors of EH. Therefore, we used regression analysis to adjust for the confounding effects of the various nongenetic covariates, including gender and family history of hypertension.
Conclusion
In conclusion, this was the first study to investigate the association between the C681G and intron CT locus of the human PPARγ gene and EH in a Han Chinese population. The present data indicated that the haplotypes GC, CT, and GT were specific genetic marker candidates for EH. While the genetics of the common variations of C681G and intron CT at the PPARγ locus appear to be complex, unraveling this complexity reveals that it has a role in the modulation of hypertension.
References
Benkirane K, Viel EC, Amiri F et al (2006) Peroxisome proliferator-activated receptorg regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension 47:102–108
Sourij H, Zweiker R, Wascher TC (2006) Effects of pioglitazone on endothelial function, insulin sensitivity, and glucose control in subjects with coronary artery disease and new-onset type 2 diabetes. Diabetes Care 29:1039–1045
Sidhu JS, Kaposzta Z, Markus HS et al (2004) Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol 24:930–934
Haffner SM, Greenberg AS, Weston WM et al (2002) Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 106:679–684
Leibovitz E, Schiffrin EL (2007) PPAR activation: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 50:120–125
Laforet M, Froelich N, Parissiadis A et al (1997) An intronic mutation responsible for a low level of expression of an HLA-A*24 allele. Tissue Antigens 50:340–346
Seber S, Ucak S, Basat O et al (2006) The effect of dual PPAR alpha/gamma stimulation with combination of rosiglitazone and fenofibrate on metabolic parameters in type 2 diabetic patients. Diabetes Res Clin Pract 71:52–58
Oliver WR, Shenk JL, Snaith MR et al (2001) A selective peroxisome proliferator -activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A 98:5306–5311
Gallicchio L, Kalesan B, Huang HY et al (2008) Genetic polymorphisms of peroxisome proliferator-activated receptors and the risk of cardiovascular morbidity and mortality in a community-based cohort in washington county, Maryland. PPAR Res 81:1–9
Zhou H, Guo ZR, Yu LG et al (2010) Evidence on the applicability of the ATPIII, IDF and CDS metabolic syndrome diagnostic criteria to identify CVD and T2DM in the Chinese population from a 6.3-year cohort study in mid-eastern China. Diabetes Res Clin Pract 90:319–325
Ding Y, Guo ZR, Wu M et al (2012) Gene-gene interaction between PPARδ and PPARγ is associated with abdominal obesity in a Chinese population. J Genet Genomics 39:625–631
World Health Organization (1999) International society of hypertension guidelines for the management of hypertension. Guidelines Subcommittee. J Hypertens 17:151–183
Meirhaeghe A, Fajas L, Helbecque N et al (1998) A genetic polymorphism of the peroxisome proliferator-activated receptor gamma gene influences plasma leptin levels in obese humans. Hum Mol Genet 7:435–440
Meirhaeghe A, Fajas L, Gouilleux F et al (2003) A functional polymorphism in a STAT5b site of the human PPAR 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler Thromb Vasc Biol 23:289–294
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hyper tension. Histochem Cell Biol 122:339–352
Yosefy C, Magen E, Kiselevich A et al (2004) Rosiglitazone improves, while glibenclamide worsens blood pressure control in treated hypertensive diabetic and dyslipidemic subjects via modulation of insulin resistance and sympathetic activity. J Cardiovasc Pharmacol 44:215–222
Benkirane K, Viel EC, Amiri F et al (2006) Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen -activated protein kinase in blood vessels in vivo. Hypertension 47:102–108
Buchanan TA, Meehan WP, Jeng YY et al (1995) Blood pressure lowering by pioglitazone. Evidence for a direct vascular effect. J Clin Invest 96:354–360
Itoh H, Doi K, Tanaka T et al (1999) Hypertension and insulin resistance: role of peroxisome proliferator-activated receptor-gamma. Clin Exp Pharmacol Physiol 26:558–560
Bernobich E, De-Angelis L, Lerin C et al (2002) The role of the angiotension system in cardiac glucose homeostasis: thetapeutic implications. Drugs 62:1295–1314
Calnek DS, Mazzella L, Roser S et al (2003) Peroxisome proliferator- activated receptor gamma ligands increase release of nitric oxide form endothelial cells. Arterioscler Thromb Vasc Biol 23:52–57
Kotchen TA, Zhang HY, Reddy S et al (1996) Effect of pioglitazone on vascular reactivity in vivo and in vitro. Am J Physiol 270:660–666
Martin ER, Lai EH, Gilbert JR et al (2000) SNPing away at complex disease: analysis of singe-nucleotide polymorphisms around APOE in Alzheimer disease. Am J Hum Genet 67:383–394
Ma HX, Xu L, Yuan J et al (2007) Tagging single nucleotide polymorphisms in excision repair cross-complementing group 1 (ERCC1) and risk of primary lung cancer in a Chinese population. Pharmacogenet Genomics 17:417–423
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Q., Guo, Z., Hu, X. et al. Haplotype analysis of PPARγ C681G and intron CT variants. Herz 39, 264–270 (2014). https://doi.org/10.1007/s00059-013-3819-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-3819-x