Abstract
Optical coherence tomography (OCT) is a novel light-based imaging modality for application in the coronary circulation. Compared to conventional intravascular ultrasound, OCT has a ten-fold higher image resolution. This advantage has seen OCT successfully applied in the assessment of atherosclerotic plaque, stent apposition, and tissue coverage, heralding a new era in intravascular coronary imaging. The present article discusses the diagnostic value of OCT, both in cardiovascular research as well as in potential clinical application.
The unparalleled high image resolution and strong contrast between the coronary lumen and the vessel wall structure enable fast and reliable image interpretation. OCT makes it possible to visualize the presence of atherosclerotic plaque in order to characterize the structure and extent of coronary plaque and to quantify lumen dimensions, as well as the extent of lumen narrowing, in unprecedented detail. Based on optical properties, OCT is able to distinguish different tissue types, such as fibrous, lipid-rich, necrotic, or calcified tissue. Furthermore, OCT is able to cover the visualization of a variety of features of atherosclerotic plaques that have been associated with rapid lesion progression and clinical events, such as thin cap fibroatheroma, fibrous cap thickness, dense macrophage infiltration, and thrombus formation. These unique features allow the use of OCT to assess patients with acute coronary syndrome and to study the dynamic nature of coronary atherosclerosis in vivo and over time. This permits new insights into plaque progression, regression, and rupture, as well as the study of effects of therapies aimed at modulating these developments.
Today’s OCT technology allows high detail resolution as well as fast and safe clinical image acquisition. These unique features have established OCT as the gold standard for the assessment of coronary stents. This technique makes it possible to study stent expansion, peri-procedural vessel trauma, and the interaction of the stent with the vessel wall down to the level of individual stent struts, both acutely as well as in the long term, where it is has proven extremely sensitive to the detection of even minor amounts of tissue coverage. These qualities render OCT indispensable to addressing vexing clinical questions such as the relationship of drug-eluting stent deployment, vascular healing, the true time course of endothelial stent coverage, and late stent thrombosis. This may also better guide the optimal duration of dual anti-platelet therapy that currently remains unclear and relatively empirical.
In the future, OCT might emerge, parallel to its undisputed position in research, as the tool of choice in all clinical scenarios where angiography is limited by its nature as a two-dimensional luminogram.
Zusammenfassung
Die intrakoronare optische Kohärenztomographie (OCT) ist ein relativ neues optisches Verfahren zur Beurteilung der Koronarmorphologie. OCT erstellt, ähnlich wie die konventionelle intrakoronare Ultraschallbildgebung, ein Querschnittsbild der Gefäßwand, mit einer 10-fach höheren Auflösung. Der Beitrag fasst die Erkenntnisse zum diagnostischen Vermögen der OCT zusammen und bewertet sie im Hinblick auf eine mögliche breitere klinische Anwendung.
OCT erlaubt eine klare und detaillierte Darstellung endoluminaler Strukturen. Atherosklerotische Plaques können schon im frühen Stadium sicher erkannt und detailliert erfasst werden. Verschiedene Plaquekomponenten können aufgrund ihrer optischen Eigenschaften verlässlich typisiert werden, in lipidreiches, nekrotisches, verkalktes und fibröses Gewebe. Darüber hinaus können morphologische Marker, die in Studien mit klinischen Ereignissen assoziiert wurden, wie ausgedehnte Fibroatherome, dünne fibröse Kappen, Makrophageninfiltrationen und Thromben diagnostiziert werden. Mit diesem diagnostischen Profil ermöglicht OCT Analysen bei akutem Koronarsyndrom und vor allem longitudinale Untersuchungen zum Verständnis von Plaqueprogression, -regression sowie -ruptur und ihrer therapeutischen Modifikation. Wegen der hohen Detailauflösung von OCT etablierte sich diese Methode rasch zum neuen Goldstandard zur Beurteilung von Koronarstents, sowohl im akuten als auch im Langzeitverlauf. OCT erlaubt die Analyse individueller Stent-Struts, ihrer Apposition und Neointimabekleidung im Lauf der Zeit. Diese Informationen sind wertvoll für die Entwicklung von Koronarstents, aber auch zur Therapie von Stentrestenose und Thrombose. In Zukunft können diese Einblicke möglicherweise auch zu einer Optimierung der antithrombozytären Therapie beitragen.
Die hohe Detailauflösung sowie die schnelle, patienten- und anwenderfreundliche Bildgebung machen die OCT, neben ihrer längst akzeptierten Rolle in der Forschung, prinzipiell attraktiv für eine breitere klinische Anwendung in all den Situationen, in denen die Angiographie aufgrund ihres zweidimensionalen Luminogrammcharakters limitiert ist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optical coherence tomography (OCT) is a novel light-based imaging modality for application in the coronary circulation. Compared to conventional intravascular ultrasound (IVUS), OCT has a ten-fold higher image resolution. This advantage has seen OCT successfully applied in the assessment of atherosclerotic plaque, stent apposition and tissue coverage, heralding a new era in intravascular coronary imaging.
The origins of OCT date back to 1990, to a technology known as optical coherence domain reflectometry (OCDR). OCDR waveform scans, however, were very hard to interpret. By using an interferometer it was possible to record reflections from various depths in a biological tissue and to create an image that was much easier for a human to interpret. The first manuscript from the Massachusetts Institute of Technology, published in 1991, describes the basic concept of an OCT imaging system and discusses its possible applications in both retinal and arterial imaging [1]. Compared to an OCT microscope, used in ophthalmology and in most experimental settings, intracoronary OCT represented a challenge, as blood needs to be cleared temporarily from the field of view during imaging. In the past, this necessity and its potential for causing coronary ischemia limited the application of OCT to selected patients, lesions and operators. Today, however, this major drawback is largely solved by the introduction of the second generation Fourier domain OCT technology. Fourier domain OCT employs an easy-to-use, short monorail OCT imaging catheter and allows for very fast data acquisition. As a result, a coronary artery can be scanned today in a user- and patient-friendly manner within a couple of seconds, and thus without introducing clinically relevant ischemia [2, 3].
In recent years, intracoronary OCT has been successfully applied as a research tool, where it has become the new gold standard for the in-vivo assessment of coronary stents, with the ODESSA [4] and LEADERS [5] trials pioneering OCT in multicenter randomized clinical settings.
The unparalleled high image resolution and strong contrast between the coronary lumen and the vessel wall structure enable fast and reliable image interpretation for research purposes. These features can be advantageous in the clinical setting as well as in real-world practice. However, given the novelty of the method, there are currently no established indications for the clinical use of intracoronary OCT. This article discusses the diagnostic value of OCT, both in cardiovascular research as well as in potential clinical application.
Physical principles
The principle of OCT is analogous to pulse-echo ultrasound imaging; however, a near infrared light source (approximately 1300 nm wavelength) is used rather than sound to create the image. Whereas ultrasound produces images from backscattered sound “echoes,” OCT uses infrared light waves that reflect off the internal microstructure within the biological tissues. Both the bandwidth of the infrared light used and the wave velocity are orders of magnitude higher than in medical ultrasound. The resulting resolution depends primarily on the ratio of these parameters, and is one order of magnitude larger than that of conventional IVUS: the axial resolution of OCT is about 15 μm. The lateral resolution is mainly determined by the imaging optics in the catheter and is approximately 25 µm. This high resolution, however, is at the expense of a reduced penetration depth, caused by the attenuation of light in the tissue and the need to create a blood-free environment for imaging. In coronary arteries, blood (erythrocytes) represents a non-transparent tissue causing multiple scattering and substantial signal attenuation. Consequently, blood must be cleared from the field of view during OCT imaging.
As the speed of light is much faster than that of sound, an interferometer is required to measure the backscattered light [6]. The interferometer splits the light source into two “arms”: a reference arm and a sample arm, which is directed into the tissue. The light from both arms is recombined at a detector, which registers the so-called interferogram, the sum of reference and sample arm fields. Because of the large source bandwidth, the interferogram is non-zero only if the sample and reference arms are of equal length, within a small window equal to the coherence length of the light source [7, 8].
The interferogram is detected as a function of wavelength, either by using a broadband source and spectrally resolved detection, or alternatively by incorporating a novel wavelength-swept laser source [9, 10]. This latter technique is also called “swept-source OCT,” or optical frequency domain imaging (OFDI), and capitalizes most effectively on the higher sensitivity and signal-to-noise ratio offered by Fourier domain detection. This development (summarized under the name second generation OCT or Fourier domain OCT) has led to fast image acquisition speeds, with greater penetration depth, without loss of vital detail or resolution, and represents a great improvement on time-domain OCT systems used in the past. From the signal received in one wavelength sweep, the depth profile can be constructed by the Fourier transform operation that is performed electronically in the data processing unit. In a Fourier domain OCT system, the wavelength range of the sweep determines the resolution of the image, while the imaging depth is inversely related to the instantaneous spectral width of the source.
Fourier domain OCT systems produce images much faster than standard video rate, so recorded data has to be replayed for analysis by the operator. Currently, OCT systems scan 200–500 angles per revolution (frame), in the order of 105 lines per second and 5–10 images/mm in a pullback. If these parameters are maintained with high-speed systems, 20 mm/s (or higher) pullback speeds are possible at the same sampling density as conventional OCT data. The high scan speeds have been employed for real-time volumetric imaging of dynamic phenomena including fast pullbacks for intracoronary imaging with minimal ischemia, and retinal scans with minimal motion artefacts [11].
Practical application in the catheterization laboratory
Currently, one Fourier domain OCT system is commercially available (St. Jude/Lightlab Imaging Inc, Westord, MA). The optical probe is integrated in a 2.7-F short monorail catheter compatible with 6-F guiding catheters and a 0.014” guidewire. The imaging catheter is part of the sample arm of the interferometer. The optical signal is transmitted by a single-mode fiber, which is fitted with an integrated lens micro-prism assembly to focus the beam and direct it towards the tissue. The focus is approximately 1 mm outside the catheter. In order to scan the vessel lengthwise, the catheter imaging tip is pulled back while rotating, inside a transparent sheath, allowing collection of a three-dimensional dataset of the coronary artery. Both rotary and pullback motion are driven proximally by a motor outside the patient.
For clinical imaging, the OCT imaging catheter is advanced distally into the coronary artery via a standard angioplasty guide wire (0.014”.) Automated OCT pullback (pullback speed is typically 20 mm/s) is performed during simultaneous flush delivery through the guide catheter, using a power injector (typical flush rate 3.0 ml/s) connected to the standard Y-piece of the guiding catheter. Although a variety of solutions, warmed to 37°C, are used as a flush medium, most authors prefer iso-osmolar contrast medium, as it has been extensively tested and optimized for cardiac use and is readily available in the catheterization laboratory.
OCT and its role in the assessment of atherosclerotic lesions
OCT makes it possible to visualize the presence of atherosclerotic plaque, to characterize the structure and extent of coronary plaque, and to quantify lumen dimensions as well as the extent of lumen narrowing in unprecedented detail [12, 13, 14]. OCT signal intensity is mapped to a color space that can be displayed on a monitor. A popular color map is the “sepia” scale, ranging from black (low OCT signal) through brown, yellow and white (high OCT signal); alternatively, grayscale (low is black, high is white) or inverted grayscale maps are used.
Normal coronary morphology
The normal coronary artery wall appears as a three-layer structure on OCT images. The media is seen as a dark band delineated by the internal elastic lamina and external elastic lamina. As the mean media thickness is 200 µm, it can be visualized easily by OCT [15, 16]. In the presence of vessel remodelling, i.e., an asymmetric expansion of the vessel wall at the sites of plaque accumulation, the media typically becomes thinner. Unfortunately, because of its limited tissue penetration (1–1.5 mm), OCT does not appear to be suited to the study of vessel remodelling, as it often does not allow visualization of the media in the presence of atherosclerotic vessel wall thickening.
The normal intima consists of a thin sub-endothelial collagen layer covered towards the lumen by a single layer of endothelial cells. This normal anatomical structure is beyond the resolution of OCT. However, OCT can detect early stages of intimal thickening, depicted as a signal-rich, homogeneous, thin rim of tissue. Thus, OCT can confirm the absence of significant atherosclerosis or indicate the degree of subclinical atherosclerotic lesion formation. Serial measurements can be performed to monitor the structural changes that occur in the vessel wall over time. This information could be clinically relevant to study the natural history of coronary atherosclerosis and the effect of different therapies on the regression/progression of the incipient plaque [17, 18].
Qualitative description of atherosclerosis
As the various components of atherosclerotic plaques have different optical properties, OCT makes it possible to differentiate them to a great extent. Identification of plaque components by OCT depends on the penetration depth of the incident light beam into the vessel wall. The depth of penetration is greatest for fibrous tissue and least for thrombi with calcium and lipid tissue having intermediate values [19, 20, 21]. Typically, OCT images are interpreted by eyeballing [17, 22]: assessing the signal intensity and geometry [23], applying the following definitions:
Fibrous plaques are typically rich in collagen or muscle cells and have an homogeneous, signal-rich OCT signal.
Calcifications within plaques are identified by the presence of well-delineated, low back-scattering, signal-poor regions. In general, superficial calcium deposits can be studied and measured using OCT unless their thickness is greater than 1.0–1.5 mm, thereby reaching the penetration limit of OCT. Under these circumstances, the diagnosis of focal calcification can be challenging. Signal-poor calcifications might be confused with signal-poor, lipid-rich tissue [24]. It can be difficult to make a diagnosis from a single still frame; therefore, it might be helpful and increase the diagnostic accuracy to review the pullback sequence.
Necrotic cores or lipid-rich tissues are less well-delineated than calcifications, appearing as diffusely bordered, signal-poor regions with overlying signal-rich bands, corresponding to fibrous caps. The superiority of OCT for lipid-rich plaque detection has been confirmed in other studies comparing OCT, IVUS, and IVUS-derived techniques for plaque composition analysis [15, 25].
Although the majority of lesions’ lipid pool thickness cannot be measured because of insufficient tissue penetration, the thickness of the fibrous cap delimiting superficial lipid pools can be measured by OCT. An estimate of the lipid/necrotic core content of a lesion can be derived, e.g., from the number of quadrants occupied by necrotic lipid pool [26, 27].
Thrombi are identified as masses protruding into the vessel lumen that are discontinuous from the surface of the vessel wall. Red thrombi consist mainly of red blood cells; relevant OCT images are characterized as high-backscattering protrusions with signal-free shadowing. White thrombi consist mainly of platelets and white blood cells and are characterized by signal-rich, low-backscattering billowing projections protruding into the lumen [28].
Macrophages are seen by OCT as signal-rich, distinct, or confluent punctate dots that exceed the intensity of background speckle noise [29]. Macrophages may often be seen at the boundary between the bottom of the cap and the top of a necrotic core.
Vasa vasorum and neovascularization. (Neo)vessels within the intima appear as signal-poor voids that are sharply delineated and usually contiguous and seen on multiple frames [30].
Generally, good accuracy as well as inter- and intra-observer agreement for visual plaque characterization was reported with TD-OCT [19, 24]. Similar results have been reported recently with second-generation FD-OCT systems [19, 31]. However, whilst eyeballing of OCT images allows for fast and reliable plaque characterization, correct image interpretation depends on the experience of the observer and on the penetration depth into the tissue. Alternatively, a variety of approaches for quantitative tissue analysis are under development to further improve diagnostic accuracy in an observer-independent way [29, 32].
Plaque rupture and intracoronary thrombosis
Acute coronary syndromes (ACS) caused by the rupture of a coronary plaque triggering coronary thrombosis are common initial and often fatal manifestations of coronary atherosclerosis in otherwise apparently healthy subjects. Detecting lesions at high risk of rupture (so-called vulnerable plaques) would be of great importance for the prevention of future ACS. OCT has emerged as one of the most promising tools to assess patients with ACS and to detect key features of plaques at high risk for rupture, due to its ability to provide unique information on plaque composition, the thickness of the fibrous cap, the presence of macrophages and thrombi, as well as tissue collagen composition, and to indentify causes of ACS. OCT allows the diagnosis of thin fibrous cap atheroma (TCFA) with a sensitivity of 90% and a specificity of 79% compared to histopathology [33]. In a study comparing OCT, IVUS, and angioscopy in patients with acute myocardial infarction, Kubo et al. reported that OCT was the only imaging technology able to estimate fibrous cap thickness (mean 49±21 µm). This ability to assess the fibrous cap indicates that OCT may be well suited for the in vivo detection of TCFA [27], to follow their phenotype over time [34], and to assess TCFA rupture [35]. Previous clinical studies revealed an association between clinical patient presentation and plaque phenotype. A higher incidence of lipid-rich plaque and TCFA was observed in acute myocardial infarction and ACS patients as compared to patients with stable angina [20, 26, 36, 37].
These clinical observations are important, since they not only confirm histopathology observations, but also offer the potential to assess atherosclerotic plaque and its dynamic changes in a longitudinal manner. This is of note since the stability of a plaque depends not only on the size of the necrotic core and the thickness of the fibrous cap, but also on its collagen content and organization. Several investigations have shown a lower collagen content, thinner collagen fibers, and fewer smooth muscle cells (SMC) in unstable plaques. Polarization-sensitive OCT (PSOCT) is a new technology that enhances OCT by measuring tissue birefringence, a property that is elevated in tissues containing proteins with an ordered structure such as collagen. It has been demonstrated that PSOCT is able to evaluate collagen content, collagen fiber thickness, and SMC density in plaques and in fibrous caps of fibroatheromas [38]. This information is related to the mechanical stability of the fibrous cap and could help improve the identification of lesions at high risk of rupture in patients.
OCT and its role in the assessment of coronary stents
For the past two decades, IVUS has been used to assess the acute result following stenting, providing valuable information on stent expansion, strut apposition and signs of vessel trauma including dissections and tissue prolapse. As OCT creates cross-sectional images of the coronary artery, similar to IVUS, it is widely assumed that the OCT information can be used for clinical decision-making analogous to IVUS. It should be noted, however, that there are currently no established OCT criteria for optimal stent expansion or prospective data proving prognostic benefit. Nevertheless, clinical experience over the last decade has demonstrated that OCT can provide valuable anatomic information to optimize stent implantation and adapt PCI strategy in individual patients. Typical examples include the true extent of atherosclerotic plaque which potentially makes it possible to select the most appropriate stent length and landing zones, the reference lumen dimensions allowing for optimal stent diameter selection, and the visualization of stent expansion, permitting safe and predictable post-dilatation in the case of (focal) under-expansion. The specific diagnostic value of OCT consists in the fact that it can provide unique information on the stent interaction with the vessel wall on the level of individual struts and, secondly, that OCT can provide detailed three-dimensional datasets, and thus allows for clarity in all situations where angiography suffers from its limitations as a two-dimensional luminogram technique.
Acute stent expansion and strut apposition
IVUS studies [39, 40] suggested that stent strut malapposition is a relatively uncommon finding, observed in approximately 7% of cases, and that strut malapposition does not increase the risk of subsequent major adverse cardiac events. In contrast, OCT can visualize the complex coronary arterial wall structure after stenting in much greater detail [41]. Similar to IVUS, OCT enables the assessment of stent areas within the stented segment as well as lumen areas in the adjacent reference segments, thus enabling assessment of stent expansion. OCT studies in the acute post-stent setting [42] have demonstrated a relatively high proportion of stent struts, not completely apposed to the vessel wall, even after high pressure post-dilatation with this phenomenon being particularly evident in regions of stent overlap. In an evaluation of OCT findings following stent implantation to complex coronary lesions, Tanigawa et al. [43] reported a strut malapposition rate per lesion of 9.1±7.4%. Univariate predictors of malapposition on multilevel logistic regression analysis where the implantation of a sirolimus-eluting stent (likely due to its increased strut thickness and closed cell design) together with the presence of overlapping stents, a longer stent length, and a type C lesion. Likely mechanical explanations for stent malapposition include strut thickness, closed cell design, or acute stent recoil [44]. While these findings are impressive and helpful for the improvement of future stent designs, the clinical relevance and potentially long-term sequelae of malapposed struts as detected by OCT are currently unknown.
Peri-procedural vessel injury and incomplete lesion coverage
High-pressure deployment is necessary for adequate stent expansion, but it can induce periprocedural vessel damage. Experimental data have related the vessel response to injury after implantation with stent restenosis. Given its high resolution, OCT allows detailed visualization of vessel damage after stent implantation and can distinguish different types of injury. Furthermore, the technique allows the quantification of these findings [45]. A recent study showed that the frequency of tissue prolapse or intra-stent dissections after stenting was high, irrespective of the clinical presentation of the patients [45]. Dedicated assessment of the reference segments at the extremities of the stents revealed a high proportion of patients with lipid-rich plaques at the stent borders [46]. Fibroatheroma was observed in 15.3% of the distal and 28.9% of the proximal edges and thin-cap fibroatheroma (TCFA) extending over the extremities of the stent in 7%. Plaque type at the stent borders affected the incidence of edge dissections with edge dissections observed more frequently when the plaque type at the border was fibrocalcific (43.8%) or lipid-rich (37.5%) than when the plaque was fibrous (10%) (p=0.009).
The role of these edge dissections that were angiographically silent in the majority of cases (κ=0.22 when compared to OCT) still needs to be evaluated. IVUS data have suggested that non-flow-limiting edge dissections are not necessarily associated with an increase in acute or long-term events or development of restenosis [47].
Assessment of long-term outcome
Interest in long-term stent strut vessel wall interaction is manifold, including an assessment of the stability of the acute result, visualization of complex anatomy that is not accessible by angiography or IVUS, and a clearer understanding of reasons for stent failures, when they do occur.
OCT can reliably detect early and very thin layers of tissue coverage on stent struts. Tissue coverage can be visualized reliably and its quantification can be performed with high reproducibility [12]. This renders OCT an ideal tool to assess subtle, but potentially relevant differences in various stents. The LEADERS trial was a multi-center, randomized comparison of a biolimus-eluting stent (BES) with biodegradable polymer and a sirolimus-eluting stent (SES) using a durable polymer. OCT demonstrated that strut coverage at 9 months follow-up was more complete in patients allocated to BESs when compared to SESs. Twenty patients were included in the analysis in the BES group (29 lesions with 4592 struts) and 26 patients in the SES group (35 lesions with 6476 struts). A total of 83 struts were uncovered in the BES group and 407 out of 6476 struts were uncovered in the SES group [weighted difference –1.4%, 95% confidence interval (CI) –3.7–0.0, p=0.04] [5]. In the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, patients with STEMI were randomized to PES or BMS implantation [48]. OCT at 13 months follow-up revealed that implantation of PES as compared with BMS significantly reduced neointimal hyperplasia but resulted in higher rates of uncovered and malapposed stent struts (1.1±2.5% in BMS lesions versus 5.7±7.0% in PES lesions; p<0.0001).
Recently, OCT was successfully employed to study tissue coverage at follow-up in bioresorbable scaffolds [49, 50, 51]. It was able to visualize the particular structure of the scaffold struts, the tissue coverage over time, as well as changes in the optical properties of the vascular tissue during the bioresorption process in a unique way. This type of information is crucial to understanding bioresorption under clinical conditions in a wide range of atherosclerotic plaques and to drive further development.
While these described observations are important to understand differences in stent design, further studies are required to determine the clinical significance of these findings. To date, no threshold for coverage has been established. Pathological data in humans suggest that neointimal coverage of stent struts could be used as a surrogate marker of endothelialization due to the good correlation between strut coverage and endothelialization thrombosis [52, 53]. However, OCT observations need to be interpreted with caution. OCT is limited by its resolution of 15 μm, which is greater than the thickness of an individual layer of endothelial cells. Therefore, coverage that is not visible by OCT does not exclude the presence of an endothelial layer. Secondly, the presence of tissue coverage does not necessarily imply the presence of a functionally intact endothelium [54]. Early experimental stent data showed that endothelial function can vary considerably and show evidence of damage when subjected to the Evan’s blue dye exclusion test, even in the presence of a well structured neointimal layer [55].
However, OCT is the only imaging modality to date that offers—within the discussed limits—the opportunity to understand tissue coverage and neointima formation in drug-eluting stents over time. Clearly, larger stent trials with OCT at different time periods are needed to obtain a representative assessment of the true time course of endothelial stent coverage of these stents. Recent improvements in OCT technology, with frequency-domain OCT, allow for a simple imaging procedure and offer the potential for large scale prospective studies, indispensable to address vexing clinical questions such as the relationship of drug-eluting stent deployment, vascular healing, the true time course of endothelial stent coverage, and late stent thrombosis. This may also better guide the optimal duration of dual anti-platelet therapy that currently remains unclear and rather empirical.
Summary and outlook
OCT generates intracoronary images with unprecedented image resolution. Fourier domain OCT technology allows for widespread, fast, and safe clinical application. These unique features have established OCT as the gold standard for the assessment of coronary stents. Furthermore, OCT is able to cover the visualization of a variety of features of atherosclerotic plaques that have been associated with rapid lesion progression and clinical events. However, no clinical indication for OCT imaging has hitherto been established. There is no controlled data supporting a prognostic role for OCT for the assessment of cardiovascular risk or in catheter-based interventions. However, there is broad agreement that the detailed and easily accessible and interpretable information from OCT on the presence of atherosclerosis, its extent, lumen narrowing, as well as on the results of any interventional measures can be of clinical value, at least in individual patients and in specific clinical scenarios. Preliminary data indicate that OCT can change the operator’s intention-to-treat and modify the overall revascularization strategy, potentially avoiding unnecessary interventional procedures. OCT might be efficient in complex interventions including treatment of left main stem, ostial left anterior descending, or left circumflex artery lesions or bifurcations, as well as in all cases of angiographically ambiguous lesions and stent failures. As such, OCT might emerge—parallel to its undisputed position in research—as the tool of choice in all clinical scenarios where angiography is limited by its nature as a two-dimensional luminogram.
Intracoronary OCT plaque characterization. a Three-layer appearance with minimal concentric intimal thickening in subclinical atherosclerosis. The intima is concentric and homogeneously signal-rich (yellow). The signal-poor dark media is circumferentially visible over the complete vessel circumference. The adventitia is visualized as an outer signal-rich layer. b Eccentric fibroatheroma with predominantly fibrous, homogeneously signal-rich plaque in a 2–9 o’clock position (arrowheads). The signal-poor media is visible as a dark band over the majority of the vessel circumference (dotted line). c Eccentric fibroatheroma with focal calcification appearing as a signal-poor, relatively dark structure with sharply delineated borders (dotted line). d Eccentric fibroatheroma with predominantly lipid-rich plaque in a 2–8 o’clock position. Typically, a fibrous cap can be recognized as signal-rich yellow structure towards the lumen with a rapid drop in signal intensity towards the abluminal plaque side, caused by the high attenuation of lipid-rich tissue (arrows). An asterisk indicates guidewire artefact. OCT: St. Jude/Lightlab Imaging C7XR
Ruptured thin cap fibroatheroma. a Intracoronary OCT: Clinical example of a ruptured thin-cap fibroatheroma in a patient undergoing primary PCI. OCT imaging was performed after intracoronary thrombus aspiration. The thin fibrous cap has ruptured in the shoulder region of the eccentric plaque. Remnants of the necrotic core are in direct contact with the blood stream (double asterisks). Asterisk indicates guidewire artefact. b Magnification (3.7×) showing the rupture site of the thin fibrous cap. The thickness of the remnants of the fibrous cap is 52 μm (arrows). OCT: St. Jude/Lightlab Imaging C7XR
Online three-dimensional (3D) rendering of an atherosclerotic coronary artery segment. a Coronary angiogram of the right coronary artery; b–e OCT image display (commercially available intracoronary Fourier domain OCT system; Lightlab Imaging Inc, Westford, MA, US). b Online 3D lumen reconstruction of the imaged artery segment, side branches, as well as the guiding catheter can be easily recognized and used as landmarks. c Automated display of the minimal lumen area and proximal and distal reference segments. d Conventional two-dimensional longitudinal view. e Conventional cross-sectional view of the minimal lumen area
Intracoronary thrombus formation, as observed by OCT. a White thrombus: A signal-rich irregular mass protruding into the lumen is visible at the 3–9 o’clock position. The vessel wall is still circumferentially visible; the thrombus does not cause significant attenuation of the signal. b Red thrombus: A signal-rich irregular mass protruding into the lumen is visible at the 9–2 o’clock position. There is rapid attenuation of the OCT signal and, as a result, the vessel wall cannot be visualized at the 9–2 o’clock position. Asterisk indicates guidewire artefact. OCT: St. Jude/Lightlab Imaging C7XR
OCT immediately after stent implantation. a Well apposed struts: Metallic stent struts are visible as signal-rich structures with typical dorsal shadowing. All stents struts are completely apposed against the vessel wall. The lumen as well as the stent area can be clearly recognized and quantified by planimetry. b Strut malapposition: Metallic stent struts are visible as signal-rich structures with typical dorsal shadowing. At the 9 o’clock position, the struts are not apposed against the vessel wall. At the 3 o’clock position, struts are not apposed against the vessel wall, as they are positioned at the ostium of a side branch (SB). Asterisk indicates guide wire artefact. OCT: St. Jude/Lightlab Imaging C7XR
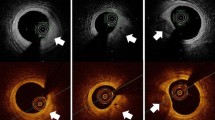
OCT observations during PCI. a Coronary angiogram demonstrating a significant lesion involving the bifurcation of the left anterior descending artery and the 2nd diagonal branch (Medina class 1.1.1). b Angiographic result after stent implantation in mini-crush technique: The left anterior descending artery was treated with a Xience V 2.25×28-mm stent followed by postdilatation with a 3.0×12-mm balloon. The 2nd diagonal branch was treated with a Xience 2.25×8-mm stent. c Intracoronary OCT of the left anterior descending artery reveals inadequate stent expansion with malapposition of stent struts at the 3–9 o’clock position. d Intravascular ultrasound image of the corresponding vessel segment fails to demonstrate strut malappostion. e Proximal stent edge immediately after stent implantation (Xience V™ 2.25×28 mm). f Proximal stent edge after post-dilatation with a Mercury NC™ balloon (3.0×15 mm). Malapposition of stent struts at the 3–5 o’clock position still can be observed. g Final result of the proximal stent edge after additional post-dilatation with a Voyager NC™ balloon (3.5×8 mm). The stent is well expanded and all struts are in complete contact with the vessel wall. Note the discrepancy between the original stent size, selected based on online QCA as compared to the final balloon diameter, safely used to optimize stent expansion based on invasive imaging. Asterisks indicate guidewire artefact (note: there are two guidewires visible; one is placed in the left anterior descending artery, another in the second diagonal branch). OCT: St. Jude/Lightlab Imaging C7XR
Example of an edge dissection after intracoronary stent implantation. a Intracoronary OCT shows eccentric fibroatheroma. A disruption of the thickened intimal layer is clearly visible at the 6 o’clock position. Asterisk indicates guidewire artefact. b Magnification (3.0×) revealing disruption of the thickened intimal layer, extending up to the media, which can be recognized as a thin, signal-poor black rim. OCT: St. Jude/Lightlab Imaging C7XR
Clinical example of serial OCT imaging in a patient participating in the ABSORB Cohort A trial. a Coronary angiography. b Three-dimensional OCT rendering. c OCT cross-sectional view. OCT demonstrates strut changes over time, At 2 years, struts are not discernible from the vessel wall anymore; the side branch ostium is wide open and easily accessible. OCT: St. Jude/Lightlab Imaging M3 & C7XR. (Modified from [56])
References
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254:1178–1181
Okamura T, Gonzalo N, Gutierrez Chico JL et al (2010) Does the second generation OCT improve safety and feasibility in clinical practice? A single center experience. J Am Coll Cardiol 55:E1906
Gonzalo N TG, Serruys PW, Soest G van et al (2010) Second generation optical coherence tomography in clinical practice. High speed data acquisition shows excellent reproducibility in patients undergoing percutaneous coronary interventions. Rev Esp Cardiol (in press)
Guagliumi G, Musumeci G, Sirbu V et al (n d) Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv 3:531–539
Barlis P, Regar E, Serruys PW et al (2010) An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J 31:165–176
Michelson A, Morley E (1887) On the relative motion of the earth and the luminiferous aether. Philos Mag S5:449–463
Schmitt JM, Knuttel A, Yadlowsky M, Eckhaus MA (1994) Optical-coherence tomography of a dense tissue: statistics of attenuation and backscattering. Phys Med Biol 39:1705–1720
Schmitt JM (1999) Optical coherence tomography (OCT): a review. IEEE J. Sel Top Quantum Electron 5:1205–1215
Yun S, Tearney G, Bouma B et al (2003) High-speed spectral-domain optical coherence tomography at 1.3 mum wavelength. Opt Express 11:3598–3604
Chinn SR, Swanson EA, Fujimoto JG (1997) Optical coherence tomography using a frequency-tunable optical source. Opt Lett 22:340–342
Lim H, Mujat M, Kerbage C et al (2006) High-speed imaging of human retina in vivo with swept-source optical coherence tomography. Opt Express 14:12902–12908
Tanimoto S, Rodriguez-Granillo G, Barlis P et al (2008) A novel approach for quantitative analysis of intracoronary optical coherence tomography: high inter-observer agreement with computer-assisted contour detection. Catheter Cardiovasc Interv 72:228–235
Gonzalo N, Garcia-Garcia HM, Serruys PW et al (2009) Reproducibility of quantitative optical coherence tomography for stent analysis. EuroIntervention 5:224–232
Okamura T, Gonzalo N, Gutierrez-Cjico JL et al (2010) Reproducibility of coronary Fourier domain optical coherence tomography: quanitative analysis of in vivo stented coronary arteries using three different software packages. EuroIntervention 6:371–379
Kawasaki M, Bouma BE, Bressner J et al (2006) Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol 48:81–88
Kume T, Akasaka T, Kawamoto T et al (2005) Assessment of coronary intima – media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J 69:903–907
Zimarino M, Prati F, Stabile E et al (2007) Optical coherence tomography accurately identifies intermediate atherosclerotic lesions – an in vivo evaluation in the rabbit carotid artery. Atherosclerosis 193:94–101
Kolodgie FD, Burke AP, Nakazawa G, Virmani R (2007) Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler Thromb Vasc Biol 27:986–989
Yabushita H, Bouma BE, Houser SL et al (2002) Characterization of human atherosclerosis by optical coherence tomography. Circulation 106:1640–1645
Kubo T, Imanishi T, Takarada S et al (2007) Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 50:933–939
Kume T, Akasaka T, Kawamoto T et al (2006) Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol 97:1172–1175
Prati F, Zimarino M, Stabile E et al (2008) Does optical coherence tomography identify arterial healing after stenting? An in vivo comparison with histology, in a rabbit carotid model. Heart 94:217–221
Prati F, Regar E, Mintz GS et al (2010) Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 31:401–415
Manfrini O, Mont E, Leone O et al (2006) Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol 98:156–159
Low AF KY, Yiong-Huak C, Tearney GJ et al (n d) In vivo characterisation of coronary plaques with conventional grey-scale intravascular ultrasound: correlation with optical coherence tomography. EuroIntervention 4:626–632
Jang IK, Tearney GJ, MacNeill B et al (2005) In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111:1551–1555
Barlis P, Serruys PW, Gonzalo N et al (2008) Assessment of culprit and remote coronary narrowings using optical coherence tomography with long-term outcomes. Am J Cardiol 102:391–395
Kume T, Akasaka T, Kawamoto T et al (2006) Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol 97:1713–1717
Tearney GJ, Yabushita H, Houser SL et al (2003) Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107:113–119
Regar E, Beusekom HM van, Giessen WJ van der, Serruys PW (2005) Images in cardiovascular medicine. Optical coherence tomography findings at 5-year follow-up after coronary stent implantation. Circulation 112:e345–e346
Gonzalo N, Tearney GJ, Serrruys PW et al (2010) Second generation optical coherence tomography. Rev Esp Cardiol (in press)
Soest G van, Goderie T, Regar E et al (2010) Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt 15:011105
Kume T, Okura H, Yamada R et al (2009) Frequency and spatial distribution of thin-cap fibroatheroma assessed by 3-vessel intravascular ultrasound and optical coherence tomography: an ex vivo validation and an initial in vivo feasibility study. Circ J 73:1086–1091
Tanaka A, Imanishi T, Kitabata H et al (2008) Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol 102:975–979
Gonzalo N, Tearney GJ, Soest G van et al (2011) Witnessed coronary plaque pupture during cardiac catheterization. JACC Cardiovascular Imaging (in press)
Kubo T IT, Kitabata H, Kuroi A et al (2008) Comparison of vascular response after sirolimus eluting stent implantation between patients with unstable and stable angina pectoris. A serial optical coherence tomography study. J Am Coll Cardiol Imaging 1:475–484
Fujii K, Kawasaki D, Masutani M et al (n d) OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. JACC Cardiovasc Imaging 3:168–175
Nadkarni SK, Pierce MC, Park BH et al (2007) Measurement of collagen and smooth muscle cell content in atherosclerotic plaques using polarization-sensitive optical coherence tomography. J Am Coll Cardiol 49:1474–1481
Hong MK, Mintz GS, Lee CW et al (2006) Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation 113:414–419
Tanabe K, Serruys PW, Degertekin M et al (2005) Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents: insights from the randomized TAXUS II trial. Circulation 111:900–905
Bouma BE, Tearney GJ, Yabushita H et al (2003) Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart 89:317–320
Tanigawa J, Barlis P, Dimopoulos K, Di Mario C (2008) Optical coherence tomography to assess malapposition in overlapping drug-eluting stents. EuroIntervention 3:580–583
Tanigawa J, Barlis P, Kaplan S, Goktekin OCD (2006) Stent strut apposition in complex lesions using optical coherence tomography. Am J Cardiol 98[Suppl 1]:97 M
Regar E, Schaar J, Serruys PW (2006) Images in cardiology. Acute recoil in sirolimus eluting stent: real time, in vivo assessment with optical coherence tomography. Heart 92:123
Gonzalo N, Serruys PW, Okamura T et al (2009) Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart 95:1913–1919
Gonzalo N, Serruys PW, Okamura T et al (2010) Relation between plaque type and dissections at the edges after stent implantation: An optical coherence tomography study. Int J Cardiol [epub ahead of print]
Futamatsu H, Sabate M, Angiolillo DJ et al (2006) Characterization of plaque prolapse after drug-eluting stent implantation in diabetic patients: a three-dimensional volumetric intravascular ultrasound outcome study. J Am Coll Cardiol 48:1139–1145
Guagliumi G, Costa MA, Sirbu V et al (2011) Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation 123:274–281
Ormiston JA, Serruys PW, Regar E et al (2008) First–in-man evaluation of a bioabsorbable-everolimus eluting coronary stent system (BVS) in the treatment of patients with single de-novo native coronary artery lesions: The ABSORB Trial. Lancet 2:371:899–907
Serruys PW, Ormiston JA, Onuma Y et al (2009) A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373:897–910
Serruys PW, Onuma Y, Ormiston JA et al (2011) Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation 122:2301–2312
Finn AV, Joner M, Nakazawa G et al (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115:2435–2441
Finn AV, Nakazawa G, Ladich E et al (2008) Does underlying plaque morphology play a role in vessel healing after drug-eluting stent implantation? JACC Cardiovasc Imaging 1:485–488
Beusekom HM van, Serruys PW (n d) Drug-eluting stent endothelium: presence or dysfunction. JACC Cardiovasc Interv 3:76–77
Beusekom HM van, Whelan DM, Hofma SH et al (1998) Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J Am Coll Cardiol 32:1109–1117
Okamura T, Serruys PW, Regar E (2010) Cardiovascular flashlight. The fate of bioresorbable struts located at a side branch ostium: serial three-dimensional optical coherence tomography assessment. Eur Heart J 31:2179
Conflict of interest
The corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regar, E., Ligthart, J., Bruining, N. et al. The diagnostic value of intracoronary optical coherence tomography. Herz 36, 417–429 (2011). https://doi.org/10.1007/s00059-011-3487-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-011-3487-7