Abstract
Objective
To investigate the effect of enamel deproteinization and air abrasion on shear bond strength (SBS), adhesive remnant index (ARI) scores, and surface topography when bonding orthodontic brackets to fluorosed enamel.
Materials and methods
The sample included 90 fluorosed and 30 normal premolars divided into four groups: group I (fluorosed premolars subjected to air abrasion before acid etching), group II (fluorosed premolars subjected to deproteinization before acid etching), group III (fluorosed premolars; control for groups I and II), and group IV (normal premolars; control for group III). Bonding procedures included etching with 37% phosphoric acid, priming with TransbondTM XT primer (3M Unitek, Monrovia, CA, USA), and application of TransbondTM XT adhesive paste (composite; 3M Unitek, Monrovia, CA, USA). Air abrasion was done using 50 µm aluminum oxide particles under 0.28 MPa pressure for 5 s with the micro-etcher held at a distance of 10 mm. Deproteinization was done for 60 s with 5% sodium hypochlorite (NaOCl).
Results
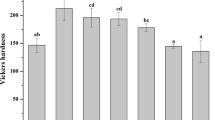
Fluorosed premolars subjected to deproteinization showed the lowest (median = 6.57 MPa) SBS among the four groups, followed by 8.14, 8.90, 8.14 MPa for groups I, III, and IV respectively. ARI scores were significantly different between the four groups (p = 0.006). Fluorosed enamel etched after air abrasion or deproteinization with NaOCl showed a predominance of type 4 etching pattern with some areas appearing unetched.
Conclusions
Shear bond strength of all groups was within the 6–8 MPa acceptable range for orthodontic purposes. Fluorosed premolars subjected to deproteinization showed the lowest values. Further studies are recommended to scrutinize the deproteinization technique.
Zusammenfassung
Zielsetzung
Untersucht werden sollte die Auswirkung der Schmelzdeproteinisierung und der Luftabrasion auf die Scherhaftung (SBS), den Adhäsivrestindex (ARI) und die Oberflächentopographie beim Kleben von kieferorthopädischen Brackets auf von Fluorose betroffenem Schmelz.
Materialien und Methoden
Die Stichprobe umfasste 90 fluorierte und 30 normale Prämolaren, die in 4 Gruppen eingeteilt wurden: Gruppe I (Prämolaren mit Fluorose, die vor der Säureätzung einer Luftabrasion unterzogen wurden), Gruppe II (Prämolaren mit Fluorose, die vor der Säureätzung deproteinisiert wurden), Gruppe III (Prämolaren mit Fluorose; Kontrolle für die Gruppen I und II) und Gruppe IV (normale Prämolaren; Kontrolle für Gruppe III). Das Bondingverfahren umfasste die Ätzung mit 37%iger Phosphorsäure, die Grundierung mit TransbondTM XT Primer (3M Unitek, Monrovia, CA, USA) und die Applikation der TransbondTM XT Adhäsivpaste (Komposit; 3M Unitek, Monrovia, CA, USA). Die Luftabtragung erfolgte mit 50 µm großen Aluminiumoxidpartikeln unter einem Druck von 0,28 MPa für 5 s, wobei der Micro-Etcher in einem Abstand von 10 mm gehalten wurde. Die Deproteinisierung wurde während 60 s mit 5% Natriumhypochlorit (NaOCl) durchgeführt.
Ergebnisse
Prämolaren mit Fluorose, die einer Deproteinisierung unterzogen wurden, zeigten den niedrigsten (Median 6,57 MPa) SBS unter den 4 Gruppen, gefolgt von 8,14, 8,90 und 8,14 MPa für die Gruppen I, III bzw. IV. Die ARI-Scores waren signifikant unterschiedlich zwischen den 4 Gruppen (p = 0,006). Von einer Fluorose betroffener Schmelz, der nach Luftabrasion oder Deproteinisierung mit NaOCl geätzt wurde, zeigte überwiegend ein Ätzmuster vom Typ 4, wobei einige Bereiche ungeätzt erschienen.
Schlussfolgerungen
Die Scherhaftung aller Gruppen lag innerhalb des kieferorthopädisch akzeptablen Bereichs von 6‑8 MPa. Prämolaren mit einer Fluorose, die einer Deproteinisierung unterzogen wurden, zeigten die niedrigsten Werte. Es werden weitere Studien empfohlen, um die Deproteinisierungstechnik zu überprüfen.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Dental fluorosis is a developmental problem resulting from overdose and chronic ingestion of fluoride during childhood [1]. Daily intake of water fluoridated with concentrations greater than 1–2 ppm can cause ameloblasts to show metabolic changes leading to reduced matrix formation and tooth maturation [2, 3]. Fluorosed enamel is characterized by a hypermineralized well-calcified outer layer and a hypomineralized porous inner layer [4]. Fluorosis severity varies, so multiple indices have been developed over the years to classify fluorosis such as the Dean’s Index [5], the Thylstrup and Fejerskov Index (TFI) [6], and the Total Surface Index of Fluorosis (TSIF) [7].
Bonding to enamel is the most commonly used technique for fixing orthodontic attachments. Investigating shear bond strength (SBS) of orthodontic brackets to fluorosed enamel. Some studies found no difference between bond strength to fluorosed and normal enamel [8, 9], while others found significantly lower bond strength to fluorosed enamel [10,11,12,13].
Cleaning and polishing followed by phosphoric acid etching is the routine method for conditioning the enamel surface before bonding. The hypermineralized surface of fluorosed enamel has been proved to be difficult to etch [14]; therefore, some authors investigated prolonged enamel etching to enhance etching results and increase bond strength [15, 16]. Several other methods have also been tried like adhesion promoters [17] and deproteinization that was used originally in root canal treatment [18, 19]. The idea was to use a deproteinizing agent such as sodium hypochlorite (NaOCl) to remove the organic elements of the enamel and the plaque layer adhered to it [20]. The dark color commonly seen in scanning electron microscope (SEM) images of enamel has been attributed to the organic pellicle that was not removed during cleaning and polishing. Why the organic pellicle is retained after pumicing enamel is not clear yet, but might reside in the presence of proteins as part of the crystals forming the normal enamel [20]. Air abrasion is another method that has been suggested to increase bond strength to fluorosed teeth. As air abrasion increases enamel roughness, the enamel surface area for bonding increases and the mechanical retention improves [15].
The objectives of the present research study were the following: (1) test the effect of enamel deproteinization and air abrasion on bond strength and adhesive remnant index (ARI) scores of orthodontic brackets bonded to fluorosed enamel and (2) compare surface topography between normal enamel, fluorosed enamel, enamel treated with NaOCl or air abrasion during the bonding/debonding process.
The null hypothesis for the present research study was that different treatments of fluorosed enamel do not affect the SBS and the ARI scores differently.
Materials and methods
The study was approved by the Research Ethics Committee of the institution.
Sample size
The sample size was calculated by G*Power software version 3.1.9.4 (Heinrich-Heine-Universität Düsseldorf, Germany). Based on previous similar studies [15, 21], a total of 120 premolars (30 premolars in each of the four groups) would achieve a power higher than 90% to detect a large effect size difference (f = 0.4) with a significance level (α) of 0.05. The sample included 90 fluorosed premolars and 30 normal premolars used as a control to the fluorosed enamel. The premolars were recently extracted for orthodontic treatment. The selected premolars had buccal surfaces free of any visible enamel cracks or caries. The fluorosed premolars had a score of 3 or 4 according to the Tooth Surface Index of Fluorosis (TSIF) [7]. The specific description of teeth with a TSIF score 3 is that chalky white fluorosis affects more than half of the visible surface, whereas in score 4 there is enamel staining in combination with any of TSIF levels 1, 2, or 3. Staining is a definitive area of discoloration that may vary from light to very dark brown [7]. Buccal enamel of the premolars was assessed visually with the naked eye under normal room illumination and the judgment was supported by florescence images (Fig. 1).
Image of a premolar specimen digital image under operating light (a) and fluorescence image (b); dark areas indicate loss of fluorescence, i.e., enamel fluorosis
Bild einer Prämolarenprobe, digitales Bild unter Operationslicht (a) und Fluoreszenzbild (b); dunkle Bereiche zeigen Fluoreszenzverlust, d.h. Schmelzfluorose
Specimen preparation
The fluorosed and normal premolars were collected and stored in saline at room temperature. After extraction and before storage, premolars were cleaned from blood or any tissue debris and disinfected using 0.1% thymol.
A well-established method [22] was used to fix the premolars. Each premolar was fixed by self-curing acrylic resin in customized blocks employing polypropylene pipes. The premolars were fixed by embedding the root and the lingual surface in the acrylic resin keeping the buccal surface uncovered. All bonded specimens were kept in distilled water for 7 days before debonding to measure the shear bond strength.
Brackets
Metal 0.022-inch premolar orthodontic brackets (Ormco, Glendora, CA, USA) were bonded to the buccal surface of the premolars. The average surface area of the base of the bracket was 9.63 mm2 [10, 12, 15].
Study design and specimen preparation
The fluorosed premolars were arbitrarily distributed into three equal groups and the normal premolars were designated as the fourth group. Accordingly, the following groups were developed and bonded:
-
Group I Fluorosed premolars subjected to air abrasion before acid etching: the buccal surface was cleaned and polished with a mixture of water and pumice and then washed thoroughly with water and dried with compressed air free of wetness and oil. Following cleaning, the buccal surface was sandblasted using 50 µm aluminum oxide fragments at 0.28 MPa pressure for 5 s with the micro-etcher held at 10 mm distance. The bonding procedures included etching with 37% phosphoric acid gel (Ivoclar, Vivadent, Schaan, Liechtenstein) for 30 s, priming with TransbondTM XT primer (3M Unitek, Monrovia, CA, USA) and finally application of TransbondTM XT adhesive paste (3M Unitek, Monrovia, CA, USA) and light curing for 20 s from the mesial and distal with a Elipar S10 LED light-curing unit (3M ESPE, St. Paul, MN, USA) for complete polymerization.
-
Group II Fluorosed premolars subjected to deproteinization before acid etching: the process started with cleaning and polishing as in group I, then the buccal surface of the premolars was deproteinized with 5% sodium hypochlorite (NaOCl) using a microbrush to apply it for 60 s, then washing and drying for 10 s. Bonding the brackets followed the same steps described in group II.
-
Group III Fluorosed premolars; control for groups I and group II: after cleaning and polishing, the fluorosed enamel was bonded following the same steps used for bonding the brackets in group I and group II.
-
Group IV Normal premolars; control for group III: the procedure was the same used in group III. All specimens were preserved in distilled water at 37 °C for 1 day and then thermocycled (SD Mechatronik thermocycler THE-1100, Feldkirchen-Westerham, Germany) between 5 and 55 °C for 500 cycles before shear bond strength testing.
Shear bond strength testing
A universal testing machine (Instron, Norwood, MA, USA) was used for shear bond strength (SBS) testing. The shearing force was applied via a sharp stainless steel blade attached to the upper part of the testing machine. The force was applied in occlusogingival direction at the bracket–tooth interface with a crosshead speed of 1 mm/min until failure of bonding. Failure load was converted to megapascal (MPa) by dividing the failure load by the surface area of the bracket base, (1 MPa = 1 N/mm2).
Adhesive remnant index (ARI) scores
The adhesive remaining on enamel after debonding the bracket was assessed at 20× magnification [23] using the Årtun and Bergland [24] four-point index:
-
0 = no adhesive remains on the enamel, i.e., bond failure happened between the resin and enamel,
-
1 = less than 50% of the resin remains on the tooth surface, i.e., bond failure happened principally at the resin–enamel interface,
-
2 = more than 50% of the resin remains on the tooth surface, i.e., bond failure happened mostly between the bracket and resin interface, and
-
3 = all resin remains on the tooth surface with a distinctive negative copy of bracket base, i.e., bond failure happened at the bracket–resin interface.
Scanning electron microscope examination
Additional specimens were used for scanning electron microscope (SEM) examination. To prepare the specimens for the examination, the premolars were fixed on aluminum plates and sputter-coated with gold to be examined with SEM (JSM-6510 LV, JEOL, Tokyo, Japan) operated at an accelerating voltage 30 kV. Images were captured at two magnifications: ×500 and ×1500. The examination was done for normal and fluorosed enamel after cleaning and polishing, treatment with air abrasion or NaOCl, acid etching, and bracket debonding (Table 1, Figs. 2–6). The etching pattern was assessed according to the 4 etching types described by Gwinnett [25]:
-
Type 1 representing an evident loss of the prism cores while the peripheries remain relatively intact,
-
Type 2 representing an evident loss of the peripheries while the cores remain intact,
-
Type 3 representing only pitting of the enamel surface layer, and
-
Type 4 representing smooth surface layer as both type 3 and type 4 show no clear prism outline.
Scanning electron microscope (SEM) images of normal enamel (a,b) and fluorosed enamel (c,d) after cleaning and polishing. ×500 and ×1500 magnification, respectively
Rasterelektronenmikroskopische (REM) Aufnahmen von normalem Schmelz (a,b), Schmelz mit Fluorose (c,d) nach Reinigung und Politur. Vergr. 500:1 bzw. 1500:1
Statistical analyses
The statistical analyses were done with the SPSS software (version 25, IBM, Armonk, NY, USA). The Shapiro–Wilk test showed that data was not normally distributed so quantitative data were conveyed as the median and interquartile range (IQR). The nonparametric Kruskal–Wallis H test was used to identify significant differences among groups. The ARI scores were compared using the Χ2 test. The significance level was set at P < 0.05.
Results
There was a statistically significant difference (p = 0.002) in the SBS among the four groups; pairwise comparisons showed a significantly lower shear bond strength of group II (fluorosed premolars subjected to deproteinization before acid etching) than in the other three groups (Table 2).
ARI scores were significantly different among the four groups (p = 0.006); pairwise comparisons showed that group III (fluorosed premolars not subjected to any treatment other than acid etching) was significantly different from group I and group IV specifically in ARI scores 1 and 3 (Table 3).
In the SEM images (×500 and ×1500), normal enamel appeared regular and considerably smooth (Fig. 2a,b), while fluorosed enamel appeared pitted and irregular with pores of different sizes and shapes scattered unevenly throughout (Fig. 2c,d). Normal enamel etched with 37% phosphoric acid showed large areas of type 1 and type 2 etching patterns with the typical histological appearance of enamel prisms (Fig. 3a,b), while etched fluorosed enamel showed a blend of type 2 and type 3 etching patterns with some areas showing type 4 pattern (Fig. 3c,d). After debonding, normal enamel (Fig. 4a,b) and fluorosed enamel (Fig. 4c,d) appeared affected by the removal of the remnant composite. After air abrasion with aluminum oxide, fluorosed enamel appeared as dull, rough-pitted surface (Fig. 5a,b), whereas etching after air abrasion resulted in type 4 etching pattern (Fig. 5c,d). When deproteinized with NaOCl, enamel appeared covered with remnants (Fig. 6a,b), and acid etching resulted in type 4 etching pattern with some areas appearing unetched (Fig. 6c,d). Fluorosed enamel in the three groups included varying degrees of unetched areas. The histological appearances of the different etching patterns were described in other research papers [26]. The debonded enamel (Fig. 4; Fig. 5e,f; Fig. 6e,f) showed remnants of composite on the surface. However, the smoothness of the surface after cleaning was different among the groups.
Scanning electron microscope (SEM) images of fluorosed enamel after air abrasion (a,b), after air abrasion followed by acid etching (c,d), and after debonding (e,f). ×500 and ×1500, respectively
Rasterelektronenmikroskopische (REM) Aufnahmen von Schmelz mit Fluorose nach Luftabrasion (a,b), nach Luftabrasion und anschließender Säureätzung (c,d) sowie nach Bracket-Debonding (e,f). Vergr. 500:1 bzw. 1500:1
Scanning electron microscope (SEM) images of fluorosed enamel after treatment with NaOCl (a,b), after treatment with NaOCl followed by acid etching (c,d), and after debonding (e,f). ×500 and ×1500, respectively
Rasterelektronenmikroskopische (REM) Aufnahmen von Schmelz mit Fluorose nach Behandlung mit NaOCl (a,b), nach Behandlung mit NaOCl mit anschließender Säureätzung (c,d) und nach Debonding (e,f). Vergr. 500:1 bzw. 1500:1
Discussion
The present research study shows median SBS among the groups that could be described as comparable to the bond strength range recommended for orthodontic purposes; however, the minimum, maximum, and IQR values show wide variations (Table 2). It has been suggested that obtaining bond strength values ranging from about 6–8 MPa is sufficient to ensure good clinical performance [27]. While very low bond strength would lead to high bracket failures, very high bond strength is not desirable in orthodontics. Bond strength is required to be just high enough to withstand the intraoral stresses; higher bond values may induce enamel fracture during bracket debonding and have consequences for the removal of the adhesive remnant at the end of the orthodontic treatment [28]. It also worth noting that reporting average values implies evenly distributed stresses; however, the debonding loading commonly produces uneven stress distribution and fracture starts at areas of peak stress [29, 30].
Comparing the groups showed similar bond strength for fluorosed and normal enamel when enamel was conditioned with phosphoric acid only without any other treatment. While these findings are in agreement with some previous studies [8, 9] that found no significant differences between SBS values when fluorosed and normal enamel were compared, they disagree with others [10,11,12,13, 21] where SBS to fluorosed enamel was significantly lower than to normal enamel.
In 2008, Espinosa et al. [20] showed that the use of 5.25% NaOCl for deproteinization before acid etching eliminated the organic substances from the enamel surface and thus increased the total etched area that was predominantly of type 1 and type 2 etching pattern. The increase in the etched area and the improvement in the quality of the etching pattern should increase bond strength. Sharma et al. [21] published similar results. Deproteinization with 5.25% NaOCl before acid etching increased bond strength of brackets to fluorosed enamel and etching patterns of type 1 and type 2 were predominant. Contrary to Espinosa et al., Ahuja et al. [31] reported in 2010 similar etching patterns in the two groups where the etching was done with 37% phosphoric acid in only one group, while in the other acid etching was preceded by deproteinization with 5.25% NaOCl. In alignment with these findings, studying the effect of deproteinization on SBS of composite resin to the enamel of permanent [32] or to the enamel of primary, and immature and mature permanent [33] teeth yielded that deproteinization with 5% NaOCl for enamel surface before etching with 37% phosphoric acid did not increase the bond strength.
In the present research study, group II (fluorosed premolars subjected to deproteinization before acid etching) showed lower bond strength compared to the other three groups. Contradictory results of different studies [20, 21, 31, 32] including the current study dictate further research on the effects of the deproteinization technique if applied in combination with different materials and on surfaces with variable morphology and degrees of fluorosis.
Searching for an explanation, previous studies [34, 35] investigated the pH of dentin surfaces deproteinized with NaOCl and found significantly higher pH values than the pH of untreated dentin surfaces even after rinsing with copious amounts of water for enough time. Thus, an alkaline effect of NaOCl seems to decrease the acidity of the etching acid. Another factor that could influence the effect of NaOCl is its high reactivity with the amino acids of the organic component that it targets. This reaction makes it highly resistant to washing which might further act on compromising the bond strength [36]. Thus, the possible effects of NaOCl on the pH of the etching acid and also on the adhesion of the organic components to the enamel during orthodontic bracket bonding should be investigated further. The SEM images in the present research study showed that the enamel after deproteinization with NaOCl was covered with remnants that may cause the enamel to resist etching. When etching enamel for bonding orthodontic brackets, these two factors would compromise the etching effect and subsequently decrease the bond strength. For the future, to support/undermine these explanations, it is recommended to conduct electron diffraction spectroscopy (EDS) analysis for chemical characterization of the enamel surface after each step of the bonding procedure.
The data distribution of the present research study showed variations implying deproteinization being a sensitive technique that needs further investigation. The sensitivity of the technique may lie in the method of application, time of application, washing and drying techniques, or even the material used for deproteinization itself. Different materials and concentrations have been tried for deproteinizing [37].
Silva-Benitez et al. [16] found that air abrasion of enamel followed by acid etching provided adequate bond strength, but did not improve this in case of moderate fluorosis. The air abrasion protocol used in the present research study resulted in a bond strength similar to that of normal and fluorosed enamel etched with phosphoric acid only. Suma et al. [38] found that combining air abrasion with acid etching created greater SBS than acid etching alone in moderate-to-severe dental fluorosis regardless of the adhesion system used. Different reasons might explain the increased bond strength with the use of air abrasion. When the enamel surface is subjected to air abrasion, it becomes a more rough-irregular surface which would increase the surface area for bonding and, thus, improve bond strength. Another likely effect of air abrasion is the removing of the outer highly mineralized layer of the fluorosed enamel which is composed of fluorapatite crystals and aprismatic enamel. Removing this acid-resistant layer might improve the result of the acid etching on the core enamel [39]. The specifications of an air abrasion protocol such as the size of the aluminum oxide particles, the level of air pressure, the duration of abrasion, and the distance between the abrasive device and the enamel surface might be the factors responsible for variations between study results. When comparing the air abrasion group with the deproteinization group, the present research study found higher bond strength with air abrasion than with deproteinization.
The main difference in brackets’ failure site between groups was seen in the fluorosed enamel group that had much higher numbers of ARI score 1 and much lower numbers of ARI score 3 indicating predominance of failures at the enamel–composite interface. Also, the group of fluorosed enamel treated with NaOCl was significantly different from the normal enamel group, and the group of fluorosed enamel treated with air abrasion presented as well higher numbers of ARI score 1 and lower numbers of ARI score 3. Differences in the distribution of ARI scores among groups do not necessarily reflect differences in bond strength [40,41,42,43] and should thus not influence the interpretation of the results related to bond strength. The interest in the ARI scores resides in the importance of the step of cleaning the enamel surface from the adhesive remnant after bracket debonding. The preferred site of failure whether at the enamel–resin interface, bracket–adhesive interface, or within the adhesive is discussed controversially regarding needed chair time for debonding and preservation of the enamel [28, 44,45,46,47,48]. The bond between composite and enamel is a mechanical bond formed by the composite infiltrating the pores formed by acid etching of the enamel. Cleaning and polishing are required after debonding to remove composite remnants and return the enamel to the original state. SEM images from the four groups for enamel after debonding in the present research study showed altered enamel evident by composite remnant still present even after the attempts to remove it with a carbide bur. Previous studies using SEM images to evaluate the enamel after bracket debonding and removal of the composite remnants reported the presence of composite remnants even after the use of different methods to clean the enamel [49].
In vitro studies bear limitations. Laboratory simulations cannot exactly replicate the oral conditions [50]. However, in vitro studies are the trailblazers for future clinical trials. Additionally, no study, including the present one, included the degree of fluorosis as an independent variable. Usually, teeth with a certain degree of fluorosis that could be mild, moderate, or severe as indicated by fluorosis scores are included in a study. The present research study tested only premolars. Different results could be obtained when testing teeth of different morphology [51].
Conclusions
-
The bond strength of all groups was within the acceptable range for orthodontic purposes (6–8 MPa).
-
Treating fluorosed enamel with air abrasion before acid etching did not increase bond strength.
-
Fluorosed premolars subjected to deproteinization showed the lowest bond strength. Further studies are recommended to scrutinize the deproteinization technique using different materials on different surface morphologies and degrees of fluorosis.
References
Clark MB, Slayton RL (2014) Fluoride use in caries prevention in the primary care setting. Pediatrics 134:626–633
Denbesten P, Wu L (2011) Chronic fluoride toxicity: dental fluorosisrter. Monogr Oral Sci 22:81–96
Aoba T, Fejerskov O (2002) Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med 13:155–170
Bronckers ALJJ, Lyaruu DM, DenBesten PK (2009) The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res 88:877–893
Dean HT (1934) Classification of mottled enamel diagnosis. J Am Dent Assoc 21:1421–1426
Thylstrup A, Fejerskov O (1978) Clinical appearance of dental fluorosis inpermanent teeth in relation to histologic changes. Community Dent Oral Epidemiol 6:315–328
Horowitz HS, Driscoll WS, Meyers RJ, Heifetz SB, Kingman A (1984) A new method for assessing the prevalence of dental fluorosis—the tooth surface index of fluorosis. J Am Dent Assoc 109:37–41
Isci D, Sahin Saglam AM, Alkis H, Elekdag-Turk S, Turk T (2011) Effects of fluorosis on the shear bond strength of orthodontic brackets bonded with a self-etching primer. Eur J Orthod 33:161–166
Bakhadher W, Talic N, Al Hezaimi K (2015) Bond strength and micro-computed tomographic evaluation of pre-coated brackets. Aust Orthod J 31:201–207
Gungor AY, Turkkahraman H, Adanir N, Alkis H (2009) Effects of fluorosis and self etching primers on shear bond strengths of orthodontic brackets. Eur J Dent 3:173–177
Adanir N, Türkkahraman H, Güngör AY (2007) Effects of fluorosis and bleaching on shear bond strengths of orthodontic brackets. Eur J Dent 1:230–235
Adanir N, Türkkahraman H, Yalçin Güngör A (2009) Effects of adhesion promoters on the shear bond strengths of orthodontic brackets to fluorosed enamel. Eur J Orthod 31:276–280
Trakinienė G, Petravičiūtė G, Smailienė D, Narbutaitė J, Armalaitė J, Lopatienė K et al (2019) Impact of fluorosis on the tensile bond strength of metal brackets and the prevalence of enamel microcracks. Sci Rep 9:5957
Denbesten PK, Thariani H (1992) Biological mechanisms of fluorosis and level and timing of systemic exposure to fluoride with respect to fluorosis. J Dent Res 71:1238
Najafi HZ, Moshkelgosha V, Khanchemehr A, Alizade A, Mokhtar A (2015) The effect of four surface treatment methods on the shear bond strength of metallic brackets to the fluorosed enamel. J Dent (Shiraz) 16:251–259
Silva-Benítez EL, Zavala-Alonso V, Martinez-Castanon GA, Loyola-Rodriguez JP, Patiño-Marin N, Ortega-Pedrajo I et al (2013) Shear bond strength evaluation of bonded molar tubes on fluorotic molars. Angle Orthod 83:152–157
Gaur A, Maheshwari S, Verma SK, Tariq M (2016) Effects of adhesion promoter on orthodontic bonding in fluorosed teeth: a scanning electron microscopy study. J Orthod Sci 5:87–91
Grandini S, Balleri P, Ferrari M (2002) Evaluation of glyde file prep in combination with sodium hypoclorite as a root canal irrigant. J Endod 28:300–303
Ercan E, Ozekinci T, Atakul F, Gul K (2004) Antibacterial activity of 2 % chlorhexidine gluconate and 5.25 % sodium hypochlorite in infected root canal: In vivo study. J Endod 30:84–87
Espinosa R, Valencia R, Uribe M, Ceja I, Saadia M (2008) Enamel deproteinization and its effect on acid etching: an in vitro study. J Clin Pediatr Dent 33:13–19
Sharma R, Verma M (2017) Deproteinization of fluorosed enamel with sodium hypochlorite enhances the shear bond strength of orthodontic brackets: an in vitro study. Contemp Clin Dent 8:20–25
Montasser MA, Taha M (2014) Effect of enamel protective agents on shear bond strength of orthodontic brackets. Prog Orthod 15:34
Montasser MA, Drummond JL (2009) Reliability of the adhesive remnant index score system with different magnifications. Angle Orthod 79:773–776
Årtun J, Bergland S (1984) Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod 85:333–340
Gwinnett AJ (1971) Histologic changes in human enamel following treatment with acidic adhesive conditioning agents. Arch Oral Biol 16:731–738
Galil KA, Wright GZ (1979) Acid etching patterns on buccal surfaces of permanent teeth. Pediatr Dent 1:230–234
Reynolds IR (1975) A review of direct orthodontic bonding. Br J Orthod 2:171–178
Al Shamsi A, Cunningham JL, Lamey PJ, Lynch E (2006) Shear bond strength and residual adhesive after orthodontic bracket debonding. Angle Orthod 76:694–699
Katona TR (1997) A comparison of the stresses developed in tension, shear peel, and torsion strength testing of direct bonded orthodontic brackets. Am J Orthod Dentofacial Orthop 112:244–251
Algera TJ, Feilzer AJ, Prahl-Andersen B, Kleverlaan CJ (2011) A comparison of finite element analysis with in vitro bond strength tests of the bracket-cement-enamel system. Eur J Orthod 33:608–612
Ahuja B, Yeluri R, Baliga S, Munshi AK (2010) Enamel deproteinization before acid etching—a scanning electron microscopic observation. J Clin Pediatr Dent 35:169–172
Harleen N, Ramakrishna Y, Munshi AK (2011) Enamel deproteinization before acid etching and its effect on the shear bond strength—an in vitro study. J Clin Pediatr Dent 36:19–23
Aras S, Küçükeçmen HC, Öaroğlu SI (2013) Deproteinization treatment on bond strengths of primary, mature and immature permanent tooth enamel. J Clin Pediatr Dent 37:275–279
Taniguchi G, Nakajima M, Hosaka K, Iwamoto N, Ikeda M, Foxton RM et al (2009) Improving the effect of NaOCl pretreatment on bonding to caries-affected dentin using self-etch adhesives. J Dent 37:769–775
Kunawarote S, Nakajima M, Shida K, Kitasako Y, Foxton RM, Tagami J (2010) Effect of dentin pretreatment with mild acidic HOCl solution on microtensile bond strength and surface pH. J Dent 38:261–268
Guentzel JL, Liang Lam K, Callan MA, Emmons SA, Dunham VL (2008) Reduction of bacteria on spinach, lettuce, and surfaces in food service areas using neutral electrolyzed oxidizing water. Food Microbiol 25:36–41
Hamama HHH (2018) Effect of smear layer deproteinization on bonding of self-etch adhesives to dentin: a systematic review and meta-analysis. Restor Dent Endod 42:1–16
Suma S, Chandra Shekar BR, Anita G, Kallury A (2012) The effect of air abrasion on the retention of metallic brackets bonded to fluorosed enamel surface. Indian J Dent Res 23:230–235
Patcas R, Zinelis S, Eliades G, Eliades T (2015) Surface and interfacial analysis of sandblasted and acid-etched enamel for bonding orthodontic adhesives. Am J Orthod Dentofacial Orthop 147:S64–75
Delport A, Grobler SR (1988) A laboratory evaluation of tensile bond strength of some orthodontic bonding resins to enamel. Am J Orthod Dentofacial Orthop 93:133–137
Buyukyilmaz T, Usumez S, Karaman AL (2003) Effect of self-etching primer on bond strength—are they reliable? Angle Orthod 73:64–70
Cacciafesta V, Sfondrini MF, De Angelis M, Scribante A, Klersy C (2003) Effect of water and saliva contamination on shear bond strength of brackets bonded with conventional, hydrophilic, and self-etching primers. Am J Orthod Dentofacial Orthop 123:633–640
Zeppieri IL, Chung CH, Mante FK (2003) Effect of saliva on shear bond strength of an orthodontic adhesive used with moisture-insensitive and self-etching primers. Am J Orthod Dentofacial Orthop 124:414–419
Britton JC, McInnes P, Weinberg R, Ledoux WR, Retief DH (1998) Shear bond strength of ceramic orthodontic brackets to enamel. Am J Orthod Dentofacial Orthop 114:243–247
Bishara SE, VonWald L, Laffoon JF, Jacobsen JR (2000) Effect of altering the type of enamel conditioner on the shear bond strength of a resin-reinforced glass ionomer adhesive. Am J Orthod Dentofacial Orthop 118:288–294
Guan G, Takano-Yamamoto T, Miyamoto M, Hattori T, Ishikawa K, Surzuki K (2000) Shear bond strengths of orthodontic plastic brackets. Am J Orthod Dentofacial Orthop 117:438–443
Mui B, Rossouw PE, Kulkarni GV (1999) Opitimization of a procedure for rebonding dislodged orthodontic brackets. Angle Orthod 69:276–281
Scougall-Vilchis RJ, Zárate-Díaz C, Kusakabe S, Yamamoto K (2010) Bond strengths of different orthodontic adhesives after enamel conditioning with the same self-etching primer. Aust Orthod J 26:84–89
Claudino D, Kuga MC, Belizário L, Pereira JR (2018) Enamel evaluation by scanning electron microscopy after debonding brackets and removal of adhesive remnants. J Clin Exp Dent 10:e248–e251
Atabek Ş, Özden AN (2019) Comparison of the effect of proanthocyanidin surface treatments on shear bond strength of different cements. Materials (Basel) 12:2676
Oztürk B, Malkoç S, Koyutürk AE, Catalbas B, Ozer F (2008) Influence of different tooth types on the bond strength of two orthodontic adhesive systems. Eur J Orthod 30:407–412
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Basalamah, A. Maher, A.H. Whba, A. Scribante, M.F. Sfondrini and M.A. Montasser declare that they have no competing interests.
Ethical standards
The study was approved by the Research Ethics Committee of the Faculty of Dentistry, Mansoura University (number 17050618).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basalamah, A., Maher, A., Whba, A.H. et al. Effects of fluorosed enamel on orthodontic bracket bonding. J Orofac Orthop 84, 88–99 (2023). https://doi.org/10.1007/s00056-021-00342-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-021-00342-x