Abstract
As is typical of chemically defended animals, poison frogs present high variability in their alkaloid-based defenses. Previous studies have shown that geographically separated color morphs of Oophaga and Dendrobates species differ in both alkaloid composition and arthropod palatability. Here, we tested the generality of that finding by studying the alkaloid composition and palatability of geographically separated blue and orange morphs of the splash-backed poison frog, Adelphobates galactonotus. We identified and quantified the alkaloid composition of each individual frog using gas chromatography–mass spectrometry and evaluated the palatability of individual secretions to arthropods conducting feeding trials with Drosophila melanogaster. Despite their conspicuous differences in color and separation on opposite sides of a large aquatic barrier, the two morphs did not differ in alkaloid composition or palatability. This result shows that both color morphs are equally chemically protected and suggests that the color variation is not driven by predator selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poison frogs have evolved the ability to sequester defensive alkaloids from dietary arthropods (Saporito et al. 2009, 2012). As is typical of chemically defended animals (Speed et al. 2012), the alkaloid-based defenses of poison frog skin secretions are highly variable within species and even among individuals of the same population (e.g. Daly et al. 2008; Jeckel et al. 2015a). Alkaloid variation is related to multiple factors, including genetic or epigenetic differences in uptake (Daly et al. 2003; Hantak et al. 2013), availability of alkaloid-containing prey (Daly et al. 1994), size and abundance of granular glands (Saporito et al. 2010a), age (Jeckel et al. 2015b), sex (Saporito et al. 2010b), season (Saporito et al. 2006), habitat type (Andriamaharavo et al. 2010), and geographic location (e.g. Saporito et al. 2006, 2007a; Daly et al. 2008).
Alkaloid variation has also been associated with variation in skin coloration in poison frogs. Most poison frog species present gaudy, presumably aposematic coloration, and chromatic polytypism is common in this group (e.g. Silverstone 1975; Myers and Daly 1976; Brusa et al. 2013; Hoogmoed and Ávila-Pires 2012; Patrick and Sasa 2009; Noonan and Comeault 2008). Variation in alkaloid composition among polytypic populations has been studied most extensively in Oophaga pumilio, a dendrobatid poison frog distributed in lowland rainforests of the Caribbean slope in southern Nicaragua, Costa Rica, and northwestern Panama (Frost 2019). Throughout most of its range, populations of O. pumilio are similar in color, but the insular populations of the Bocas del Toro Archipelago, Panama are characterized by highly localized chromatic polytypism. Populations located on different islands differ wildly in coloration (Maan and Cummings 2012), alkaloid profiles (Saporito et al. 2006), toxicity (Daly and Myers 1967; Maan and Cummings 2012), and palatability (Bolton et al. 2017). Similar findings have also been reported for other polytypic species of Oophaga, such as O. histrionica (Myers and Daly 1976) and O. granulifera (Wang 2011).
Although Oophaga is the most well-studied polytypic genus of poison frog, chromatic polytypism also occurs in several other poison frog lineages and is especially common among the toothless dendrobatines (Dendrobatini; Grant et al. 2017) of the ADO clade, composed of Adelphobates, Dendrobates, and Oophaga (Grant 2019). Lawrence et al. (2019) recently found that two morphs of the chromatically polytypic species D. tinctorius differ in both alkaloid composition and palatability, thereby matching previous findings in Oophaga. However, no studies have examined polytypic species of Adelphobates. As such, to test the generality of findings in Oophaga and Dendrobates in this clade, we investigated the defensive alkaloids of two geographic color morphs of the splash-backed poison frog, Adelphobates galactonotus, a chromatically polytypic species distributed south of the Amazon River in Brazil (Hoogmoed and Ávila-Pires 2012). Specifically, to determine if geographically separated color morphs differ in skin alkaloid composition and palatability, we compared the alkaloid composition and arthropod palatability of secretions from the blue morph, known exclusively from the eastern side of Caxiuanã Bay, and the widespread orange morph, collected on the western side of the bay.
Materials and methods
Sample collection
We collected adult individuals of A. galactonotus in January 2017 in Pará state, Brazil (Fig. 1), including 5 (2 males, 3 females) of the orange morph collected on the western side of Caxiuanã Bay at a locality inside a protected area (Caxiaunã National Forest, 1°48′16.87ʺ S, 51°26′45.31ʺ W), and 5 (2 males, 3 females) of the blue morph collected on the eastern side of the bay, near riverside plantations (1°57′43ʺ S, 51°25′09ʺ W). We based our sample size on the results of previous studies that analyzed differences in alkaloid composition among populations (Saporito et al. 2006, 2007a; Andriamaharavo et al. 2010; Grant et al. 2012). To avoid interference of anesthetics commonly used to euthanize amphibians (Saporito and Grant 2018), we euthanized frogs by cooling followed by flash freezing in liquid nitrogen (e.g. Lillywhite et al. 2017). Following euthanasia, we removed and weighed the entire skin to 0.1 mg and examined gonads to confirm sex and maturity. We stored skins in individual 4-mL glass vials containing 100% methanol and sealed with Teflon-coated lids and deposited specimens in the amphibian collection of the Museum of Zoology of the University of São Paulo under voucher numbers MZUSP A158924–33.
Alkaloid extract preparation
We isolated alkaloids from individual methanol extracts using an acid–base extraction following Saporito et al. (2010b) and Jeckel et al. (2015a). For each individual frog skin, we performed two extractions: one for alkaloid analysis and another for palatability assays. For the extractions used in alkaloid analyses, we added 100 μL of nicotine (10 μg nicotine/100 μL methanol) as an internal standard and resuspended the alkaloids in 100 μL of 100% methanol. For the extractions used in palatability assays, we resuspended the alkaloids in 100 μL of 20% sucrose/50% ethanol solution without adding nicotine.
Alkaloid identification and quantification
We identified alkaloids by comparing the observed mass spectrometry (MS) properties and gas-chromatography (GC) retention times (Rt) with those of previously reported anuran alkaloids (e.g. Daly et al. 2005). Most anuran alkaloids have been assigned code names that consist of a bold-face number corresponding to the nominal mass and a bold-face letter to distinguish alkaloids of the same nominal mass (Daly et al. 2005). We tentatively identified isomers of previously characterized alkaloids on the basis of their electron impact (EI) and chemical ionization (CI) mass spectral data and GC retention times. Following the methods of Garraffo et al. (2012), we considered alkaloids to be new isomers if they shared identical EI–MS data with a previously identified alkaloid but differed in Rt by at least 0.15 min (Daly et al. 2005). We analyzed each individual frog skin extract in three chromatographic replicates and determined the average quantity of defensive compounds by comparing the observed alkaloid peak areas to the peak area of the nicotine internal standard, using Varian MS Workstation v.6.9 SPI.
Palatability test
In addition to visually oriented vertebrate predators, chemically oriented arthropods also predate poison frogs (Fritz et al. 1981; Szelistowski 1985; Gray et al. 2010; Santos and Cannatella 2011; Stynoski et al. 2014a, b; Murray et al. 2016). Drosophila melanogaster is commonly used as a model to study arthropod taste perception and specifically to understand alkaloid perception by arthropods (Devambez et al. 2013; Lee et al. 2015; Meunier et al. 2003; Sellier et al. 2011), making it a suitable proxy to assess how arthropod predators might perceive variation in alkaloid defenses (Bolton et al. 2017).
To evaluate the palatability of A. galactonotus secretions to arthropods, we conducted feeding trials in which common fruit flies (Drosophila melanogaster) were allowed the option to feed on two different sucrose solutions (Bolton et al. 2017). In this assay, we added red food coloring to the control solutions (sucrose without alkaloids) and blue food coloring to the treatment solutions (sucrose with alkaloids) to distinguish between feeding preferences during trials. Previous studies have used D. melanogaster in multiple choice feeding trials and have demonstrated that fruit flies show no preference for different colored solutions (Meunier et al. 2003; Sellier et al. 2011; Bolton et al. 2017). Fruit fly abdomens are transparent, which enabled us to determine which colored solution they fed on or if they consumed a mixture of both colored solutions.
Following the procedures of Bolton et al. (2017), we made two stock solutions for use in the palatability assays, one for the control solution (no alkaloids) and one for the treatment solution (alkaloids). Each stock solution contained 20 mL of 20% sucrose/50% ethanol. For the control solution, we added 100 µL of red food coloring (Market Pantry®) to one stock solution. For the alkaloid treatment solution, we added 50 µL of blue food coloring (Market Pantry®) to the other stock solution. We ran separate experiments for each of the 10 frog skins so that each treatment solution reflected an individual frog’s naturally occurring alkaloid defenses. To determine if alkaloid palatability is dose-dependent, we tested three alkaloid concentrations for each individual frog, comprising, respectively, 2.5%, 1.25%, and 0.625% of the total quantity of the alkaloids present in each individual frog skin samples.
Each fruit fly palatability assay used 10 individual D. melanogaster (wingless, wild type, Carolina Science) that were 3–11 days old (average 5 days), grown on standard fruit fly media (Formula 4–24® Plain, Carolina Science), and starved for 24 h prior to the experiment. We placed these 10 fruit flies in a 9-cm Petri dish (Fisherbrand, 100 mm × 15 mm, sterile, Polystyrene) lined with filter paper dampened with deionized water (to provide moisture for the fruit flies) and containing 10 µL each of the control and treatment solutions on plastic cover slips (22 mm Fisherbrand® 2R Plastic Cover Slips). Following the methods of previous studies (Sellier et al. 2011; Devambez et al. 2013; Bolton et al. 2017), we allowed the fruit flies to feed on the solutions for 2 h in the dark, and then euthanized them by freezing.
To quantify feeding preference, we used a dissecting microscope to examine the fruit flies and counted the individuals with red, blue, and purple (mixed) solutions in their abdomens. From this count, we calculated a palatability index for each assay to determine the relative palatability of each alkaloid solution. The palatability index is a value that ranges from − 1 to + 1, where zero and positive values represent a palatable alkaloid solution and negative values indicate an unpalatable alkaloid solution relative to the control (Bolton et al. 2017). This index was calculated as followed: (# of blue fruit flies−# of red fruit flies − 0.5 * # of purple fruit flies)/(total # of fruit flies). We included each alkaloid extract from an individual frog in four independent replicate assays at each of the three concentrations (n = 12 for each individual frog skin extract).
Statistical analysis
We used non-metric multidimensional scaling (nMDS) to visualize and compare alkaloid composition (richness, type, and quantity of alkaloids) and one-way analysis of similarity (ANOSIM) to test for differences. Both nMDS and ANOSIM analyses were based on Bray–Curtis similarity matrices. We tested for differences in the quantity and richness of sequestered alkaloids between color morphs, sizes (SVL and mass), and sexes using Wilcoxon rank sum tests, and examined the relationship between alkaloid quantity and richness using linear regression. To test if frog alkaloids were considered unpalatable to fruit flies at each of the three concentrations, we performed one-tailed independent samples t-tests. Palatability index scores of zero or greater are considered palatable, and therefore average palatability indices for frogs were compared to a hypothesized mean of zero (Dyer et al. 2003; Bolton et al. 2017). To determine if there was a dose response in palatability among alkaloid concentrations, we used linear regression. To test for differences in alkaloid palatability, we performed an independent samples t-test. To examine the relationship between palatability and alkaloid quantity and alkaloid richness, we used linear regression. nMDS and ANOSIM were performed in PRIMER-E version 6, comparisons of alkaloid composition between morphs and sexes were performed using the statistical package R-3.6.0 (R Core Team 2019), and statistical analyses for the palatability assays were conducted using GraphPad Prism Software version 8.0.0 for Windows.
Results
Alkaloid composition
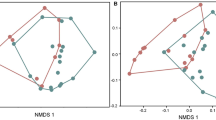
Alkaloid composition did not differ significantly between the two color morphs of A. galactonotus (alkaloid quantity [W = 15, p = 0.69], skin mass corrected alkaloid quantity [W = 14, p = 0.84], richness [W = 14, p = 0.84], and total composition analysis [Global R = 0.12, p = 0.198]; Fig. 2a–d). The total number and quantity of dietary alkaloids varied among individual skin extracts, including among individuals of the same population (Table 1). Females and males did not differ in size (W = 18, p = 0.26), and there was no difference in alkaloid quantity (W = 12, p = 1), skin mass corrected alkaloid quantity (W = 11, p = 0.91), and richness (W = 19, p = 0.17; Fig. 2e–f). Total quantity and richness of alkaloids were not significantly related (F1,8 = 4.5, R2 = 0.4, p = 0.07), even when corrected by wet skin mass (F1,8 = 2.4, R2 = 0.2, p = 0.16).
Comparison of alkaloid composition between morphs and sexes of Adelphobates galactonotus. a nMDS plot of alkaloid composition between blue and orange morphs. Each circle represents an individual frog, and the distance between symbols represents the relative difference in alkaloid composition. The diameter of each circle is proportional to the quantity of alkaloids present in that frog (μg per frog skin). b–d Comparison of alkaloid quantity (μg), mass corrected alkaloid quantity, and alkaloid richness, respectively, between blue and orange morphs. e–g Comparison of alkaloid quantity (μg), mass corrected alkaloid quantity, and alkaloid richness, respectively, between males and females of both color morphs
We identified 89 alkaloids (including isomers) representing 16 structural classes (Table 2). Seven alkaloids are new, and we also identified several tentatively new isomers of previously characterized alkaloids. The MS data and Rt for all seven new alkaloids are shown in Supplementary material 1 and Rt for all of the new isomers are included in Supplementary material 2.
Overall, the most abundant alkaloid in A. galactonotus was histrionicotoxin (HTX) 259A (348.8 ± 413.1 μg per skin), with 3 times the amount of the second most abundant alkaloid, HTX 261A (103.6 ± 81.5 μg per skin). Both alkaloids were present in all individuals of both color morphs. Allopumiliotoxin (aPTX) 337D and 5,6,8-indolozidine (5,6,8-I) 259C were also present in all individuals of both populations, but in smaller amounts (26.1 ± 22.5 μg and 52.1 ± 43.7 μg per skin, respectively). 5,6,8-I 231B and 249C, aPTX 305A, decahydroquinoline (DHQ) trans-243A, HTX 285B and Izidine 211C were present in all but one individual, and aPTX 323B was present in all but two individuals.
Among the 89 alkaloids in total, 46 are shared between the two populations. Among the 26 alkaloids that are unique to the blue morph, 17 are present in only one individual, 6 are present in two individuals and 2 are present in three individuals. The only alkaloid present exclusively in all individuals of the blue morph is DHQ 5-epi-trans-243A, which is an isomer of DHQ trans-243A found in all individuals of both morphs. In the orange morph, we found 15 unique alkaloids, 10 of which are present in only one individual, 3 in two individuals, and 2 in three individuals. All alkaloids present in each of the color morphs are listed in Table 2 (alkaloids and quantities for each individual are provided in Supplementary material 3). Although only 46 of the 89 alkaloids are shared among the two populations, the amount of the exclusive alkaloids in each color morph add up to only 7.2% of the total alkaloids found in the blue morph population and 3.2% of the total alkaloids in the orange morph population.
Palatability test
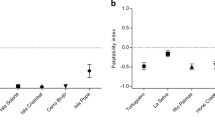
Frog alkaloids were significantly unpalatable to fruit flies at all three concentrations (p ≤ 0.001 for all comparisons). There was no statistically significant dose response in palatability among concentrations for either morph (Orange: F1,13 = 3.43, p = 0.087; Blue: F1,13 = 1.37, p = 0.264); however, on average, the higher concentrations of alkaloids were more unpalatable (Fig. 3). Being conservative, the lowest concentration of 0.6% was used for all of the remaining analyses. There were no significant differences in palatability between the orange and blue populations of A. galactonotus (t = 0.43, p = 0.681; Fig. 4). There was no relationship between alkaloid palatability and alkaloid quantity for either morph (Orange: F1,3 = 5.27, p = 0.106, R2 = 0.637; Blue: F1,3 = 0.477, p = 0.539, R2 = 0.137; Fig. 5a) or alkaloid richness (Orange: F1,3 = 0.763, p = 0.447, R2 = 0.203; Blue: F1,3 = 1.43, p = 0.318, R2 = 0.322; Fig. 5b); however, there was a trend towards a decrease in palatability with an increase in alkaloid quantity and richness (Fig. 5).
Discussion
In the only previous study that examined the alkaloids of wild-caught A. galactonotus, Daly et al. (2009) analyzed a single specimen from Tucuruí (reported as “Tucurvi”), Pará. Although Daly et al. (2009) did not provide color information, only orange frogs are known from that region (Hoogmoed and Ávila-Pires 2012). Among the four alkaloids we observed in all individuals, Daly et al. (2009) also detected HTX 259A (trace amount), HTX 261A (minor constituent), and 5,6,8-I 259C (major constituent); however, they did not detect any aPTX 337D. Daly et al. (2009) also reported HTX 291A, aPTX 253A and 267A, DHQ trans-243A, and 5,6,8-I 249C as major constituents; in our results, HTX 291A was present in both blue and orange populations, aPTX 253A and 267A were absent in both populations, and DHQ trans-243A and 5,6,8-I 249C were present in both populations. We did not perform statistical comparisons of the Tucuruí population with the blue and orange populations we studied because only one individual from that population has been analyzed. However, given that the orange morph has a broad distribution and additional morphs exist (Hoogmoed and Ávila-Pires 2012), future studies should compare multiple populations to test the generality of our findings in this species.
Alkaloid composition does not differ between the orange and blue populations of A. galactonotus, despite their conspicuously different coloration and geographic separation on opposite sides of a significant aquatic barrier. This result contrasts with those of previous studies of other species of poison frog that found significant differences (Saporito et al. 2006, 2007a; Daly et al. 2008; Grant et al. 2012; McGugan et al. 2016; Lawrence et al. 2019). The two main reasons for the lack of significant differences are: 1) the high amount of shared alkaloids between the populations, including the two most abundant alkaloids (HTX 259A and HTX 261A), and 2) the low amounts and occurrence of unshared alkaloids. The difference between results found in A. galactonotus and other species could be due to lack of variation in alkaloid-containing arthropods on either side of Caxiuanã Bay and variation among localities of other species. For example, in a study of Oophaga sylvatica, McGugan et al. (2016) attributed differences among populations to differences in arthropod availability. Unfortunately, data on arthropod availability are lacking for our study sites. Alternatively, the difference could be due to genetically or epigenetically determined alkaloid uptake being the same in orange and blue morphs of A. galactonotus but differing among populations of other poison frog species. The physiological and genetic aspects of alkaloid sequestration are not understood, but experiments suggest that they play a role in the variation of alkaloid composition (Daly et al. 1994, 2003; Hantak et al. 2013).
The unpalatability of alkaloid defenses in A. galactonotus is consistent with previous studies of alkaloid palatability in poison frogs (Schulte et al. 2017; Bolton et al. 2017; Lawrence et al. 2019). Given the lack of differences in alkaloid composition between blue and orange morphs, the lack of differences between the two color morphs in palatability is expected. Previous studies have found that conspicuousness of dorsal skin coloration in poison frogs is an honest indicator of alkaloid presence for visually oriented predators (Stuckert et al. 2014, 2018), and native predators are able to recognize and avoid aposematic coloration (e.g. Saporito et al. 2007b; Noonan and Comeault 2008). However, variation in visual cues and alkaloid levels is not necessarily correlated (Daly and Myers 1967; Wang 2011; Stuckert et al. 2014, 2018; Crothers et al. 2016; Bolton et al. 2017) and do not predict differences in predation risk (Hegna et al. 2011; Stuckert et al. 2014). In A. galactonotus, the orange morph is brighter than the blue morph (Rojas et al. 2015); however, the lack of differences in alkaloid composition and palatability illustrate that this difference in brightness and hue is not a qualitative indicator of toxicity in this species.
The lack of differences in the defensive chemicals and palatability of the orange and blue morphs of A. galactonotus suggests that the color polytypism in this species is not related to predation. Indeed, our results are consistent with previous findings that the frequency of attacks by vertebrates on paraffin models representing these two color morphs does not differ in either population (Rojas et al. 2015). Similarly, accumulation of dietary carotenoids has been shown to be unrelated to defensive chemicals in other poison frogs (e.g. Crothers et al. 2016), and captive breeding experiments with A. galactonotus have shown that orange and blue morphs are not diet dependent (AMJ and TG, unpublished data). Instead, we suggest that the different color morphs in A. galactonotus might be related to female mate preference. Nothing is known about mate preferences in this species; however, females of O. pumilio prefer males of the same color morph (Maan and Cummings 2008) through parental imprinting (Yang et al. 2019). If female A. galactonotus also imprint on parental coloration and prefer males of the same color morph, then assortative mating could be the main driver of the distinctive color polytypism in A. galactonotus.
References
Andriamaharavo NR, Garraffo HM, Saporito RA, Daly JW, Razafindrabe CR, Andriantsiferana M, Spande TF (2010) Roughing it: a mantellid poison frog shows greater alkaloid diversity in some disturbed habitats. J Nat Prod 73:322–330. https://doi.org/10.1021/np900721r
Bolton SK, Dickerson K, Saporito RA (2017) Variable alkaloid defense in the dendrobatid poison frog (Oophaga pumilio) are perceived as differences in palatability to arthropods. J Chem Ecol 43:273–289
Brusa O, Bellati A, Meuche I, Mundy NI, Pröhl H (2013) Divergent evolution in the polymorphic granular poison-dart frog, Oophaga granulifera: genetics, coloration, advertisement calls and morphology. J Biogeogr 40:394–408. https://doi.org/10.1111/j.1365-2699.2012.02786.x
Crothers L, Saporito RA, Yeager J, Lynch K, Friesen C, Richards-Zawacki CL, McGraw K, Cummings M (2016) Warning signal properties covary with toxicity but not testosterone or aggregate carotenoids in a poison frog. Evol Ecol 30:601–621. https://doi.org/10.1007/s10682-016-9830-y
Daly JW, Myers CW (1967) Toxicity of Panamanian poison frogs (Dendrobates): some biological and chemical aspects. Science 156:970–973
Daly JW, Secunda S, Garraffo HM, Spande TF, Wisnieski A, Cover JF Jr (1994) An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32:657–663
Daly JW, Garraffo HM, Spande TF, Clark VC, Ma J, Ziffer H, Cover JF (2003) Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates. Proc Natl Acad Sci USA 100:11092–11097. https://doi.org/10.1073/pnas.1834430100
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds. J Nat Prod 68:1556–1575. https://doi.org/10.1021/np0580560
Daly JW, Garraffo HM, Spande TF, Giddings LA, Saporito RA, Vieites DR, Vences M (2008) Individual and geographic variation of skin alkaloids in three species of Madagascan poison frogs (Mantella). J Chem Ecol 34:252–279. https://doi.org/10.1007/s10886-007-9396-9
Daly JW, Ware N, Saporito RA, Spande TF, Garraffo HM (2009) N-methyldecahydroquinolines: an unexpected class of alkaloids from Amazonian poison frogs (Dendrobatidae). J Nat Prod 72:1110–1114. https://doi.org/10.1021/np900094v
Devambez I, Ali Agha M, Mitri C, Bockaert J, Parmentier ML, Marion-Poll F, Grau Y, Soustelle L (2013) Gαo is required for L-Canavanine detection in Drosophila. PLoS One. https://doi.org/10.1371/journal.pone.0063484
Dyer LA, Dodson CD, Gentry G (2003) A bioassay for insect deterrent compounds found in plant and animal tissues. Phytochem Analysis 14:381–388. https://doi.org/10.1002/pca.734
Fritz G, Rand AS, dePamphilis CW (1981) The aposematically colored frog, Dendrobates pumilio, is distasteful to the large, predatory ant, Paraponera clavata. Biotropica 13:158–159
Frost DR (2019) Amphibian species of the world: an online reference. Version 6.0. American Museum of Natural History http://research.amnh.org/herpetology/amphibia/index.html. Accessed 12 Aug 2019
Garraffo HM, Andriamaharavo NR, Vaira M, Quiroga MF, Heit C, Spande TF (2012) Alkaloids from single skins of the Argentinian toad Melanophryniscus rubriventris (Anura, Bufonidae): an unexpected variability in alkaloid profiles and a profusion of new structures. SpringerPlus 1:51. https://doi.org/10.1186/2193-1801-1-51
Grant T (2019) Outgroup sampling in phylogenetics: severity of test and successive outgroup expansion. J Zool Syst Evol Res. https://doi.org/10.1111/jzs.12317
Grant T, Colombo P, Verrastro L, Saporito RA (2012) The occurrence of defensive alkaloids in non-integumentary tissues of the Brazilian red-belly toad Melanophryniscus simplex (Bufonidae). Chemoecology 22:169–178. https://doi.org/10.1007/s00049-012-0107-9
Grant T, Rada M, Anganoy-Criollo M, Batista A, Dias PH, Jeckel AM, Machado DJ, Rueda-Almonacid JV (2017) Phylogenetic systematics of Dart-Poison frogs and their relatives revisited (Anura: Dendrobatoidea). S Am J Herpetol 12:S1–S90. https://doi.org/10.2994/SAJH-D-17-00017.1
Gray HM, Kaiser H, Green DM (2010) Does alkaloid sequestration protect the green poison frog, Dendrobates auratus, from predator attacks? Salamandra 46:235–238
Hantak MM, Grant T, Reinsch S, Mcginnity D, Loring M, Toyooka N, Saporito RA (2013) Dietary alkaloid sequestration in a poison frog: an experimental test of alkaloid uptake in Melanophryniscus stelzneri (Bufonidae). J Chem Ecol 39:1400–1406. https://doi.org/10.1007/s10886-013-0361-5
Hegna RH, Saporito RA, Gerow KG, Donnelly MA (2011) Contrasting colors of an aposematic poison frog do not affect predation. Ann Zool Fenn 48:29–38
Hoogmoed MS, Ávila-Pires TCS (2012) Inventory of color polymorphism in populations of Dendrobates galactonotus (Anura: Dendrobatidae), a poison frog endemic to Brazil. Phyllomedusa 11:95–115
Jeckel AM, Grant T, Saporito RA (2015a) Sequestered and synthesized chemical defenses in the poison frog Melanophryniscus moreirae. J Chem Ecol 41:505–512. https://doi.org/10.1007/s10886-015-0578-6
Jeckel AM, Saporito RA, Grant T (2015b) The relationship between poison frog chemical defenses and age, body size, and sex. Front Zool 12:27. https://doi.org/10.1186/s12983-015-0120-2
Lawrence JP, Rojas B, Fouquet A, Mappes J, Blanchette A, Saporito RA, Bosque RJ, Courtois EA, Noonan BP (2019) Weak warning signals can persist in the absence of gene flow. Proc Nat Acad Sci USA. https://doi.org/10.1073/pnas.1901872116
Lee Y, Moon SJ, Wang Y, Montell C (2015) A drosophila gustatory receptor required for strychnine sensation. Chem Senses 40:525–533. https://doi.org/10.1093/chemse/bjv038
Lillywhite HB, Shine R, Jacobson E, Denardo DF, Gordon MS, Navas CA, Wang T, Seymour RS, Storey KB, Heatwole H, Heard D, Brattstrom B, Burghardt GM (2017) Anesthesia and euthanasia of amphibians and reptiles used in scientific research: should hypothermia and freezing be prohibited? Bioscience 67:53–61. https://doi.org/10.1093/biosci/biw143
Maan ME, Cummings ME (2008) Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 62:2334–2345. https://doi.org/10.1111/j.1558-5646.2008.00454.x
Maan ME, Cummings ME (2012) Poison frog colors are honest signals of toxicity, particularly for bird predators. Am Nat 179:1–14. https://doi.org/10.1086/663197
McGugan JR, Byrd GD, Roland AB, Caty SN, Kabir N, Tapia EE, Trauger SA, Coloma LA, Connell LAO (2016) Ant and mite diversity drives toxin variation in the little devil poison frog. J Chem Ecol 42:537–551. https://doi.org/10.1007/s10886-016-0715-x
Meunier N, Marion-Poll F, Rospars JP, Tanimura T (2003) Peripheral coding of bitter taste in Drosophila. J Neurobiol 56:139–152. https://doi.org/10.1002/neu.10235
Murray EM, Bolton SK, Berg T, Saporito RA (2016) Arthropod predation in a dendrobatid poison frog: does frog life stage matter? Zoology 119:169–174
Myers CW, Daly JW (1976) Preliminary evaluation of skin toxins and vocalizations in taxonomic and evolutionary studies of poison-dart frogs (Dendrobatidae). B Am Mus Nat Hist 157:173–262
Noonan BP, Comeault AA (2008) The role of predator selection on polymorphic aposematic poison frogs. Biol Lett 5:51–54. https://doi.org/10.1098/rsbl.2008.0586
Patrick LD, Sasa M (2009) Phenotypic and molecular variation in the green and black poison-dart frog Dendrobates auratus (Anura: Dendrobatidae) from Costa Rica. Rev Biol Trop 57:313–321
Rojas D, Stow A, Amézquita A, Simões PI, Lima AP (2015) No predatory bias with respect to colour familiarity for the aposematic Adelphobates galactonotus (Anura: Dendrobatidae). Behaviour 152:1–21. https://doi.org/10.1163/1568539X-00003297
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Santos JC, Cannatella DC (2011) Phenotypic integration emerges from aposematism and scale in poison frogs. Proc Natl Acad Sci USA 108:6175–6180. https://doi.org/10.1073/pnas.1010952108
Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW (2006) Geographic and seasonal variation in alkaloid-based chemical defenses of Dendrobates pumilio from Bocas del Toro, Panama. J Chem Ecol 32:795–814. https://doi.org/10.1007/s10886-006-9034-y
Saporito RA, Donnelly MA, Jain P, Garraffo HM, Spande TF, Daly JW (2007a) Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50:757–778. https://doi.org/10.1016/j.toxicon.2007.06.022
Saporito RA, Zuercher R, Roberts M, Gerow KG, Donnelly MA (2007b) Experimental evidence for aposematism in the dendrobatid poison frog Oophaga pumilio. Copeia 2007:1006–1011
Saporito RA, Spande TF, Garraffo HM, Donnelly MA (2009) Arthropod alkaloids in poison frogs: a review of the ‘Dietary Hypothesis’. Heterocycles 79:277–297. https://doi.org/10.3987/REV-08-SR(D)11
Saporito RA, Donnelly MA, Madden AA, Garraffo HM, Spande TF (2010a) Sex-related differences in alkaloid chemical defenses of the dendrobatid frog Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. J Nat Prod 73:317–321. https://doi.org/10.1021/np900702d
Saporito RA, Isola M, Maccachero VC, Condon K, Donnelly MA (2010b) Ontogenetic scaling of poison glands in a dendrobatid poison frog. J Zool 282:238–245. https://doi.org/10.1111/j.1469-7998.2010.00732.x
Saporito RA, Donnelly MA, Spande TF, Garraffo HM (2012) A review of chemical ecology in poison frogs. Chemoecology 22:159–168. https://doi.org/10.1007/s00049-011-0088-0
Saporito RA, Grant T (2018) Comment on Amézquita et al. (2017) “Conspicuousness, color resemblance, and toxicity in geographically diverging mimicry: the pan-Amazonian frog Allobates femoralis”. Evolution 72:1009–1014. https://doi.org/10.1111/evo.13468
Schulte LM, Saporito RA, Davison I, Summers K (2017) The palatability of Neotropical poison frogs in predator-prey systems: do alkaloids make the difference? Biotropica 49:23–26. https://doi.org/10.1111/btp.12404
Sellier MJ, Reeb P, Marion-Poll F (2011) Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chem Senses 36:323–334. https://doi.org/10.1093/chemse/bjq133
Silverstone PA (1975) A revision of the poison-arrow frogs of the genus Dendrobates Wagler. Nat Hist Mus L A Sci Bull 21:1–55
Speed MP, Ruxton GD, Mappes J, Sherratt TN (2012) Why are defensive toxins so variable? An evolutionary perspective. Biol Rev 87:874–884. https://doi.org/10.1111/j.1469-185X.2012.00228.x
Stuckert AM, Saporito RA, Venegas PJ, Summers K (2014) Alkaloid defenses of co-mimics in a putative Müllerian mimetic radiation. BMC Evol Biol 14:1–8. https://doi.org/10.1186/1471-2148-14-76
Stuckert AM, Saporito RA, Summers K (2018) An empirical test indicates only qualitatively honest aposematic signaling within a population of vertebrates. J Herpetol 52:201–208. https://doi.org/10.1670/17-047
Stynoski JL, Shelton G, Stynoski P (2014a) Maternally derived chemical defences are an effective deterrent against some predators of poison frog tadpoles (Oophaga pumilio). Biol Lett 10:20140187. https://doi.org/10.1098/rsbl.2014.0187
Stynoski J, Torres-Mendoza Y, Sasa-Marin M, Saporito RA (2014b) Evidence of maternal provisioning of alkaloid-based chemical defenses in the strawberry poison frog Oophaga pumilio. Ecology 95:587–593
Szelistowski WA (1985) Unpalatability of the poison arrow frog Dendrobates pumilio to the ctenid spider Cupiennius coccineus. Biotropica 17:345–346
Wang IJ (2011) Inversely related aposematic traits: reduced conspicuousness evolves with increased toxicity in a polymorphic poison-dart frog. Evolution 65:1637–1649. https://doi.org/10.1111/j.1558-5646.2011.01257.x
Yang Y, Servedio MR, Richards-Zawacki CL (2019) Imprinting sets the stage for speciation. Nature 574:99–102. https://doi.org/10.1038/s41586-019-1599-z
Acknowledgements
We thank M. Rada, J.J. Ospina-Sarria, C.A. Lopes, S. Andrade, the São Sebastião and Vila do Bravo communities, and the crew of Estação Científica Ferreira Penna (Museu Paraense Emílio Goeldi, Universidade Federal do Pará) for assistance during fieldwork. Specimen collection (license numbers 13173-2 and 54640-1) and export (authorization number 17BR025049/DF) permits were issued by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO)/Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). This study was approved by the Ethics Committee on Animal Use of the Institute of Biosciences, University of São Paulo (CEUA Protocol 268/2016). We thank M. Nichols for his assistance in maintaining the GC–MS and Kresge Foundation and Colleran-Weaver for funding. This research was supported by the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Proc. 306823/2017-9) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Proc. 2012/10000-5, 2016/09999-9, 2018/15425-0).
Author information
Authors and Affiliations
Contributions
AMJ, RAS, and TG contributed to the study conception and design. Specimens were collected by AMJ and TG. Chemical analysis was performed by AMJ and RAS. Palatability tests were performed by SK and RAS. The first draft of the manuscript was written by AMJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Marko Rohlfs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeckel, A.M., Kocheff, S., Saporito, R.A. et al. Geographically separated orange and blue populations of the Amazonian poison frog Adelphobates galactonotus (Anura, Dendrobatidae) do not differ in alkaloid composition or palatability. Chemoecology 29, 225–234 (2019). https://doi.org/10.1007/s00049-019-00291-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-019-00291-3