Abstract
The mutualistic relationships between certain ant and aphid species are well known, the primary benefits being protection for the aphids and carbohydrate-rich honeydew for the ants. Questions remain, however, as to the exact semiochemical factors that establish and maintain such relationships. In this study, we used a series of treatments and associated controls placed at the end of a two-way olfactometer to determine the degree of attractiveness of a complete plant–aphid–honeydew system as well as individual components of that system. Both the olfactometer branch selected by the black garden ant (Lasius niger) and the linear speed with which ants moved through the device were measured. Study results showed that ants were attracted not just to the complete plant system and the honeydew itself, but also to the microbial flora in the absence of plant or honeydew, and specifically to a bacterium from the black bean aphid (Aphis fabae) honeydew, Staphylococcus xylosus. This bacterium produces a blend of semiochemicals that attract the ant scouts. This information suggests the presence of a naturally occurring, reliable biotic cue for detection of potential aphid partners. This would have to be confirmed in natural conditions by further field experiments. Rather than being opportunistic species that coincidentally colonize a sugar-rich environment, microorganisms living in aphid honeydew may be able to alter emissions of volatile organic compounds (VOCs), thus significantly mediating partner attraction. A bacterial involvement in this mutualistic relationship could alter the manner in which these and similar relationships are viewed and evaluated. Future studies into mutualism stability and function among macroscopic partners will likely need for transition from a two-partner perspective to a multiple-partner perspective, and consider the microbial component, with the potential for one or more taxa making significant contributions to the relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food-related mutualisms with ants that are based on sugar-rich production by one partner are numerous and diverse, ranging from within-insect cooperation, like many honeydew-producing insects, to inter-kingdom relations, like extrafloral nectaries produced by several plant species (Beattie 1985; Stadler and Dixon 2005). Aphid–ant interactions have long been considered the paradigm of mutualism and the subject of considerable historical and contemporary research. Small and defenseless, aphids are easy prey for numerous predators and parasitoids. However, some aphid species are frequently found in association with ants that tend and protect the aphids in exchange for honeydew, which is a reliable and abundant carbohydrate source (Bristow 1991; Dixon 1985; Stadler and Dixon 2005). It has been estimated that myrmecophily, i.e., ants tending, is mandatory for one-third of aphid species, facultative for another third, and never observed for the final third (Stadler 1997).
In Europe, three ant genera appear to be primarily involved in these mutualistic interactions: Lasius, Myrmica and Formica (Guénard 2007; Stadler and Dixon 1999). Among mutualistic ant species, the black garden ant, Lasius niger L. (Hymenoptera, Formicidae), is well known to tend several aphid species, including the black bean aphid, Aphis fabae Scopoli (Homoptera, Aphididae) (El-Ziady and Kennedy 1956). These two species were selected as model taxa for this research as they are widespread in their natural environment and are frequently used as model species for the study of mutualistic relationships.
Honeydew, excreted in large amounts when aphids feed on the plant phloem sap, is a complex mixture of sugars, organic acids, amino acids and some lipids (Hussain et al. 1974; Leroy et al. 2011b; Mittler 1958). Its composition varies according to several factors, such as species, physiological state of the host plant, season and aphid endosymbionts (Fischer and Shingleton 2001; Fischer et al. 2002, 2005; Leroy et al. 2011b; Woodring et al. 2007). Phloem sap composition is reflected in honeydew sugars and amino acids, the latter also being produced by endosymbiotic bacteria to palliate the very low and unbalanced concentrations of these compounds in the phloem sap (Douglas 1998; Febvay et al. 1999; Leroy et al. 2011b).
Excreted and deposited on the host plant or falling onto close surroundings, honeydew constitutes an excellent growth medium for diverse microorganisms, including potential aphid pathogens. Volatile compounds found in honeydew, mainly bacterial in origin, are known to attract aphid enemies such as the hoverfly Episyrphus balteatus (De Geer) (Leroy et al. 2011a).
Despite the potential negative biotic threats generated by honeydew, its benefits to the aphids include firming the mutualistic relationship with ant species that utilize the stable sugar source (Bristow 1991), in exchange for diverse services. Ant attendance is known to improve aphid fitness by increasing their reproductive rate and fecundity, extending life span and providing better protection against pathogens and enemies (El-Ziady and Kennedy 1956; Fischer et al. 2001; Guénard 2007; Stadler et al. 2001; Way 1963). Therefore, while honeydew volatiles may attract aphid enemies, the compounds may also attract the species that benefit the aphids. Little is known about the specific role or magnitude of the beneficial contribution of the volatile chemical component to the mutualistic relationship. This paper describes the results of a study designed to investigate the role of these compounds in the attraction of potential ant partners and the origins of those relationships.
Materials and methods
Plants and insects
In a climate-controlled room (16 h light/8 h dark photoperiod; 20 ± 2 °C), black bean aphids, A. fabae (Scopoli), were reared for several generations on broad beans, Vicia faba L., cultivated on a 1/1 mix of perlite and vermiculite substrate. Plants used in experiments measured around 15 cm high. All substrates used in behavioral assays were previously sterilized. Lasius niger (Linne) colonies were collected in the region surrounding Gembloux (Belgium) and kept under the same climatic conditions, but in separate chambers. Nests were placed in plastic containers coated with polytetrafluoroethylene (Fluon®, Whitford, UK) to prevent escape. Test tubes covered with a red transparent foil were used as laboratory rearing nests; a water and aqueous brown sugar solution (342 g/L) was provided in excess, and dead insects (fruit flies and mealworms) were provided weekly as an additional food source. All nests used in the bioassays were comprised of a queen, brood, and a minimum of 500 foragers.
Identification of the honeydew volatile compounds
The composition of the volatile organic compounds (VOCs) was determined using 250-µL glass inserts and solid-phase microextraction (SPME, 10-mm fiber with a 50/30 µm carboxen–divinylbenzene–polydimethylsiloxane coating—Supelco). The material analyzed was A. fabae honeydew (10 µL), dripping from aphid-infested V. faba plants, that was immediately collected on sterilized plastic foil under sterile conditions using 2 µL microcapillaries. Empty inserts were used for controls. Four replicates were conducted.
For each analysis, the same fibers were conditioned at 250 °C for 1 h in a split–splitless injector before sampling. Volatile collections were performed at 25 °C for 24 h. Desorption and GC–MS analyses were conducted on a Thermo Trace GC coupled with a Trace MS (Thermo Electron Corporation, Interscience, Louvain-la-Neuve, Belgium) equipped with an Optima 5 Accent (Macherey–Nagel, Düren, Germany) capillary column (30 m × 0.25 mm I.D.; 0.25 µm film thickness) under the following conditions: splitless injector at 230 °C; vector gas was helium, at a 1 mL/min flow rate; oven temperature program: 40 °C held for 2 min, raised at 5 °C/min to 150 °C, then at 10 °C/min to 210 °C, and finally at 120 °C/min to 280 °C held for 1 min; transfer line was at 250 °C. Mass spectra were acquired at 70 eV on a mass range from m/z 35 to 450 amu and analyzed using the NIST05 and Wiley8 libraries. Identifications were confirmed either by comparison with retention times of synthetic standards (Sigma-Aldrich, Steinheim, Germany) or by determination of retention indices. The relative proportions of each of the identified components are expressed in percent of total sample-related peak area.
Choice tests with two-way olfactometer
The degree of attractive effect on ants was assessed for different samples by using a two-way olfactometer, consisting of a Y-shaped glass tube (diameter 1.5 cm, entrance length 20 cm; length of each arm 30 cm). Samples and controls were placed in 4-L glass jars. Filtered air was forced into the jars at 200 mL/min and delivered to the olfactometer’s branches via Teflon® tubing.
Ants were starved for 3 days prior to an assay. The olfactometer’s entrance was placed in the ant’s rearing box, allowing the insects to enter the system. Only one ant worker at a time was allowed in the olfactometer; the entrance was closed to prevent additional ants from entering after the first ant went in. For each tested ant, the following parameters were recorded: (1) the first branch it entered (initial orientation), (2) the choice, i.e., the branch selected to reach the end of the olfactometer arm and (3) the average linear speed while passing through the branch. The test ended when the ant reached a point located 25 cm from where the two arms branched (“choice point”). The linear speed was calculated by measuring the time spent to pass through an olfactometer branch and was expressed in cm/s. The attractiveness of a sample was expressed by the relative number of ants (%) choosing the particular sample side as their final choice.
All assays were conducted at 20 °C in a dark-walled chamber presenting no visual cues that may influence ant choices. To prevent ants from laying trails, they were never allowed to reach the actual samples. Moreover, in order to palliate any potential bias induced by the environment or by any marking of the substrate by exploring ants, sample and control sides were switched every five ants. The olfactometer was completely cleaned every 20 ants.
Several sample–control couples were tested following this protocol (Table 1) and changed every 20 ants. The first group of samples included naturally occurring elements. The goal was to assess to what extent each element that characterizes an aphid-infested plant contributes to its global attractiveness to the ants, specifically, the aphids themselves, the attacked plant and the honeydew. Since those elements cannot be completely dissociated, assays have been conducted by successive elimination of each element in order to assess their relative impact on ants’ attraction. The first sample was an aphid-infested plant (a pot holding nine V. faba infested by 50 A. fabae for three days); its attractiveness was compared to that of a healthy plant without aphids. The second sample was prepared similar to the first one, but all aphids were completely removed before the assay. The last sample consisted of only honeydew collected for 3 days from a heavily infested plant onto wet substrate (perlite/vermiculite 1:1) to avoid desiccation. In this case, the control was a clean pot of substrate with the same humidity.
The second group of samples included only bacterial cultures to assess the degree to which ants would be attracted to honeydew microflora. The first sample of this group was an 863 liquid culture medium, containing 20 g of glucose and 10 g of both yeast extract and casein peptone per liter of distilled water, inoculated with 20 µL of fresh honeydew collected as previously described and incubated for 2 days at 20 °C. Sterile 863 medium was used as the control for this test. The second sample was the same medium inoculated solely with Staphylococcus xylosus, which is the only bacterial strain from A. fabae honeydew that was shown to have a significant attraction potential for the ants. This S. xylosus medium was incubated under the same conditions as the whole bacterial culture treatment, and the control was also sterile 863 culture medium. Sample volume for each test was 60 mL.
The significance of the ant preferences was assessed with binomial tests. Average linear speeds of ants in the two branches were compared, assay by assay, with t tests. Global mean speeds observed for naturally occurring samples were compared by Kruskal–Wallis tests, which were also used to assess significance in the bacterial culture sample studies. If no differences were observed within a group, values within a group were pooled for between-group comparisons using Kruskal–Wallis tests. Nonparametric tests were used when parametric assumptions were not observed. Differences were considered significant at p ≤ 0.05. Statistical analyses were conducted using Minitab 15.1 (State College, PA, USA).
Isolation of honeydew microbial content
Several bacteria which may influence the attractive effect of honeydew were isolated. Collected Aphis fabae honeydew (20 µL) was diluted and plated on 868 agar medium (containing, per liter of distilled water, 17 g of agar and 10 g each of glucose, yeast extract and casein peptone). Colonies were visible after 24 h of incubation at 25 °C, and the strains were isolated on the same medium based on morphological growth characteristics. The isolated strains were screened for their relative attractiveness.
In order to investigate the occurrence of additional fungal microorganisms potentially hidden by bacteria, the same process was repeated using chloramphenicol-treated plates. Chloramphenicol is a broadband antibiotic specific against bacteria but not fungi, thus leaving only the latter as potentially influencing attractiveness.
Screening of microbial attractiveness
Once isolated, strains were cultured at 25 °C in 863 liquid medium. After 48 h, attractiveness was tested by the same olfactometry protocol. These preliminary screenings were run with 20 ants per bacterium. Significance of the observed attractions was assessed with binomial tests using Minitab 15.1 software.
Identification of the attractive bacterium
For bacterial identification, genomic DNA was extracted from cells grown at 20 °C for 48 h on 868 agar plates using a Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
The 16S rRNA gene was PCR-amplified using the universal primers P0 (5′-GAA GAG TTT GAT CCT GGC TCA G-3′) and P6 (5′-CTA CGG CTA CCT TGT TAC GA-3′) (Ventura et al. 2001). The PCRs were completed using 1x ReadyMix Taq PCR Reagent Mix (Sigma-Aldrich, St. Louis, MO, USA), 0.5 µM of each primer and ~50 ng of genomic DNA as the template. The PCR program included a 5-min initial denaturation step at 95 °C, followed by 26 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min, and a final extension for 10 min at 72 °C. The PCR product was purified using a GFX PCR DNA and Gel Band Kit (GE Healthcare, Buckinghamshire, UK), then sequenced using the Big Dye v3.1 Kit and an ABI 3730 DNA Analyzer (Applied Biosystems/Life Technology, Carlsbad, CA, USA) at the GIGA Center at the University of Liege. The same P0 and P6 primers were also used for sequencing. Electropherograms of both 16S gene strands were aligned, analyzed, and edited with BioEdit (version 5.0.9; Hall 1999) in order to determine the correct 16S sequence.
Strain identification was conducted by comparing the 16S rRNA gene sequence determined here with all those in the GenBank database, using BLASTN (Altschul et al. 1997). The 16S sequence obtained here was deposited in the GenBank database under accession number KP668813.
In order to discriminate between the two identified Staphylococcus species, a diagnostic PCR was performed with primers that were specific for each of the two Staphylococcus species. Three PCR primer sets were used, one that was specific for S. saprophyticcus, 5′-TCA AAA AGT TTT CTA AAA AAT TTA C-3′ and 5′-ACG GGC GTC CAC AAA ATC AAT AGG A-3′(Martineau et al. 2000), yielding a 210 bp fragment, and two sets that were specific for S. xylosus, XYL F (5′-AAC GCG CAA CGT GAT AAA ATT AAT G-3′) and XYL R (5′-AAC GCG CAA CAG CAA TTA CG-3′) (Morot-Bizot et al. 2003), yielding a 539 bp fragment, and xylBF (5′-CGT CTC AAG AAG TTG AAG ACA-3′) and xylBR (5′-CTC CAC CAC CAA TTG ATA CA-3′) (Blaiotta et al. 2003), yielding a 899 bp fragment. The PCRs were prepared as described above, and the PCR program was the same as for amplification of the 16S rRNA gene, except that the elongation time was 1 min for the 899 bp fragment and 40 s for the two smaller fragments. The PCR products were resolved by electrophoresis on 1 % (w/v) agarose gels stained with 1 µg/mL ethidium bromide.
Identification of S. xylosus origin
To determine whether S. xylosus is excreted with honeydew or already present in the aphid environment, the whole bodies of 20 A. fabae adults were surface-sterilized by dipping into 70 % ethanol for three minutes followed by washing in a sterile 9 g/L NaCl solution following the protocol proposed by Leroy et al. (2011a). Surface-sterilized aphids were crushed under sterile conditions in the same solution [using a previously described method (Fischer and Lognay 2012)], diluted and plated. Based on the morphology of the colonies, catalase activity and cell morphology, S. xylosus was isolated and its identification confirmed using API® Staph galleries (BioMérieux, Marcy l’Etoile, France).
Characterization of the S. xylosus VOC emission profile
In order to determine the contribution of S. xylosus in the emission of honeydew volatile compounds, SPME and GC–MS analyses were performed on bacterial cultures using a protocol proposed by Leroy et al. (Leroy et al. 2011a) and successfully applied to Acyrthosiphon pisum (Harris) bacteria VOC sampling (Table 2).
The bacterium was grown in 863 liquid culture medium at 20 °C for 48 h. A 1 mL aliquot of the culture medium was placed in a 20-mL SPME vial. Control samples were 1 mL aliquots of sterile medium placed in similar vials. Volatile compounds were sampled by 10-mm DVB-CAR-PDMS SPME fibers (50/30 µm film thickness) (Supelco) for 3 h at 25 °C. GC–MS analyses were conducted under the same conditions as previously described. Three replicates were analyzed.
Results
Aphid honeydew and ant attraction
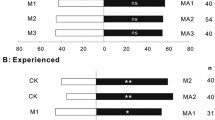
Sixty-eight percent of the tested foragers were attracted by the complete infested plant system (host plant + aphids + honeydew; binomial test, n = 100, p < 0.001) (Fig. 1); 64 % of tested ants were attracted to a broad bean plant covered with honeydew (binomial test, n = 100, p = 0.007). Finally, 64 % of the tested ants were attracted to honeydew alone (binomial test, n = 120, p = 0.002). The attraction toward honeydew alone was not statistically different from the complete system (χ 2 test, p = 0.391).
Behavioral response of L. niger foragers to aphid-related VOCs. Five preference tests of L. niger foragers in a two-way olfactometer presented with VOCs originating from (1) A. fabae infested plants (n = 100), (2) infested plants with aphids just removed (n = 100), (3) A. fabae honeydew (n = 120), (4) 863 medium inoculated with all honeydew microflora (n = 60) or (5) 863 medium inoculated with only S. xylosus (n = 60). Controls for these five treatments were (treatments 1 and 2) healthy plants, (treatment 3) sterile wet culture substrate (perlite/vermiculite 1:1), and (treatments 4 and 5) sterile 863 medium. 1 Ants that choose either the sample or control branches as their final destination in the olfactometer (%). 2 and 3 Relative number of ants changing their initial choice (%) and leaving the olfactometer (%), respectively. 4 Linear speed (cm/s, X ± SEM) of ants in each olfactometer branch. ** and *** indicate significant differences from the control at p < 0.01 and p < 0.001, respectively. ns not significantly different (p > 0.05)
While no ant reversed course and tried to leave the olfactometer once they were inside, there were a few that changed their initial choice (Fig. 1). The proportion of ants that altered their choices appears to be higher for ants initially choosing the control side. During all those assays, ants’ mean speed was monitored in both branches of the olfactometer (Fig. 1). No significant differences in speed were observed between sample and control branches within a test (t test, all p > 0.440), nor between the three tests described above (Kruskal–Wallis test, p = 0.187).
Attractiveness of honeydew: the role of microorganisms
The biological effects of microbial VOCs (mVOCs) produced by honeydew bacteria were tested using the same olfactometer. Liquid 863 culture medium inoculated with freshly collected honeydew attracted a significantly higher number of ants, with 78 % of the ant foragers following the sample branch (binomial test, n = 60, p < 0.001) (Fig. 1). Microbes were the only possible emitters of attractive VOCs since the control sample was sterile 863 culture medium inoculated with honeydew filtered through 0.2-µm membrane filters. It is highly unlikely, therefore, that honeydew enzymes would have played a role in attracting the ants.
Once in the olfactometer, some ants left the system before reaching the end. The proportion of exiting ants appears higher for the control (11 %) than for the sample (4 %) branch. Moreover, as previously observed, the proportion of ants changing their choices appeared higher when the ants initially started down the control arm (36 vs. 7 %). Compared to the earlier experiments, the average linear speed achieved by the ants decreased significantly (Kruskal–Wallis test, p < 0.001), which is due to a more sinuous route in the olfactometer with more brief stops. However, there remained no significant difference between control and sample sides (t test, p = 0.442).
Further behavioral assays performed with S. xylosus (as discussed in the next paragraph), and conducted using the same protocol as previously described, confirmed the attractiveness of this bacterium found in honeydew. Indeed, 75 % of tested ants showed an attraction to the bacterium (n = 60, binomial test, p < 0.001). Tendencies observed with the honeydew-inoculated medium remained the same. However, the cases where ants reversed course still occurred more frequently when ants initially chose the control branch. Average linear speeds toward the bacterial samples were not statistically different (Kruskal–Wallis test, p = 0.275), but were slower than the speeds exhibited by ants moving toward the complete infested plant system and its components (first three tests described above) (Kruskal–Wallis test, p < 0.001). In addition to a slower (although not statistically slower) speed, ants were apparently more hesitant, with more brief stops. This speed remained equivalent for both branches of each assay (t test, all p > 0.360).
Identification and origin of attractive bacteria identified from honeydew
Six morphologically different bacteria were isolated from honeydew on 868 culture media. Yeasts and fungi were not present on this medium, either before or after adding the broadband antibacterial chloramphenicol.
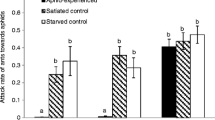
Preliminary attraction potential screening showed a significant attractiveness for “bacterium 4” only (binomial test, p < 0.001) (Fig. 2), with 90 % of ants attracted. That bacterium, found at a concentration of 2 × 105 CFU/mL of honeydew, was identified as Staphylococcus sp. (potentially saprophyticcus or xylosus) by 16S ribosomal RNA sequences based on 100 % nucleotide sequence identity with these two species over a 100 % sequence coverage. The diagnostic PCR amplification was negative with the S. saprophyticcus-specific primers and positive with the two sets of S. xylosus-specific primers, confirming that the isolated strain was S. xylosus.
The occurrence of the bacterium in surface-sterilized aphid bodies was demonstrated by an API Staph test (BioMérieux) after isolation of the strain.
Origin of the honeydew attractive VOCs
Twenty-two volatile chemicals, including ketones, esters, alcohols, aldehydes and acids, were identified from freshly collected honeydew (Table 2). Most of them were also detected in S. xylosus cultures and were similar to the mVOCs produced by S. sciuri, an A. pisum bacterium (Leroy et al. 2011a) (Table 2).
Discussion
Honeydew is the keystone factor leading to ant–aphid partnerships, strongly influencing the relationship through its composition and abundance (Fischer et al. 2001; Kiss 1981; Völkl et al. 1999; Way 1963). The data presented here represent the first evidence of a distant attraction of ants that is driven by aphid honeydew and mediated by the associated microflora through release of VOCs, in laboratory conditions. Even though mVOCs emitted from honeydew have been reported to attract aphid enemies (Leroy et al. 2011a), the potential extent of their role in ant–aphid mutualism was unexpected. Indeed, when considering the plant–aphid–honeydew system, neither the removal of plants nor the aphids themselves significantly influenced ant behavior. Honeydew VOCs alone have the same biological effects as the complete system (Fig. 1). However, previous studies have shown an effect from diverse concentrations of E-β-farnesene (EBF), an aphid pheromone, on ant’s social behavior (Nault et al. 1976; Verheggen et al. 2012). In combination with our results, this suggests either that VOCs emitted by the aphid itself (EBF) present a relatively low attractiveness for the ants compared to honeydew or that their effect is not additive with that of the honeydew, honeydew alone being able to elicit the entire response.
Honeydew-inoculated, as well as S. xylosus-inoculated, culture media remained attractive, confirming the high importance of honeydew microorganisms.
When comparing results from behavioral assays, two groups can be distinguished: one composed of naturally occurring samples (the aphid-infested plant and its separate components), and one comprised of bacterial cultures (Fig. 1). While attractiveness remains constant throughout all assays, for the last group the number of ants leaving the initially selected branch was higher and their average speed was lower. Those results suggested a reduction in the efficiency of ant attraction which is likely a result from the differences of VOC profiles observed between honeydew and bacterial cultures (Table 2), which probably originates from the differences between those two groups in terms of bacterial growth conditions.
When isolated from A. fabae honeydew microflora and from surface-sterilized aphids, S. xylosus emits a mVOC pattern qualitatively similar to that produced by genuine honeydew excreted by A.fabae colonies or to that emitted by cultures of S. sciuri (Table 2). The latter, isolated from the non-myrmecophilous pea aphid Acyrtosiphon pisum and closely related to S. xylosus, is known to release mVOCs which guide hoverflies toward their aphid prey (Leroy et al. 2011a). Of the 10 compounds described by these authors, nine have also been found in S. xylosus mVOCs, and 10 new compounds have been identified for the latter species (Table 2). Several of them have been found in diverse glands of various ant species, mostly Myrmicinae. The identified compounds include propanone (Attygalle et al. 1983) and 2-methyl and 3-methlybutanoic acid (Wood et al. 2002), which have been found in Dufour glands as well as in mandibular glands (Cammaerts et al. 1981; Wood et al. 2011). These glands have also been found to contain 3-methyl-1-butanol, butanoic acid (Wood et al. 2002) and phenylethanol (Wood et al. 2011), the latter having also been found in poison glands, along with 2, 5-dimethylpyrazine; phenylethanol is involved in recruitment ant trail laying (Attygalle and Morgan 1984; Liu and Liu 2002; Plowes et al. 2014). Benzaldehyde has been found in pygidial glands (Hölldobler et al. 2013). However, none of these compounds have previously been found in the genus Lasius, nor identified as semiochemicals for this genus. Aphid gut microflora is partially acquired during probing by the insect on the host leaf surface or feeding in vascular tissues. Bacteria are able to pass through the stylets’ food canal, colonize the luminal surface of intestinal epithelia, and be partially excreted in honeydew (Davidson et al. 2000; Grenier et al. 1994; Harada and Ishikawa 1997; Leroy et al. 2011a). Since honeydew had been collected in sterile conditions immediately after its excretion, S. xylosus, found at a concentration of 2 × 105 CFU/mL of fresh honeydew, can be considered a host-associated bacterium excreted by the aphid rather than a bacterium colonizing honeydew after its excretion. Staphylococcus xylosus has been found in surface-sterilized aphids following a protocol proposed by Leroy et al. (2011a), thus confirming the host origin of this bacterium. Moreover, S. xylosus is a quite ubiquitous species, found in various natural environments like soil, beach sand, water, grass and the skin of cattle and birds (Kloos 1980). Aphids are likely hosts of this bacterium, which is probably acquired during probing (Haynes et al. 2003). Aphid physical and biochemical mechanisms, as well as the composition of honeydew, could be effective filters, allowing only a few bacterial taxa to develop and colonize the gut; similar to what occurs for yeasts in flower nectar (Herrera et al. 2010). This kind of partner selection is based on the microbes’ abilities to survive in this rather hostile environment (high osmotic pressure, low oxygen, antimicrobial compounds produced by the host) and on the affinity of the microorganism for its host (Álvarez-Pérez et al. 2012; González-Teuber and Heil 2010; Herrera et al. 2010; Kirzinger and Stavrinides 2012).
The volatile compounds identified from plain honeydew or honeydew-isolated bacteria probably originate from degradation processes and/or modifications of sugars and amino acids of the substrate. It has been shown that direct modifications, especially those made by Staphylococcus sp. on several amino acids such as leucine, isoleucine and valine, can produce a whole set of C4 and C5 components with different chemical functions, from aldehydes to their corresponding alcohols, acids and esters (Schulz and Dickschat 2007; Thibout et al. 1993). This would explain the origin of most identified non-cyclic compounds reported in Table 2. For example, this pathway is probably responsible for the production of 2-methylbutanal and 3-methylbutanal, as well as their corresponding alcohols (2-methyl-1-butanol and 3-methyl-1-butanol) and acids (2-methylbutanoic acid and 3-methylbutanoic acid). Also synthesized by S. sciuri isolated from the aphid A. pisum, these last two compounds are known to attract and induce egg-laying by the aphid predator hoverfly Episyrphus balteatus (Leroy et al. 2011a). They may also be potentially involved in ant attraction, as other species use them to signal the presence of aphids. In situations of amino acid scarcity, which is the case for honeydew, S. xylosus is also able to form acids from glucose via another biosynthetic pathway involving pyruvate (Beck et al. 2004). Aromatic compounds, like benzeneethanol, are produced by degradation of aromatic amino acids. Along with 3-hydroxy-2-butanone, 2,3-butanedione and 3-methyl-3-buten-1-ol, these are typical fermentation-associated substances (Leroy et al. 2011a; Schulz and Dickschat 2007; Thibout et al. 1993). Therefore, despite widespread distribution in the environment, S. xylosus remains a specific indicator of honeydew presence through its mVOC emissions modulated by the growth substrate.
The ability to associate these mVOC patterns and quantities with potential aphid partners is likely to aid ant scouts by providing information about the aphid species, their location and productivity through the amount of mVOCs and the modifications of mVOC patterns of aging honeydew. Ant scouts may then exploit this information to refine their search patterns and selection of new aphid partners.
Many widespread cases of mutualism are based on the ability of one partner to produce sugar-rich secretions, exploited by the other. For example, nectaries-bearing plants attract ants and thus enhance their protection against other insects (González-Teuber and Heil 2010) and flowers that are rich in nectar attract various pollinators (e.g., insects, birds and small mammals). Sugary secretions, like nectars, also constitute a suitable growth medium for diverse microorganisms. Indeed, yeasts and bacteria have been repeatedly isolated from the nectar of flowers pollinated by various organisms including insects, birds and small mammals (Álvarez-Pérez et al. 2012; Herrera et al. 2009; Vannette et al. 2013; Wiens et al. 2008). Microbial presence alters the composition of the nectar and can modify its VOC production, thus influencing pollination. Effects on the plant (e.g., sugar modification, ethanol production) are either positive or negative for the relationship, depending on the extent of these modifications, the microorganisms involved and the pollinators considered (Herrera et al. 2008, 2009; Vannette et al. 2013; Wiens et al. 2008). While yeasts do not seem to be detrimental for plant–pollinator mutualisms at low infestation levels, bacteria are able to weaken these relationships (Vannette et al. 2013).
The data presented here indicated that honeydew, apart from serving as a source of nutrients, contains certain microbes that are attractive to ants. This first evidence of the influence of microbes in ant–aphid mutualism contributes to an altered perspective of the importance of bacterial volatiles in animal interactions. Microorganisms can impact mutualistic relationships through both direct substrate modifications as well as through emissions of volatile compounds. These results emphasize the importance of investigating the presence and potential effects of microbes in insect symbioses.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Álvarez-Pérez S, Herrera CM, de Vega C (2012) Zooming-in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol Ecol 80:591–602. doi:10.1111/j.1574-6941.2012.01329.x
Attygalle AB, Morgan ED (1984) Identification of trail pheromone of the ant Tetramorium caespitum L. (Hymenoptera: Myrmicinae). J Chem Ecol 10:1453–1468
Attygalle AB, Evershed RP, Morgan ED, Cammaerts MC (1983) Dufour gland secretions of workers of the ants Myrmica sulcinodis and Myrmica lobicornis, and comparison with six other species of Myrmica. Insect Biochem 13:507–512
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press
Beck HC, Hansen AM, Lauritsen FR (2004) Catabolism of leucine to branched-chain fatty acids in Staphylococcus xylosus. J Appl Microbiol 96:1185–1193. doi:10.1111/j.1365-2672.2004.02253.x
Blaiotta G, Pennacchia C, Parente E, Villani F (2003) Design and evaluation of specific PCR primers for rapid and reliable identification of Staphylococcus xylosus strains isolated from dry fermented sausages. Syst Appl Microbiol 26:601–610. doi:10.1078/072320203770865918
Bristow CM (1991) Why are so few aphids ant-tended? In: Cutler DF, Huxley C (eds) Ant/plant interactions. Oxford University Press, pp 104–119
Cammaerts MC, Evershed RP, Morgan ED (1981) Comparative study of the mandibular gland secretion of four species of Myrmica ants. J Insect Physiol 27:225–231
Davidson EW, Rosell RC, Hendrix DL (2000) Culturable bacteria associated with the whitefly, Bemisia Argentifolii (Homoptera: Aleyrodidae). Fla Entomol 83:159–171
Dixon AFG (1985) Aphid ecology. Blackie, Glasgow
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria. Buchnera Annu Rev Entomol 43:17–37. doi:10.1146/annurev.ento.43.1.17
El-Ziady S, Kennedy JS (1956) Beneficial effects of the common garden ant, Lasius niger L., on the black bean aphid, Aphis fabae Scopoli. Proc R Ent Soc Lond 31:5
Febvay G, Rahbe Y, Rynkiewicz M, Guillaud J, Bonnot G (1999) Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J Exp Biol 202(Pt 19):2639–2652
Fischer CY, Lognay GC (2012) Simple and automatic closed grinding and extraction system. J Chem Educ 89:1611–1612. doi:10.1021/ed2007907
Fischer MK, Shingleton AW (2001) Host plant and ants influence the honeydew sugar composition of aphids. Funct Ecol 15:544–550
Fischer MK, Hoffmann KH, Völkl W (2001) Competition for mutualists in an ant-homopteran interaction mediated by hierarchies of ant attendance. Oikos 92:531–541
Fischer MK, Volkl W, Schopf R, Hoffmann KH (2002) Age-specific patterns in honeydew production and honeydew composition in the aphid Metopeurum fuscoviride: implications for ant-attendance. J Insect Physiol 48:319–326
Fischer MK, Volkl W, Hoffmann KH (2005) Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera : Aphididae) on various host plants and implications for ant-attendance. Eur J Entomol 102:155–160
González-Teuber M, Heil M (2010) Pseudomyrmex ants and acacia host plants join efforts to protect their mutualism from microbial threats. Plant Signal Behav 5:890–892
Grenier AM, Nardon C, Rahbé Y (1994) Observations on the micro-organisms occurring in the gut of the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 70:91–96. doi:10.1111/j.1570-7458.1994.tb01762.x
Guénard B (2007) Mutualisme fourmis pucerons et guilde aphidiphage associée : le cas de la prédation furtive. Université du Québec à Montréal
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Harada H, Ishikawa H (1997) Experimental pathogenicity of Erwinia aphidicola to pea aphid, Acyrthosiphon pisum. J General Appl Microbiol 43:363–367
Haynes S et al (2003) Diversity of bacteria associated with natural aphid populations. Appl Environ Microbiol 69:7216–7223. doi:10.1128/aem.69.12.7216-7223.2003
Herrera CM, García IM, Pérez R (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89:2369–2376. doi:10.1890/08-0241.1
Herrera CM, de Vega C, Canto A, Pozo MI (2009) Yeasts in floral nectar: a quantitative survey. Ann Bot 103:1415–1423. doi:10.1093/aob/mcp026
Herrera CM, Canto A, Pozo MI, Bazaga P (2010) Inhospitable sweetness: Nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc R Soc B Biol Sci 277:747–754. doi:10.1098/rspb.2009.1485
Hölldobler B, Plowes NJR, Johnson RA, Nishshanka U, Liu C, Attygalle AB (2013) Pygidial gland chemistry and potential alarm-recruitment function in column foraging, but not solitary, Nearctic Messor harvesting ants (Hymenoptera: Formicidae: Myrmicinae). J Insect Physiol 59:863–869
Hussain A, Forrest JM, Dixon AF (1974) Sugar, organic acid, phenolic acid and plant growth regulator content of extracts of honeydew of the aphid Myzus persicae and of its host plant, Raphanus sativus. Ann Appl Biol 78:65–73. doi:10.1111/j.1744-7348.1974.tb01486.x
Kirzinger MWB, Stavrinides J (2012) Host specificity determinants as a genetic continuum. Trends Microbiol 20:88–93. doi:10.1016/j.tim.2011.11.006
Kiss A (1981) Melezitose, aphids and ants. Oikos 37:382
Kloos WE (1980) Natural populations of the genus Staphylococcus. Annu Rev Microbiol 34:559–592
Leroy PD et al (2011a) Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nature Commun 2:7. doi:Artn 348
Leroy PD et al (2011) Aphid-host plant interactions: does aphid honeydew exactly reflect the host plant amino acid composition? Arthropod Plant Interact 5:193–199. doi:10.1007/s11829-011-9128-5
Liu Y, Liu Y (2002) Identification of recruitment pheromones in the harvester ant genus Pogonomyrmex. Fenxi Huaxue 30:298–300
Martineau F, Picard FJ, Ménard C, Roy PH, Ouellette M, Bergeron MG (2000) Development of a rapid PCR assay specific for Staphylococcus saprophyticus and application to direct detection from urine samples. J Clin Microbiol 38:3280–3284
Mittler TE (1958) The feeding and nutrition of large willow aphid (Tuberolachnus salignus). II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. J Exp Biol 35:74–84
Morot-Bizot S, Talon R, Leroy-Setrin S (2003) Development of specific PCR primers for a rapid and accurate identification of Staphylococcus xylosus, a species used in food fermentation. J Microbiol Methods 55:279–286. doi:10.1016/S0167-7012(03)00159-3
Nault LR, Montgomery ME, Bowers WS (1976) Ant-aphid association: role of aphid alarm pheromone. Science 192:1349–1351
Plowes NJR, Colella T, Johnson RA, Hölldobler B (2014) Chemical communication during foraging in the harvesting ants Messor pergandei and Messor andrei. J Comp Physiol A Neuroethol Sensory Neural Behav Physiol 200:129–137
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Product Rep 24:814–842. doi:10.1039/b507392h
Stadler B (1997) The relative importance of host plants, natural enemies and ants in the evolution of life-history characters in aphids. In: Dettner K, Bauer G, Völkl W (eds) Vertical food web interactions: evolutionary patterns and driving forces. Springer, Berlin, pp 241–256. doi:citeulike-article-id:1351088
Stadler B, Dixon AFG (1999) Ant attendance in aphids: why different degrees of myrmecophily? Ecological Entomology 24:363–369
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Stadler B, Fiedler K, Kawecki TJ, Weisser WW (2001) Costs and benefits for Phytophagous myrmecophiles: when ants are not always available. Oikos 92:467–478
Thibout E, Guillot JF, Auger J (1993) Microorganisms are involved in the production of volatile kairomones affecting the host seeking behaviour of Diadromus pulchellus, a parasitoid of Acrolepiopsis assectella. Physiol Entomol 18:176–182
Vannette RL, Gauthier MPL, Fukami T (2013) Nectar bacteria, but not yeast, weaken a plant—pollinator mutualism. Proc R Soc B Biol Sci 280. doi:10.1098/rspb.2012.2601
Ventura M, Elli M, Reniero R, Zink R (2001) Molecular microbial analysis of Bifidobacterium isolates from different environments by the species-specific amplified ribosomal DNA restriction analysis (ARDRA). FEMS Microbiol Ecol 36:113–121. doi:10.1016/S0168-6496(01)00123-4
Verheggen FJ, Diez L, Sablon L, Fischer C, Bartram S, Haubruge E, Detrain C (2012) Aphid alarm pheromone as a cue for ants to locate aphid partners. PLoS One 7:e41841. doi:10.1371/journal.pone.0041841
Völkl W, Woodring J, Fischer M, Lorenz MW, Hoffmann KH (1999) Ant-aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118:483–491
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Wiens F et al (2008) Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci USA 105:10426–10431. doi:10.1073/pnas.0801628105
Wood WF, Palmer TM, Stanton ML (2002) A comparison of volatiles in mandibular glands from three Crematogaster ant symbionts of the whistling thorn acacia. Biochem Syst Ecol 30:217–222
Wood WF, Hoang TT, McGlynn TP (2011) Volatile components from the mandibular glands of the turtle ants, Cephalotes alfaroi and Cephalotes cristatus. Biochem Syst Ecol 39:135–138
Woodring J, Wiedemann R, Volkl W, Hoffmann KH (2007) Oligosaccharide synthesis regulates gut osmolality in the ant-attended aphid Metopeurum fuscoviride but not in the unattended aphid/i Macrosiphoniella tanacetaria. J Appl Entomol 131:1–7. doi:10.1111/j.1439-0418.2006.01091.x
Acknowledgments
The authors wish to thank Dr. M. Vanderplanck, MSc H. Cawoy, Mr O. Piraux and Mr P. Hacourt for their support during this project. Christophe Fischer is financially supported by a PhD grant from the Fonds pour la formation à la Recherche dans l’Industrie et l’Agriculture (FRIA). This project is also financially supported by a Fonds de la Recherche Fondamentale Collective (F.R.F.C.–F.N.R.S.) research project (2.4600.09).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Schmitt.
Rights and permissions
About this article
Cite this article
Fischer, C.Y., Lognay, G.C., Detrain, C. et al. Bacteria may enhance species association in an ant–aphid mutualistic relationship. Chemoecology 25, 223–232 (2015). https://doi.org/10.1007/s00049-015-0188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-015-0188-3