Abstract
The South American fruit fly is one of the most destructive polyphagous pests in South America. In this species, males gathered in aggregations emit volatiles that attract females; however, the compounds involved in this task remain unknown. In this study, we investigated the chemical composition of the volatile blend emitted by males aiming to identify the specific compounds within this blend that elicit behavioral responses in conspecific females. For this purpose, we performed chemical and electrophysiological analyses and bioassays. The chemical analyses revealed the presence of 29 compounds in headspace samples of A. fraterculus males, of which six compounds, i.e. α-pinene, limonene, (Z)-3-nonen-1-ol, (E,Z)-3,6-nonadien-1-ol, α-farnesene and (S,S)-(−)-epianastrephin, triggered antennal depolarization in conspecific females. In laboratory bioassays, five out of eight synthetic compounds tested individually elicited more behavioral responses than a hexane control, but only the synthetic mixture composed of all EAD-active compounds triggered behavioral responses in females similar to the responses to the headspace samples of conspecific males. In an experiment under semi-natural conditions, the synthetic mixture was more attractive to females than a hexane control and equally attractive to headspace extracts of males. This study reports the identification of male volatile compounds that act as attractant for A. fraterculus females, which may be useful for the control of this pest in infested orchards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastrepha fraterculus Wiedemann (Diptera: Tephritidae) is economically one of the most problematic pests in South America. This polyphagous species has a strong negative impact on commercial fruit production, causing quarantine restrictions on fruit exports to many countries (Aluja and Norrbom 2001; Norrbom and Korytkowski 2011). The damages are caused by the Anastrepha females, puncturing the fruits during oviposition and by the feeding larvae inside the fruit that generally result in premature fruit abortion. Moreover, the puncture facilitates the infection with microorganisms, also causing spoilage of the fruit (Nascimento and Carvalho 2000).
The mating system of A. fraterculus is very complex and involves the use of auditory, visual and chemical signals (Aluja 1994). Males release volatile compounds that attract conspecifics to a mating site. At first, only males are attracted to the mating site, forming leks, and their concerted calling attracts females. Because these chemicals are involved in the attraction of both males and females to the same site, they are to be classified as aggregation pheromones; since they act as attractants for females, they can also be considered as male sex pheromone. Calling males inflate their pleural pouches and fan their wings, thereby producing sounds and spreading emitted volatiles (Lima et al. 2001; Nation 1989).
The volatile blend released by A. fraterculus males is very complex and includes compounds belonging to different classes, such as terpenoids, unsaturated alcohols, aldehydes, lactones, and pyrazines (Břízová et al. 2013; Cáceres et al. 2009; Lima et al. 2001). In a previous work, headspace extracts of A. fraterculus calling males (Lima et al. 2001) were shown to be attractive to conspecific females. However, the identity of the compounds involved in the attraction of females remained unknown. Elucidating the identity of the male sex pheromone constituents of A. fraterculus is crucial for the development of effective methods using attractants to control these flies in infested orchards. In the present study, we aimed to identify the compounds in headspace extracts of A. fraterculus males involved in the attraction of conspecific females. We used gas chromatography coupled with an electroantennographic detector (GC-EAD) and two-dimensional gas chromatography coupled to mass spectrometry (GC × GC-TOFMS) to detect and identify the compounds in the headspace samples of A. fraterculus males that elicit antennal depolarization in conspecific females. Furthermore, we performed a series of bioassays under laboratory and semi-natural conditions to test whether EAD-active compounds (either individually or as a blend) elicit behavioral responses in A. fraterculus females.
Materials and methods
Insect collection
Larvae of a wild population of A. fraterculus were collected directly from infested guava fruits that had been harvested in an orchard located in the town of Coruripe (10°8′1″S; 36°10′34″W) in the state of Alagoas, Brazil. The larvae were placed for pupation in separate boxes (44 cm × 35 cm × 25 cm) made of expanded polystyrene and containing a mixture of washed sand and vermiculite. The flies were separated by sex within 24 h after emergence and placed into glass tanks (30 cm × 20 cm × 15 cm), which were kept at the laboratory of Chemical Ecology at Universidade Federal de Alagoas (Maceió, Alagoas, Brazil) under a 14:10 h light:dark photoperiod, at a temperature of 25 ± 1 °C and relative humidity of 60 %. The flies were fed a diet consisting of a mixture of muscovado sugar and brewer’s yeast (3:1 w/w), along with water in a separate container. In the experiments, 9–15 days old virgin flies were used. The identification of the species was based on the morphological characteristics of the female ovipositor (Zucchi 2000).
The sampling of male volatiles
Groups of 20 sexually mature virgin males of A. fraterculus were placed in a glass desiccator (180 mm high; 200 mm diameter) and the volatiles emitted were collected using dynamic headspace methods. The inlet of the desiccator had been modified by the addition of an inlet tube containing Tenax® (100 mg; Chromapack) to adsorb the released volatiles. The air inside the desiccator, enriched with the volatiles of the males, was sucked using an air pump (Resun® AC 2600) coupled to a flow meter (Supelco®) and absorbed in the Tenax filter. The air flow through the filter was 500 mL/min for 24 h. The volatiles trapped in the filter were then eluted with 500 µL of redistilled trace analysis grade hexane (Sigma-Aldrich). The headspace collection was performed in ten replicates (N = 10). The samples were stored in 2 mL vials, which were placed in the freezer (−5 °C) for chemical analyses and behavioral assays.
Electrophysiology
To detect the compounds from the headspace samples of A. fraterculus males that are potentially involved in the attraction of conspecific females, electrophysiological analyses were performed on a gas chromatograph (Thermo GC Ultra, Thermo Scientific, Milan, Italy) equipped with a flame ionization detector (FID) and a VB-5 column (30 m × 0.25 mm i.d. × 0.25 µm film, ValcoBond) and coupled to an EAD setup (heated transfer line, two-channel universal serial bus acquisition controller) provided by Syntech (Kirchzarten, Germany). Electronic flow control was used to maintain a constant helium carrier gas flow of 1 mL/min. An aliquot of the solvent headspace samples (2 µL) was injected in splitless mode at an oven temperature of 40 °C and an inlet temperature of 150 °C, followed by opening the split valve after 1 min and increasing the oven temperature at a rate of 6 °C/min to 200 °C. The final temperature was held for 5 min. The column was split at the end by a splitter into two pieces of deactivated capillary (length 40 cm, i.d. 0.25 mm). One capillary was led to the FID and the other to the head preparation. Virgin females of A. fraterculus (10–20 days old, N = 6) were used for the analyses. The head of an individual was excised from the thorax using micro-scissors. The base of the head and the tip of one antenna were mounted between two glass capillary electrodes, which were filled with insect Ringer solution (8.0 g/L NaCl, 0.4 g/L KCl, 0.4 g/L CaCl2) and connected to silver wires, closing an electric circuit. The amplified antennal signal was led to the EAD interface. A compound was considered to be EAD-active when it elicited a depolarization response in at least three of the six antennae.
Gas chromatography–mass spectrometry analyses
The headspace extracts of A. fraterculus males were analyzed on a LECO Pegasus 4D GC × GC-TOFMS instrument (LECO Corporation, St. Joseph, MI, USA) equipped with a cryogenic modulator. The first-dimension chromatographic column (column 1) was 30 m × 250 µm i.d. × 0.25 µm ZB-5MS (Phenomenex, CA, USA). The second-dimension chromatographic column (column 2) was 2 m × 100 µm i.d. × 0.20 µm RTX-50 (Restec, Bellefonte, PA, USA). Helium (1 mL/min) was used as the carrier gas in a constant flow mode. The Agilent 7683 autosampler (Agilent, Palo, Alto, CA, USA) injected 1 µL of the sample in the splitless mode in a 220 °C inlet onto column 1. The column 1 was held at 40 °C for 2 min, then ramped at 5 °C/min to 270 °C, then at 20 °C/min to 320 °C and held for 2 min. The temperature of the column 2 oven was constantly 10 °C higher than that of the column 1 oven. The transfer line was 260 °C and the ion-source set point was 220 °C. The detector voltage was 1,750 V and the first filament bias was −70 V. Mass spectra were collected from m/z 10 to 600 at 100 spectra/s. The data were processed and consecutively visualized on 2D and 3D chromatograms using the LECO ChromaTOF software. A series of n-alkanes (C8–C22; Sigma-Aldrich) were co-injected with authentic samples to determine their retention indices. The compounds were identified by a comparison of their mass-spectra fragmentation patterns, retention times, and retention indices with previously published data (Adams 2007; Vaníčková 2012; Vanícková et al. 2012) and synthetic standards (see below).
Chemicals
Except for (S,S)-(−)-epianastrephin, which was provided by Prof. Jim Nation (University of Florida, Gainesville, USA), and the mixture of (E,E):(Z,E)-α-farnesene in a proportion of 4:1, (hereafter called α-farnesene), which was provided by Dr. Blanka Kalinová (IOCB, Czech Republic), all chemicals were purchased either from Sigma-Aldrich, Brazil [(1R)-(+)-α-pinene, (1S)-(−)-α-pinene, β-pinene, camphene, ethyl hexanoate, (1R)-(+)-limonene, (1S)-(−)-limonene, linalool, camphor, (Z)-3-nonen-1-ol] or from Penta, USA [(E,Z)-3,6-nonadien-1-ol]. The purities were >95 % based on the results from capillary gas chromatography.
Laboratory bioassays
To test the attractiveness of male headspace extracts to the virgin females of A. fraterculus as well as of the individual synthetic compounds and mixture composed of EAD-active compounds, bioassays were performed in a glass container (28 cm × 10 cm × 15 cm) covered on the upper open side by clear nylon screen. For each replicate, three virgin females were marked with odorless, non-toxic dyes of different colors and placed in the bioassay arena 1 h before testing. Solutions of the synthetic standards were prepared according to the relative proportions of each compound in natural headspace male extracts [1.5:1.5:30:30:0.1:2:26:9 (1R)-(+)-α-pinene:(1S)-(−)-α-pinene:(1R)-(+)-limonene:(1S)-(−)-limonene:(Z)-3-nonen-1-ol:(E,Z)-3,6-nonadien-1-ol:α-farnesene:(S,S)-(−)-epianastrephin, respectively] at total concentration of 100 ng/mL hexane. This particular concentration was chosen based on preliminary laboratory assays, in which we tested different concentrations of the synthetic mixtures (c = 0.1, 10, 100 ng/mL). One eppendorf tube containing 0.01 mg of a biopolymer {poly[β-(1 → 4)-2-acetamido-2-deoxy-d-glucose]} and 100 µL of each treatment to be tested was placed in the central top position of the bioassay arena, resulting in headspace concentrations of four males’ equivalent (4ME) or 10 ng of the individual compounds or synthetic mixture of compounds per eppendorf tube, respectively. The impregnation of the biopolymer microspheres with the respective solutions was performed 24 h before the experiments. This method controls the release of the compounds according to their volatilities (Shailaja et al. 1997). The following compounds and mixtures have been tested: 1 = (1R)-(+)-α-pinene, 2 = (1S)-(−)-α-pinene, 3 = (1R)-(+)-limonene, 4 = (1S)-(−)-limonene, 5 = (Z)-3-nonen-1-ol, 6 = (E,Z)-3,6-nonadienol, 7 = α-farnesene, 8 = (S,S)-(−)-epianastrephin, 9 = the synthetic mixture of EAD-active compounds, 10 = male headspace extract (4ME), 11 = hexane (control). In total, ten replicates for each compound or mixture, respectively, were performed. Each bioassay lasted 20 min and the behaviors exhibited by each group of three marked females per replicate were recorded and characterized using a video camera (Nikon®, D5000, 18–55 VR) together with the data of two simultaneous observers. The behavioral response of females constitutes the two observed variables: (1) agonistic and searching behavior ≤1 cm around the odor source (walking around with antennation, short flight and fight among females) and (2) touching the odor source and remaining there for more than 2 min. These behaviors have been previously described by Robacker (1988) for Anastrepha ludens. After each assay, we cleaned the arena with acetone, methanol and distillated water, respectively. All behavioral assays were performed between 07.00 a.m. and 10.00 a.m. with a completely randomized design.
Semi-field bioassays
The attractiveness of the treatment that elicited response in females similarly to male headspace extracts in the laboratory assays was performed under semi-natural conditions. We used a square clear nylon screen cage (4 m2 width × 2 m high), which was mounted in the field of the campus of Universidade Federal de Alagoas (09°39′57″S; 35°44′06″W). To partially provide the natural conditions required by flies, four small trees (1.5 m high) in plastic pots (30 cm high, 50 cm diam.) were placed within the cage: two Araçá trees (Psidium araca Raddi) and two Fig trees (Ficus ficus L.). The potted trees were arranged equidistantly to each other in the corners of the cage. Two-choice assays were performed using McPhail traps (Isca Technologies, Brazil). The traps were hung at 15 cm from the top of cage above of the Araçá trees. To prevent flies from escaping, 250 mL of water with Tween-20 (Sigma-Aldrich, Brazil) (1 mL of Tween-20 in 1 L water) was added to each trap. After each replicate, the position of the traps was rotated clockwise thus averaging out possible positional effects. For each treatment, the eppendorf tube containing 0.01 mg of microencapsulated biopolymer (as described above) was placed in the field cages 1 h before of the release of flies. The synthetic mixture was offered at doses of 100 µg. This particular concentration was chosen based on the preliminary semi-field bioassays, in which we tested different concentrations of the synthetic mixtures (c = 10, 100, 500 µg/mL). In the first set of experiments, we evaluated the attractiveness of the synthetic mixture against a control (traps without baits) and in the second the synthetic mixture against male headspace extracts. Thirteen females of A. fraterculus were released into the field cage daily at 07:00 h, and the number of flies caught per trap was counted and recorded at 17:00 h of the same day. The uncaught flies were removed from the field cages and used only once during the experiments. The first and second sets of experiments were repeated five times each. Bioassays were performed on sunny days, with mean temperature and relative humidity of 30 ± 2 °C and 77 ± 5 %, respectively.
Statistical analyses
For the laboratory bioassays, the One-way-ANOVA test was used to compare the mean responses of females among the treatments regarding the two behavioral modalities observed (P < 0.05). Normality of data and homogeneity of variances were assessed using the Liliefors and Levene’s tests, respectively. The Tukey-HSD test was used for a posteriori pairwise comparisons (α < 0.05). The analyses were performed in BioEstat 5.0 (Ayres et al. 2007).
For the field-cage bioassays, a Wilcoxon signed-rank test was used to compare: (1) the number of females caught by the trap baited with the synthetic mixture composed of all EAD-active vs. the trap baited with a hexane control and (2) the number of females caught by the trap baited with the synthetic mixture composed of all EAD-active vs. the trap baited with male headspace samples. The tests were performed using the spreadsheet provided by http://www.biostathandbook.com/wilcoxonsignedrank.html (accessed 12 September 2014; see also McDonald 2009).
Results
Chemical analyses of headspace extracts of A. fraterculus males
The GC × GC-TOFMS analyses revealed the presence of 29 compounds in the headspace extracts of A. fraterculus males. The compounds represent a complex mixture of diverse chemical structures including terpenoids, alcohols, aldehydes, ketones and esters (Table 1). The major components (>19 %) were identified as limonene and (E,E)-α-farnesene. Minor components (1–7 %) included 2-hexanone, α-pinene, 2-ethylhexan-1-ol, p-cymene, indane, (E)-β-ocimene, (E,Z)-3,6-nonadien-1-ol, (Z,E)-α-farnesene, suspensolide, anastrephin and epianastrephin. Some volatiles were detected only in trace amounts (<0.1 %), including (Z)-3-nonen-1-ol. The stereochemistry of limonene and α-pinene could not be determined.
Electrophysiological analyses of headspace extracts of A. fraterculus males
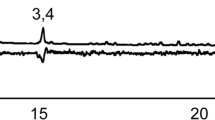
The GC-EAD analyses with the headspace samples of A. fraterculus males revealed six compounds in this complex blend eliciting consistent depolarization in the antennae of conspecific females (Table 1; Fig. 1). The compounds eliciting antennal depolarization were α-pinene, limonene, (Z)-3-nonen-1-ol and (E,Z)-3,6-nonadien-1-ol, α-farnesene and (S,S)-(−)-epianastrephin, the latter eliciting the strongest antennal response in females of A. fraterculus.
Behavioral response of Anastrepha fraterculus females to synthetic compounds in the laboratory assays
The bioassays showed that the headspace extract of A. fraterculus males, as well as the individual compounds and their mixture eliciting antennal depolarization, mediated behavioral responses in conspecific virgin females (Fig. 2a, b). With regards to behavior 1 (agonistic and searching behavior ≤1 cm around the odor source), the mean number of responses by females also differed significantly among treatments (F 10,99 = 230.4, P < 0.0001). Post hoc comparisons indicated that all treatments tested elicited more responses in conspecific virgin females than a hexane control. In relation to behavior 2 (touching the odor source and remaining there for more than 2 min), the mean number of responses by females differed significantly among treatments (F 10,99 = 123.6, P < 0.0001). Post hoc comparisons revealed that, with the exception of the two α-pinene enantiomers and (Z)-3-nonen-1-ol, all treatments tested elicited more positive responses of females than the hexane control. However, only the mixture of all EAD-active compounds was as effective as the male headspace extracts in eliciting contact by females (Fig. 2a). Again, only the synthetic mixture of all EAD-active compounds was as efficient as the male headspace extracts in this respect (Fig. 2b).
Mean responses of A. fraterculus females to EAD-active synthetic compounds (tested individually or as a blend), male headspace extracts, and a hexane control in laboratory assays (N = 10). a Agonistic and searching behavior ≤1 cm around the odor source and b touching the odor source and remain for more than 2 min. Box and whisker represent standard error and deviation, respectively. Distinct letters indicate significant differences among treatments (Tukey’s Post Hoc HSD test, P < 0.05)
Behavioral responses of A. fraterculus to synthetic compounds in a field cage
The traps baited with the mixture of synthetic EAD-active compounds caught significantly more A. fraterculus females than the control traps (Fig. 3a). However, females did not show any preference for the mixture of EAD-active compounds over the headspace male extract (Fig. 3b).
Median percentage of A. fraterculus females caught in semi-field two-choice bioassays (N = 5). Traps baited with a synthetic mixture of EAD-active compounds vs. either hexane (a) or male headspace extracts (b). Boxes and whiskers represent the first and third percentiles and maximum and minimum values, respectively. Differences between samples in each bioassay were assessed by Wilcoxon signed-rank test. n.s. not significant, ***P < 0.001
Discussion
This study represents a first step to identify and evaluate the volatiles released by A. fraterculus males, which act as attractant to conspecific females. Several compounds identified in the headspace extracts of sexually mature males of A. fraterculus in the present study (e.g. 2-ethylhexan-1-ol, limonene, (Z)-3-nonen-1-ol, (E,Z)-3,6-nonadien-1-ol, α-farnesene, (E,E)-suspensolide, anastrephin and epianastrephin) were previously reported as components of volatile blends emitted by males of the A. fraterculus from eight different populations from Brazil, one from Argentina and one from Peru (Břízová et al. 2013; Cáceres et al. 2009; Lima et al. 2001). The use of GC × GC-TOFMS technique, however, with its significant higher sensitivity and a better separation of co-eluting compounds, allowed the identification of further components, which had not been previously reported for Anastrepha species (Lima-Mendonça et al. 2014). From the twenty-nine compounds identified here, eleven are reported for the first time as constituents of the volatile blend emitted by A. fraterculus males, namely α-pinene, camphene, β-pinene, myrcene, camphor, bornyl acetate, caryophyllene oxide, 5-ethenyldihydro-5-methyl-2(3H)-furanone, 3-hexanone, hexanal, and indane. Most of these compounds have previously been identified as plant volatiles (El-Sayed 2014) and may derive from residues of the larval diet, as reported for other Tephritidae species, such as Ceratitis capitata (Vaníčková 2012; Vanícková et al. 2012).
The results provide strong evidence that the volatile blend released by calling males of A. fraterculus includes components acting as female attractants. These findings are in agreement with previously published studies on A. suspensa, A. obliqua, and A. serpentina, which show that females of these species are attracted to headspace extracts of conspecific calling males (López-Guillén et al. 2011; Nation 1975; Robacker et al. 2009). By using the GC-EAD technique, we were able to determine the compounds of the complex blend that are potentially involved in the attraction of females. The results of the bioassays do not only show that the EAD-active compounds are involved in female attraction, but also that different compounds might play different roles in the mating behavior of A. fraterculus. For example, as regards agonistic and searching behavior, all tested compounds attracted more females than a hexane control. These behaviors may be interpreted as a first step in the complex mating process of this species, i.e. attracting females to the mating site. Since mate is strongly associated with host plants, the use of plant-typical compounds (e.g. limonene, pinene, among others) would help females to find simultaneously mating and brood sites (Robacker and Hart 1986). However, after approaching a mating site, females must rely on specific compounds, which would signalize potential mate partners. Indeed, as regards behavior 2 (i.e. touch to the odor source and remaining there for more than 2 min), which seems to be more linked with mating per se, male-borne compounds [e.g. (E,Z)-3,6-nonadien-1-ol and (S,S)-(−)-epianastrephin] are highly attractive to females, whereas some plant-typical compounds (e.g. α-pinene and (Z)-3-nonen-1-ol) are not. This indicates that (E,Z)-3,6-nonadien-1-ol and (S,S)-(−)-epianastrephin are key pheromone components, which allow females to recognize males. Although our results suggest distinct roles for individual compounds, it is noteworthy to mention that, for both behavior modalities measured, only the synthetic mixture was as effective as the male headspace extracts in eliciting behavioral responses by A. fraterculus females. These results are in agreement with the unit model of female attractant proposed for Anastrepha ludens (Robacker 1988), in which pheromone components act as a unit to elicit each and/or all repertoire of behaviors.
Interestingly, except for α-pinene, all EAD-active compounds found in A. fraterculus were previously identified in the blend of volatiles released by males of three other sister species, i.e. A. suspensa (Rocca et al. 1992), A. ludens (Rocca et al. 1992, Robacker and Garcia 1990; Robacker and Hart 1985), and A. obliqua (López-Guillén et al. 2011), which were collected using the headspace technique. Among the EAD-active compounds, (S,S)-(−)-epianastrephin was responsible for the strongest antennal depolarization in A. fraterculus females. This lactone has already been identified in other species of Anastrepha, such as A. ludens (Robacker and Hart 1985) and A. suspensa (Rocca et al. 1992). In the females of A. ludens, (S,S)-(−)-epianastrephin also elicited the strongest antennal depolarization among the compounds tested (Robacker and Hart 1986). However, it only triggered behavioral responses when offered in combination with either (Z,Z)-3,6-nonadien-1-ol or (Z)-3-nonen-1-ol (Robacker 1988). The observed synergistic activity of compounds might play a crucial role as reproductive barrier among Anastrepha species, since the volatiles released by males of phylogenetically related species are quite similar (López-Guillén et al. 2011). Thus, specific combinations of compounds, rather than individual components might mediate intraspecific and interspecific recognition unambiguously. Indeed, many insect pheromones are multi-component blends (Ayasse et al. 2001; Kroiss 2008). In A. fraterculus, quantitative and qualitative variation of male pheromone blends of distinct populations has been shown (Břízová et al. 2013). Besides, males and females of the chemically distinct populations were reported to be partially or fully incompatible in mate choice tests (Dias 2012; Vera et al. 2006). The qualitative and/or quantitative differences in the composition of the multi-component pheromone might influence the decision of the female for conspecific or heterospecific males. Future experimental studies integrating electrophysiological analyses and behavioral assays and testing the attraction of females to the volatiles released by males of different species or populations are necessary to better understand the significance of variable volatile blends as reproductive barriers in the genus Anastrepha.
During the last decades, several studies have demonstrated a successful implementation of semiochemicals in the effective control of some tephritid species using the host marking pheromone (HMP), host plant kairomones, and allomones (Aluja et al. 2009; Howse 1989; Nigg et al. 1994; Shelly and Villalobos 2004). However, to date, no approach has been undertaken in A. fraterculus, using male sex pheromones as a strategy for pest control. Our bioassays performed under semi-field conditions showed that traps baited with synthetic mixtures captured more flies than a hexane control and that the synthetic mixture captured females as effectively as male headspace extracts. Furthermore, the high number of females captured in the bioassays (at least 30 %) indicates that the use of male-borne attractants could be an interesting alternative for controlling (or at least reducing) the infestation by A. fraterculus in fruit crops. The behavioral activity of the compounds identified here will be evaluated in future field tests.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Illinois
Aluja M (1994) Binomics and management of Anastrepha. Annu Rev Entomol 39:155–178
Aluja M, Norrbom AL (2001) Fruit flies (Tephrtitidae) phylogeny and evolution of behavior. CRC Press LLC, Boca Raton
Aluja M, Díaz-Fleischer F, Boller EF, Hurter J, Edmunds AJF, Hagmann L, Patrian B, Reyes J (2009) Application of feces extracts and synthetic analogues of the host marking pheromone of Anastrepha ludens significantly reduces fruit infestation by A. obliqua in tropical plum and mango backyard orchards. J Econ Entomol 102:2268–2278
Ayasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the order Hymenoptera. Annu Rev Entomol 46:31–78
Ayres M, Ayres Jr M, Ayres DL, dos Santos AS (2007) BioEstat 5.0—Aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém, Sociedade Civil Mamirauá, p 364
Břízová R, Mendonça AL, Vaníčková L, Lima-Mendonça A, da Silva CE, Tomčala A, Paranhos BAJ, Dias VS, Joachim-Bravo IS, Hoskovec M, Kalinová B, do Nascimento RR (2013) Pheromone analyses of the Anastrepha fraterculus (Diptera: Tephritidae) cryptic species complex. Flo Entomol 96:1107–1115
Cáceres C, Segura DF, Vera MT, Wornoayporn V, Cladera JL, Teal P, Sapountzis P, Bourtzis P, Zacharopoulou A, Robinson AS (2009) Incipient speciation revealed in Anastrepha fraterculus (Diptera; Tephritidae) by studies on mating compatibility, sex pheromones, hybridization, and cytology. Biol J Linn Soc 97:152–165
Dias V (2012) Compatibilidade de acasalamento de populações do complexo Anastrepha fraterculus (Diptera: Tephritidae) do Brasil. Dissertation, Universidade Federal da Bahia
El-Sayed AM (2014) The Pherobase: Database of Pheromones and Semiochemicals. http://www.pherobase.com
Howse PE (1989) Insect attractant. University of Southampton, United Kingdom
Kroiss J (2008) Chemical attraction and deception Intra- and interspecific communication in Hymenoptera. Dissertation, Universität Regensburg
Lima IS, House PE, do Nascimento RR (2001) Volatile substances from male Anastrepha fraterculus Wied. (Diptera: Tephritidae): identification and behavioural activity. J Braz Chem Soc 12:196–201
Lima-Mendonça A, AdL Mendonça, SanťAn AEG, do Nascimento RR (2014) Semiochemicals of fruit flies of the genus Anastrepha. Quim Nova 37:293–301
López-Guillén G, López LC, Malo EA, Rojas JC (2011) Olfactory responses of Anastrepha obliqua (Diptera: Tephritidae) to volatiles emitted by calling males. Fla Entomol 94:874–881
McDonald JH (2009) Handbook of biological statistics. Sparky House Publishing, Baltimore
Nascimento AS, Carvalho RS (2000) Manejo Integrado de Moscas das frutas. In: Malavasi A, Zucchi RA (eds) Moscas das Frutas de importância econômica: Conhecimento básico e aplicado. Holos, São Paulo, p 169
Nation JL (1975) The sex pheromone blend of Caribbean fruit fly males: isolation biological activity, and partial chemical characterization. J Environ Entomol 4:27–30
Nation JL (1989) The role of pheromones in the mating system of Anastrepha fruit flies. In: Robinson AS, Hooper G (eds) Fruit flies: Their biology, natural enemies and control. Elsevier Science Publisher, Amsterdam, pp 189–205
Nigg HN, Mallory LL, Simpson SE, Callaham SB, Toth JP, Fraser S, Klim M, Nagy S, Nation JL, Attaway JA (1994) Carribean fruit fly, Anastrepha suspensa (Loew), attraction to host fruit and host kairomones. J Chem Ecol 20:727–743
Norrbom AL, Korytkowski CA (2011) New species of and taxonomic notes on Anastrepha (Diptera: Tephritidae). Zootaxa 2740:1–23
Robacker DC (1988) Behavioral response of female Mexican fruit flies, Anastrepha ludens, to components of male-produced sex pheromone. J Chem Ecol 14:1715–1726
Robacker DC, Garcia JA (1990) Responce of laboratory strain Mexican fruit flies, Anastrepha ludens, to combination of fermenting fruit odor and male-produced pheromone in laboratory bioassays. J Chem Ecol 16:2027–2038
Robacker DC, Hart WG (1985) (Z)-3-nonenol, (Z, Z)-3,6-nonadienol and (S, S)-(−)-epianastrephin: male produced pheromones of the Mexican fruit fly. Entomol Exp Appl 39:103–108
Robacker DC, Hart WG (1986) Behavioral response of male and female Mexican fruit flies, Anastrepha ludens, to male-produced chemicals in laboratory experiments. J Chem Ecol 12:39–47
Robacker DC, Aluja M, Cossé AA, Sacchetti P (2009) Sex pheromone investigation of Anastrepha serpentina (Diptera: Tephritidae). Ann Entomol Soc Am 3:560–566
Rocca JR, Nation JL, Strekowski L, Battiste MA (1992) Comparison of volatiles emitted by male Caribbean and Mexican fruit flies. J Chem Ecol 18:223–244
Shailaja D, Ahmed SM, Yaseen M (1997) Comparative study of release kinetics of pheromone from polymer dispensers. J Appl Polym Sci 64:1373–1380
Shelly TE, Villalobos EM (2004) Host plant influence on the mating success of male Mediterranean fruit flies: variable effects within and between individual plants. Anim Behav 68:417–426
Vaníčková L (2012) Chemical ecology of fruit flies: genera Ceratitis and Anastrepha. Dissertation, Institute of Chemical Technology
Vaníčková L, do Nascimento RR, Hoskovec M, Ježková Z, Břízová R, Břízová R, Tomčala A, Kalinová B (2012) Are the wild and laboratory insect populations different in semiochemical emission? The case of medfly sex pheromone. J Agric Food Chem 60:7168–7176
Vera MT, Cáceres C, Wornoayporn V, Islam A, Robinson AS, De La Vega MH, Hendrichs J, Cayol JP (2006) Mating incompatibility among populations of the South American fruit fly Anastrepha fraterculus (Diptera: Tephritidae). Ann Entomol Soc Am 99:387–397
Zucchi RA (2000) Espécies de Anastrepha, sinonímias, plantas hospedeiras e parasitóides. In: Malavasi A, Zucchi RA (eds) Moscas-das-frutas de importância econômica no Brasil: Conhecimento básico e aplicado. Holos Editora, Ribeirão Preto, pp 41–48
Acknowledgments
We thank the valuable comments of the two anonymous referees in an earlier version of this manuscript. This work was supported by: FAO/IAEA, Vienna, Austria (the funding was provided to Research Contracts No. 16051 as part of the Coordinated Research Project on the Resolution of the Cryptic Species Complexes of Tephritid Pests to Overcome Constrains to SIT Application and International Trade), and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, Proc. Nos. 555094/2010-3, 308695/2011-9 and 376348/2012-7), and the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Prague (RVO: 61388963). A patent application has been filled for this discovery.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Schmitt.
Rights and permissions
About this article
Cite this article
Milet-Pinheiro, P., Navarro, D.M.A., De Aquino, N.C. et al. Identification of male-borne attractants in Anastrepha fraterculus (Diptera: Tephritidae). Chemoecology 25, 115–122 (2015). https://doi.org/10.1007/s00049-014-0180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0180-3