Abstract
Analysis of the pheromone gland extract of females of the click beetle Agriotes sordidus (Illiger), revealed the presence of geranyl hexanoate (GH) and (E,E)-farnesyl hexanoate (FH) in an approximate ratio of 1:1. In the female-released volatiles collected by headspace extraction, GH was a dominant component with FH present only in trace amounts. In field trapping tests GH on its own captured high numbers of A. sordidus adults, whereas the addition of FH in various proportions had no effect on captures. A closer scrutiny of adults caught in GH-baited traps revealed that 10–40 % of them were females. Significantly higher numbers of both female and male beetles were attracted to traps baited with GH as compared to unbaited controls showing a clear dose–response relationship with higher doses catching more beetles. In electroantennogram (EAG) tests responses of female and male antennae to a number of known click beetle pheromone components showed the same trend in both sexes, giving the highest answers to GH. This suggests that female and male antennae are similar with respect to the perception of pheromone components, and that GH shows some activity as an aggregation pheromone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implementation of IPM strategies against wireworms, the larvae of click beetles (Coleoptera, Elateridae) have been hampered due to the technical difficulties of traditional detection and monitoring methods. Following robust research efforts in the 1980s and 1990s mainly by scientists of the former Soviet Union, pheromones for a number of pest elaterids (Agriotes spp.) were elucidated (Siirde et al. 1993; Yatsynin et al. 1996). Pheromone trapping has been suggested as an easy, effective, and cheap method to precisely determine the distribution of click beetles (Furlan et al. 1997). During the past decade, the use of pheromone- or attractant-baited traps for the detection and monitoring of adults of several of the economically most important Agriotes species in Western and Central Europe has become widespread (Furlan et al. 2007).
Agriotes sordidus (Illiger) is an economically important pest in the southwestern part of Europe, but it is present in northern countries like France and Germany (Furlan et al. 2007; Lehmhus and Niepold 2013). In fact it can be considered a key pest of maize and other crops in Italy (Platia 1991; Furlan 1999, 2004, 2014; Furlan et al. 2000, 2004).
In the course of systematic field screening campaigns of synthetic compounds known from the literature as pheromone components for various click beetle species, synthetic geranyl hexanoate (GH) was found to be highly attractive to adult A. sordidus (Tóth et al. 2002a). At the same time, GH proved to be highly attractive also to the closely related A. rufipalpis Brullé (Tóth et al. 2002a), which is abundant in Central and Eastern European countries (Furlan et al. 2007).
The present study was undertaken to unequivocally characterize the chemical composition of the female-produced pheromone of A. sordidus i.e. to confirm the presence of GH as a naturally occurring behavior mediating compound. Electrophysiological reactions and behavioral responses of adult male and female beetles to selected compounds were studied to gain more knowledge on pheromone communication of click beetles in general.
Methods and materials
Insects Adult A. sordidus specimens were collected from populations reared at the Italian laboratory according to described methods (Furlan 1998, 2004).
Rearing in cages and vials started from feral females or larvae; the females obtained in overwintering cells were kept isolated in single vials with humid soil and some rye grass leaves. Solvent extraction of beetles was performed at the same time when swarming was observed in the field.
Live insects were taken to the Budapest laboratory. Female pheromone gland extracts were prepared as described (Oleshchenko et al. 1976; Ivaschenko and Adamenko 1980), by carefully piercing the pheromone gland with a fine glass capillary and collecting the liquid into the capillary. The liquid samples obtained were dissolved in hexane (a.g., MERCK KGaA, Darmstadt, Germany) to make up stock solutions.
One extract was prepared from 28 glands from females (insect culture originated from a north Italian population) using 30 µl of hexane yielding 0.93 female equivalents (FE) per µl. For a second extract, the gland contents of 33 females (the insect culture originated from a south Italian population) were extracted in 25 µl of hexane, yielding 1.32 FE per µl.
The collection of headspace volatiles was performed in an all glass/Teflon® closed-loop system (CLSA; Boland et al. 1984). Fifty female beetles were placed into a ca. 200 ml glass container for headspace collections. The air flow was 18 ml/s. Volatiles released by the beetles were collected on a CLSA carbon filter (5 mg; Brechbühler AG, Schlieren, Switzerland) for 48 h, after which the filters were extracted with ca. 20 µl dichloromethane [yielding 120 female hour equivalents (FHE) per µl]. For analysis, 1–2 µl of these samples were injected into a gas chromatograph.
Chemical analysis Gas chromatography (GC) was performed on a Hewlett-Packard 5890 instrument (Hewlett-Packard, Palo Alto, USA) equipped with a flame ionization detector, using the following columns:
-
SP 2340 fused silica (Supelco Inc., Bellefonte, USA), 30 m × 0.32 mm id; 0.20 um; 1.5 m ret. gap at the beginning of the column; split/splitless injector; 210°; carrier He 1.5 ml/min; septum purge 2 ml/min; temperature program: 60° 1 min; 10°/min to 100°; 5°/min to 220°; 20 min.
-
HP Ultra 1 crosslinked methyl silicone (Hewlett-Packard, Palo Alto, USA), 25 m × 0.2 mm id; 0.33 μm; 2.5 m ret. gap at the beginning of the column; dedicated on column injector; carrier He 1.5 ml/min; supply flow 30 ml/min; temperature program: 60°1 min; 10°/min to 100°; 5°/min to 220°; 40 min.
Gas chromatography coupled with mass spectrometry (GC/MS) was carried out by electron impact at 70 eV using a double focusing VG 70/250 SE mass spectrometer (Vacuum Generators, Manchester, UK) linked to a HP 5890 gas chromatograph. Separations were performed with a 50 m × 0.25 mm id FS capillary column coated with FFAP as the stationary phase (Macherey & Nagel, Düren, Germany). Using He as the carrier gas, the column was operated under temperature program: 3 min at 60 °C; 3 °C/min to 220 °C. Structure assignments were based on comparison of mass spectra of natural products with those reported in the literature (Bergström and Tengö 1974; McLafferty and Stauffer 1989). Unambiguous identifications were secured by comparing analytical data of the target compounds with those of authentic reference samples.
Quantification of the dominant component GH was based on calibration with synthetic standards.
Chemicals Geranyl hexanoate (GH) was purchased from Bedoukian Inc. (Danbury, USA) and was >98 % pure by GC.
(E,E)-Farnesyl hexanoate (FH) was synthesized in a purity of >96 % from (E,E)-farnesol (96 %; Bedoukian Inc.) and hexanoyl chloride (98 %; Sigma-Aldrich Kft, Budapest, Hungary) as described earlier (Tóth et al. 2003).
Traps Bottle traps were funnel traps, homemade from 2 l transparent plastic bottles by cutting the bottle below the neck (ca 10 cm), and placing the cut part upside down into the remaining bottom of the bottle (Tóth et al. 2002b). The resulting funnel entrance diameter was ca. 8 cm; the funnel hole diameter was ca. 2 cm. A transparent plastic sheet (10 × 10 cm) was placed in a vertical position immediately above the opening of the funnel. The bait dispenser was attached to the vertical plastic sheet so that the attractant-containing part was hanging into the large opening of the funnel.
YATLORf traps (Furlan et al. 2001; Furlan and Gnes 2003) (produced by RO-SA Micromecanica, San Donà di Piave, Venice, Veneto, Italy) (Fig. 1) were used in some experiments. Briefly, the bottom part of the traps was made of plastic and was similar in shape and size to the “Estron” trap described earlier (Oleschenko et al. 1987; Kudryavtsev et al. 1993) for the capture of crawling click beetles. The upper part was made of a plastic funnel attached to the bottom part at the narrow end, with two transverse vertical plastic sheets placed immediately above the opening of the funnel, thereby enabling the YATLORf trap to also catch flying individuals.
Bait Dispensers for Traps Bait dispensers for the tests were prepared by adding the required amounts of synthetic compounds (dissolved in 200 µl of hexane) into 0.7 ml polyethylene vials with lid (No. 730, Kartell Co., Italy). After evaporation of the hexane, the lids were closed, and the dispensers were wrapped individually in pieces of alufoil. All dispensers were stored at −10 °C until use.
Field trapping Field tests were conducted at multiple sites in Italy (Veneto region) and Hungary during several seasons.
Traps were set up in blocks. Each block was comprised of one of each treatment. The distance of traps within a block was 5–10 m. The distance between blocks ranged between 50–100 m. Traps were rotated within a block at each inspection, when captured beetles were recorded and removed.
The catch of a trap during the entire experimental period was taken as a replicate for statistics. As suggested by Roelofs and Cardé (1977) for trapping tests of similar nature, the catch data were transformed using (x + 0.5)1/2 and analyzed by ANOVA. If the ANOVA yielded significance, then treatment means were separated by Student–Newman–Keuls test. In case one of the treatments caught no insects, the Bonferroni–Dunn test (Dunn 1961) was used to check that mean catches in other treatments were not significantly different from zero catch (see also Table and Figure legends).
All statistical procedures were conducted using the software packages StatView® v4.01 and SuperANOVA® v1.11 (Abacus Concepts, Inc., Berkeley, USA).
Description of single field experiments
Testing the addition of FH to GH in different ratios
Experiment 1A. The objective was to check whether the addition of FH influenced the field activity of GH on A. sordidus. Treatments included traps with 30 µl of GH or FH alone, and traps with blends of 30:3, 30:30 and 3:30 µl of GH:FH. Unbaited traps were not included, since a conclusive field activity of GH (at the dose of 30 µl) had already been demonstrated (Tóth et al. 2002a). The test was run from May 20 to June 20, 1998; farm Greggio, Eraclea, Veneto Region, Italy (45° 37.98′ N 12° 38.51′ E); alfalfa field; six blocks of bottle traps.
Experiment 1B. This test was aimed at investigating the effect of the addition of FH to GH on catches of A. rufipalpis, a closely related species also known to respond to GH (Tóth et al. 2002a), to check for possible interspecific effects of FH (identified in pheromone extracts of A. sordidus during this study). Treatments were identical to those of Experiment 1A. The test was run from May 8 to June 19, 1998, Debrecen, Hajdú-Bihar county, Hungary (47° 36.3′ N 21° 39.15′ E); alfalfa field; four blocks of bottle traps.
Experiment 2. The objective was to study the effects of the addition of FH in low amounts to GH. Treatments consisted of traps baited with GH (30 µl) alone, or with blends of 30:1 µl and 30:0.3 µl of GH:FH. The test was performed from May 7 to June 8, 1999; farm Greggio, Eraclea, Veneto Region, Italy; maize field, three blocks of bottle traps.
Female-to-Male Ratios in Traps Baited with GH The objective of the following two experiments was to check for the possible presence of female beetles in catches of monitoring traps baited with 30 µl of GH. No other treatments were tested.
Experiment 3. March 15–May 17; 2004 Concordia Sagittaria, Veneto Region, Italy (45° 37.98′ N 12° 40.01′ E); bare soil; single YATLORf trap.
Experiment 4. May 1–25, 2009; San Donà di Piave, Veneto Region, Italy (45° 37.99′ N 12° 38.51′ E); bare soil; four YATLORf traps.
Testing different doses of GH on female and male catches
Experiment 5. This test aimed at checking the effect of increasing doses of GH on catches of male and female A. sordidus. The treatments of 10, 30 and 100 µl of GH and unbaited controls were set out. The test was run from May 1 to 26, 2011; San Donà di Piave, Veneto Region, Italy; bare soil; four blocks of YATLORf traps.
Testing traps baited with alive male beetles
Experiment 6. The objective was to see whether live males used as bait in traps attract conspecifics. Treatments included 20, 50 and 100 males, traps with synthetic GH and unbaited traps. For bait insects, fresh males from pheromone traps were collected and kept alive by providing fresh Festuca leaves and water soaked up in cotton wool. The test was run from May 16 to 26, 2007; San Donà di Piave, Veneto Region, Italy; bare soil; four blocks of YATLORf traps.
Electroantennography (EAG) The objective was to compare the antennal response of male and female A. sordidus to a range of synthetic standards of known click beetle pheromone compounds (Tóth 2013), to obtain an EAG response pattern (Roelofs 1977). Pure air (ambient, with no chemical stimulus) was tested as control.
A stainless steel tube (Teflon-coated inside) with a constant humidified airflow of ca. 0.7 l/min was set up. An antenna from a live beetle was amputated at the base and quickly mounted between two glass capillaries containing 0.1 M KCl solution. The mounted antenna was then placed at ca. 3 mm distance from the exiting airflow. One of the electrodes was grounded while the other was connected to a high-impedance DC amplifier (IDAC-232; Syntech, Hilversum, The Netherlands). Test compounds (10 µg each) were administered in hexane solution onto a 10 × 10 mm piece of filter paper inside a Pasteur pipette. This dosage was assumed to exceed the saturation level for all compounds, thereby balancing for differences in volatility of the compounds as suggested for similar EAG screening studies by Roelofs (1977). Stimulus treatments consisted of pushing 1 ml of air through the Pasteur pipette into the air stream flowing towards the antenna. Response amplitudes were normalized against the means of responses to the standard (E,E)-farnesyl acetate which was tested before and after the test compounds. Stimuli were administered at ca. 20–30 s intervals.
Experimental insects originated from the rearing of L. Furlan (Italy) as described earlier.
Results
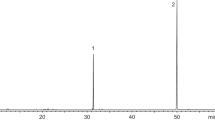
Results of Chemical Analysis of Pheromone Extracts Gas chromatographic (GC) analysis revealed two major peaks in the gland extract of female A. sordidus in proportions of ca. 1:1 (see Fig. 2). The mass spectra of the two compounds matched those of geranyl hexanoate (GH) and farnesyl hexanoate (FH), respectively (Bergström and Tengö 1974). On both the SP-2340 and the Ultra-1 columns, retention times of the natural products proved to be identical to synthetic samples of these terpenyl esters. The amount of the major pheromone component GH was estimated to range between 0.6 and 1.2 µg/female gland, and there were no significant differences in the quantitative compositions of extracts from north or south Italian populations: the arithmetic means of 977.5 ng resp. 1.085 ng (50 individual analyses from beetles at both places) equals approximately 1:1.
Gas chromatogram of a pheromone gland extract from female Agriotes sordidus (50 m FS-FFAP, 3 min 60 °C, 3 °C/min to 220 °C. For identity of peaks with numbers refer to Table 1)
Apart from GH and FH, GC/MS-analyses revealed the presence of small amounts of geraniol and farnesol and traces of some additional terpenyl esters (Fig. 2; Table 1) including 6,7-epoxygeranyl hexanoate that had been also found in A. obscurus L. (Tolasch 2004).
In contrast to results obtained with gland extracts, GC analysis of head space collections from females showed GH to be highly dominating, whereas the much less volatile FH could be detected only in traces (Fig. 3; Table 1).
Gas chromatographic analysis of gland extracts (a) and volatile collections (b) from female Agriotes sordidus [HP Ultra 1 crosslinked methyl silicone capillary column; int. std. internal standard n-dodecyl acetate; GH geranyl hexanoate; FH (E,E)-farnesyl hexanoate]. a 0.05 FE of extract and 50 ng int. std. injected; b 60 FHE of extract and 25 ng int. std. injected
Field Trapping Tests with Selected Compounds Identified in Pheromone Extracts In Experiment 1A traps containing only GH caught numerically the highest number of A. sordidus beetles, not differing from catches with blends of GH and FH in ratios of 10:1 or 1:1 (Table 2). The blend of GH:FH in a ratio of 1:10 caught significantly less beetles than any other treatments containing GH. All lures containing GH caught significantly more than traps with FH alone, which caught nil.
In Experiment 2, there was again no difference between catches of traps baited with GH alone or with 100:3 or 100:1 GH:FH mixtures (Table 2). All treatments containing GH caught significantly more than unbaited controls.
Experiment 1B was carried out in an area where A. sordidus was absent but the closely related A. rufipalpis was present. It aimed at studying possible interspecific effects of the addition of FH to GH on the pheromone of A. rufipalpis. As a result, GH alone or with blends containing GH and FH in ratios of 10:1 or 1:1 caught similarly high numbers of A. rufipalpis (Table 2). Traps with the 1:10 blend caught significantly less beetles, but still more than FH on its own, which caught the lowest mean number.
Female-to-Male Ratio of Catch in Traps with GH In Experiment 3, out of 57 beetles caught in a single GH-baited monitoring trap ca 40 % were females. In Experiment 4, from a total of 1,292 beetles caught, ca 10 % were females.
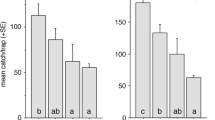
When a dose range of GH was tested (Experiment 5), even 10 µl, the lowest dose tested caught significantly more male A. sordidus than unbaited traps (Fig. 4a). Higher doses caught increasing numbers of males. Female catches showed a very similar pattern (Fig. 4b). Traps with all dosages of GH caught significantly more females than unbaited traps, and female catches also showed an increasing trend with increasing dose.
Mean (+SE) catches of male and female A. sordidus in traps baited with different doses of geranyl hexanoate (GH) in Experiment 5. a Males (total caught 709 beetles); b females (total caught 109 beetles). Means with same letter within one diagram are not significantly different at p = 5 % by ANOVA, Student–Newman–Keuls and Bonferroni–Dunn posthocs
Testing traps baited with alive male beetles
In Experiment 6, totals of 39, 0, 1, 0 and 2 female A. sordidus were caught in traps baited with synthetic GH, with 20, 50, or 100 alive males and unbaited traps, resp. Traps also caught 301, 0, 1, 0 and 0 males (for GH, 20, 50, 100 bait males and unbaited, resp.).
EAG Responses Antennal responses from male and female beetles showed remarkable similarities. While high antennal responses to GH were seen for both sexes, lower amplitude responses were noted in the same tendency in both males or females to geraniol and farnesol and some additional terpenyl esters (Fig. 5). As expected, low responses were recorded to stimulation by pure air.
Mean (+ SE) normalized electroantennogram (EAG) responses of Agriotes sordidus to selected click beetle pheromone components and air (control with no stimulus). Reponses were normalized against response to the standard (E,E)-farnesyl acetate (=100 %). Means with same letter within one diagram are not significantly different at p = 5 % by ANOVA, Student–Newman–Keuls
Discussion
It has been reported that synthetic GH attracted A. sordidus males in the field (Tóth et al. 2002a). The present study confirmed that this compound is indeed produced and emitted by female beetles, and thus, GH can be regarded as the main component of the sex pheromone of A. sordidus. First identified as a volatile compound released by click beetles (Borg-Karlson et al. 1988), GH appears to be widespread among pheromones of Agriotes spp. and has been identified in pheromone gland extracts of 6 Agriotes spp. (Tóth 2013). In A. obscurus, its behavior mediating activity as a pheromone component in the field is also well proven (see Tóth et al. 2003 and references therein).
FH, the second compound identified from gland extracts in the present study, has been found in glands of 4 other Agriotes spp. (Tóth 2013). However, to our knowledge its behavioral activity has not been proven in any click beetle species so far. The present study also failed to demonstrate any behavioral responses evoked by FH in A. sordidus, and FH had no apparent function in influencing interspecific chemical communication between A. rufipalpis and A. sordidus either.
This lack of an apparent communication function of FH may indicate that it serves other functions. Terpenes such as farnesol and its derivatives (farnesyl acetate), are known to show various physiological activities including antibiotic properties (Meigs et al. 1995; Brasch et al. 2014), and in the present case FH may well play a role as a component of “sex gland hygiene”. It is noteworthy that the ratio of compounds obtained by direct solvent extraction of pheromone glands does not necessarily reflect the ratio of components released into the air by a calling female (Millar and Sims 1998), and in fact, there has been another such documented case in pheromone studies on elaterids (Vuts et al. 2012). Geranyl butanoate was present in only traces (as compared to the highly dominant geranyl octanoate) in gland extracts of A. lineatus L. and A. proximus Schwarz, while much larger proportions of the (much more volatile) butanoate were trapped in collections of volatiles from females (Vuts et al. 2012). Optimal synthetic blends in field tests contained 1:3 to 1:1 butanoate : octanoate (Tóth et al. 2008).
Interestingly, FH was found to be present in the Dufour’s gland secretion of female Andrena bees (Hymenoptera, Andrenidae) (Bergström and Tengö 1974; Tengö and Bergström 1975), and in the cephalic secretion of many male Nomada bees (Hymenoptera, Anthophoridae) (Tengö and Bergström 1977). Females of Nomada parasitize the nests of Andrena, and many Nomada species confine their attacks to a single host species. In two such host-parasite pairs (A. haemorrhoa F.–N. bifida Thomson (=N. ruficornis Panzer) and A. carantonica Pérez–N. marshamella Kirby), FH was found to be the dominant component in respective secretions. This pairwise odor correspondence evidently relates to critical points of contact in the life cycles of host and parasite, male and female (Tengö and Bergström 1977).
FH has also been detected in the orchid Ophrys sphegodes Mill. (Asparagales, Orchidaceae) (Schiestl et al. 1997; Schiestl and Ayasse 2001). In its pollinator, the solitary bee Andrena nigroaenea Kirby, it has been found as a major constituent of the Dufour’s gland secretion and is present on the cuticle surface of unattractive females (Fernandes et al. 1981; Tengö and Bergström 1975). In A. nigroaenea, FH is electrophysiologically active on male antennae and inhibits the copulation behavior of males. Thus, it appears to also have a pheromonal function enabling males to discriminate between virgin and breeding females (Schiestl and Ayasse 2001).
At present nothing is known about the role of other minor components identified in the extracts of A. sordidus. Their possible pheromone functions (if any) should be the object of future studies.
With regard to A. sordidus, it is a most surprising result of the present study that females were also caught in traps baited with the pheromone GH. Since females—similarly to males—showed a dose–response reaction, we conclude that female catches in traps were indeed due to the GH containing bait in the traps, and this finding appeared not to be an artifact. Further indirect evidence for the effect of GH on females became evident in the EAG tests, suggesting that the antennae of females have a perception system similar to that of males. This is in contrast to moths, where male antennae are highly responsive to female-produced pheromone components, which, however, usually do not evoke significant EAG responses from female antennae.
Our results (Exp. 6.) clearly disprove the hypothesis that trapping of females could be due to a possible male signal emitted by already trapped males.
Earlier, general consent supposed that click beetles have a “classical” type of sex pheromone, with females producing and only males responding to the signal. In the view of the present results this should be re-evaluated. It will be interesting to investigate how general the phenomenon is among click beetles, and whether females of species other than A. sordidus also respond to their respective pheromone components.
To the best of our knowledge there are no previous reports in the literature on the courtship and mating behavior of A. sordidus. Future in-depth behavioral and ecological studies may clarify why females of A. sordidus would benefit from being attracted to their own pheromone. A probable hypothesis can be that—similar to other beetles using aggregation pheromones—females may be attracted by other females to a suitable food source or mating place in the biotope. In the bark beetle Dendroctonus brevicomis LeConte aggregation pheromones are active on both sexes. However, the female-released frontalin predominantly attracts males, whereas the male-produced exo-brevicomin attracts more females than males, indicating that the aggregation pheromone partially also acts as a sex pheromone (Wood 1982).
Based on the results described in this paper, traps baited with GH have been introduced into practical use in the detection and population monitoring of A. sordidus in Europe (Furlan et al. 2007; Burgio et al. 2012).
As for practical application of GH as a pheromone lure to trap A. sordidus for detection and monitoring, it is highly advantageous that it also attracts females. Traps capturing females could provide more reliable data on the timing of oviposition and thus result in more precise pest control decisions (Wall 1989; Witzgall et al. 2010). In fact, all other practical applications using the pheromone (mass trapping, lure and kill, mating disruption, etc.) may benefit from the phenomenon that GH attracts females, albeit at much lower intensity as it does males. Further research should go in the direction of increasing female catches.
References
Bergström G, Tengö J (1974) Studies on natural odoriferous compounds. Chemica Scripta 5:28–38
Boland W, Ney P, Jaenicke L, Gassmann G (1984) A “closed-loop-stripping” technique as a versatile tool for metabolic studies of volatiles. In: Schreier P (ed) Analysis of Volatiles. Walter de Gruyter, Berlin, pp 371–380
Borg-Karlson AK, Ågren L, Dobson H, Bergström G (1988) Identification and electroantennographic activity of sex specific geranyl esters in an abdominal gland of female Agriotes obscurus (L.) and A. lineatus (L.) (Coleoptera, Elateridae). Experientia 44:531–534
Brasch J, Horter F, Fritsch D, Beck-Jendroschek V, Tröger AG, Francke W (2014) Acyclic sesquiterpenes released by Candida albicans inhibit growth of dermatophytes. Med Mycol 52:46–55
Burgio G, Ragaglini G, Pestacchi R, Ferrari R, Pozzati M, Furlan L (2012) Optimization of Agriotes sordidus monitoring in northern Italy rural landscape, using a spatial approach. Bull Insect 65:123–131
Dunn OJ (1961) Multiple comparisons among means. J Am Stat Assoc 56:52–64
Fernandes A, Duffield RM, Wheeler JW, Laberge WE (1981) Chemistry of the Dufour’s gland secretion of North American andrenid bees (Hymenoptera: Andrenidae). J Chem Ecol 7:453–463
Furlan L (1998) The biology of Agriotes ustulatus Schaller (Col., Elateridae). II. Larval development, pupation, whole cycle description and practical implications. J Appl Ent 122:71–78
Furlan L (1999) Elateridi ed altri insetti terricoli: metodi di previsione. Il divulgatore 7:17–26
Furlan L (2004) The biology of Agriotes sordidus Illiger (Col., Elateridae). J Appl Ent 128:696–706
Furlan L (2014) IPM thresholds for Agriotes wireworm species in maize in Southern Europe. J Pest Sci. doi:10.1007/s10340-014-0583-5
Furlan L, Gnes C (2003) Improved trap for insects. EP 1334660 A1
Furlan L, Tóth M, Ujváry I (1997) The suitability of sex pheromone traps for implementing IPM strategies against Agriotes populations (Coleoptera: Elateridae). In: Proceedings of XIX IWGO Conference, Guimaraes, August 30–September 5, 1997, pp 173–182
Furlan L, Curto G, Ferrari R, Boriani L, Bourlot G, Turchi A (2000) Le specie di Elateridi dannose alle colture agrarie nella Pianura Padana. Informatore Fitopatologico 5:53–59
Furlan L, Tóth M, Yatsynin VG, Ujváry I (2001) The project to implement IPM strategies against Agriotes species in Europe: what has been done and what is still to be done. In: Proceedings of XXI IWGO Conference, Venice, October 27–November 3, 2001, pp 253–261
Furlan L, Garofalo N, Tóth M (2004) Biologia comparata di Agriotes sordidus Illiger nel Nord e Centro-sud d’Italia. Informatore Fitopatologico 11:32–37
Furlan L, Tóth M, Cooperators (2007) Occurrence of click beetle pest spp. (Coleoptera, Elateridae) in Europe as detected by pheromone traps: survey results of 1998–2006. IOBC/WPRS Bull 30:19–25
Ivaschenko II, Adamenko EA (1980) Site of pheromone production in females of the click beetle Selatosomus latus (Coleoptera, Elateridae) (in Russian). Zool Zh 59:225–228
Kudryavtsev I, Siirde K, Lääts K, Ismailov V, Pristavko V (1993) Determination of distribution of harmful click beetle species (Coleoptera, Elateridae) by synthetic sex pheromones. J Chem Ecol 19:1607–1611
Lehmhus J, Niepold F (2013) New finds of the click beetle Agriotes sordidus (Illiger, 1807) and an overview on its current distribution in Germany. J fur Kulturpflanzen 65:309–314
McLafferty FW, Stauffer DB (1989) The Wiley/NBS Registry of Mass Spectral Data. Wiley, New York
Meigs TE, Sherwood SW, Simone RD (1995) Farnesyl acetate, a derivative of an isoprenoid of the mevalonate pathway, inhibits DNA replication in hamster and human cells. Exp Cell Res 219:461–470
Millar JG, Sims JJ (1998) Preparation, cleanup, and preliminary fractionation of extracts. In: Millar JG, Haynes KF (eds) Methods in chemical ecology I: chemical methods. Kluwer Academic Publishers, Boston, pp 1–37
Oleschenko IN, Ismailov VY, Soone JH, Lääts KV, Kudryavtsev IB (1987) A trap for pests (in Russian). USSR Author’s Cer. No. 1233312. Byull Izobretenii 11:299 (in Russian)
Oleshchenko IN, Ivashchenko II, Adamenko EA (1976) Biological activity of sex pheromones of female click beetles. Selskokhozyaistvennaya Biologiya 11:256–258 (in Russian)
Platia G (1991) Coleoptera, Elateridae. In: Fauna d’Italia, 33-Edizioni Calderini, Bologna
Roelofs WL (1977) The scope and limitations of the electroantennogram technique in identifying pheromone components. In: McFarlane NR (ed) Crop protection agents: their biological evaluation. Academic Press, New York, pp 147–165
Roelofs WL, Cardé RT (1977) Responses of Lepidoptera to synthetic sex pheromone chemicals and their analogues. Ann Rev Entomol 22:377–405
Schiestl FP, Ayasse M (2001) Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximizing reproductive success? Oecologia 126:531–534
Schiestl FP, Ayasse M, Paulus HF, Erdmann D, Francke W (1997) Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J Chem Ecol 23:2881–2895
Siirde K, Lääts K, Erm A, Kogerman A, Kudryavtsev I, Ismailov V, Pristavko V (1993) Structure-activity relationships of synthetic pheromone components in sex communication of click beetles (Coleoptera, Elateridae). J Chem Ecol 19:1597–1606
Tengö J, Bergström G (1975) All-trans-farnesyl hexanoate and geranyl octanoate in the dufour gland secretion of Andrena (Hymenoptera: Apidae). J Chem Ecol 1:253–268
Tengö J, Bergström G (1977) Cleptoparasitism and odor mimetism in bees: do Nomada males imitate the odor of Andrena females? Science 196:1117–1119
Tolasch T (2004) Identifizierung und Synthese flüchtiger Inhaltsstoffe und neuer Pheromone aus Käfern (Coleoptera). PhD Thesis, Hamburg University, p 242
Tóth M (2013) Pheromones and attractants of click beetles: an overview. J Pest Sci 86:3–17
Tóth M, Furlan L, Szarukán I, Ujváry I (2002a) Geranyl hexanoate attracting male click beetles Agriotes rufipalpis Brullé and Agriotes sordidus Illiger (Col., Elateridae). J Appl Ent 126:312–314
Tóth M, Furlan L, Yatsynin V, Ujváry I, Szarukán I, Imrei Z, Subchev M, Tolasch T, Francke W (2002b) Identification of sex pheromone composition of click beetle Agriotes brevis Candeze. J Chem Ecol 28:1641–1652
Tóth M, Furlan L, Yatsynin VG, Ujváry I, Szarukán I, Imrei Z, Tolasch T, Francke W, Jossi W (2003) Identification of pheromones and optimization of bait composition for click beetle pests in Central and Western Europe (Coleoptera: Elateridae). Pest Manag Sci 59:417–425
Tóth M, Furlan L, Xavier A, Vuts J, Toshova T, Subchev M, Szarukán I, Yatsynin V (2008) New sex attractant composition for the click beetle Agriotes proximus: similarity to the pheromone of Agriotes lineatus. J Chem Ecol 34:107–111
Vuts J, Tolasch T, Furlan L, Bálintné-Csonka É, Felföldi T, Márialigeti K, Toshova TB, Subchev M, Xavier A, Tóth M (2012) Agriotes proximus and A. lineatus (Coleoptera: Elateridae): a comparative study on the pheromone composition and Cytochrome C oxidase subunit I gene sequence. Chemoecology 22:23–28
Wall C (1989) Evaluation and use of behaviour-modifying chemicals. In: Jutsum AR, Gordon RFS (eds) Insect pheromones in plant protection. Wiley, Chicester, pp 39–60
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol 27:411–446
Yatsynin VG, Rubanova EV, Okhrimenko NV (1996) Identification of female-produced sex pheromones and their geographical differences in pheromone gland extract composition from click beetles (Col., Elateridae). J Appl Ent 120:463–466
Acknowledgments
The present study was partially supported by OTKA grant K81494 to MT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Schmitt.
In memoriam Tibor Jermy-his scientific achievements keep him among us.
Rights and permissions
About this article
Cite this article
Tóth, M., Furlan, L., Vuts, J. et al. Geranyl hexanoate, the female-produced pheromone of Agriotes sordidus Illiger (Coleoptera: Elateridae) and its activity on both sexes. Chemoecology 25, 1–10 (2015). https://doi.org/10.1007/s00049-014-0170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0170-5