Abstract
Ecometabolomics, which aims to analyze the metabolome, the total number of metabolites and its shifts in response to environmental changes, is gaining importance in ecological studies because of the increasing use of new technical advances, such as modern HNMR spectrometers and GC-MS coupled to bioinformatic advances. We review here the state of the art and the perspectives of ecometabolomics. The studies available demonstrate ecometabolomic techniques have great sensitivity in detecting the phenotypic mechanisms and key molecules underlying organism responses to abiotic environmental changes to biotic interactions. But such studies are still scarce, and in most cases they are limited to the direct effects of a single abiotic factor or of biotic interactions between two trophic levels under controlled conditions. Several exciting challenges remain to be achieved through the use of ecometabolomics in field conditions, involving more than two trophic levels, or combining the effects of abiotic gradients with intra- and inter-specific relationships. The coupling of ecometabolomic studies with genomics, transcriptomics, ecosystem stoichiometry, community biology and biogeochemistry may provide a further step forward in many areas of ecological sciences, including stress responses, species lifestyle, life history variation, population structure, trophic interaction, nutrient cycling, ecological niche and global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The possibility of using progressively improved metabolomic techniques in ecophysiological and ecological studies has opened up a new way to advance knowledge of the structure and function of organisms and ecosystems. Metabolomics is the analysis of the complete metabolome (all the metabolites that one organism produces) at one moment (Fiehn 2002). It provides the phenotypical response at the metabolic level in a particular environmental circumstance. Moreover, it is also a powerful tool to monitor the phenotypic variability of one genotype in response to environmental changes in drought (Fumagalli et al. 2009), nutrient availability (Hirai et al. 2004, 2005), pollutants (Jones et al. 2007; Bundy et al. 2008), salinity (Fugamalli et al. 2009), temperature (Michaud and Delinger 2007) and biotic interactions (Choi et al. 2006), among other ecological factors. These studies are especially adequate in plants because metabolomic studies enable the simultaneous analysis of primary compounds together with secondary compounds, which have a defensive and protective function.
Metabolomics provides a better analysis of the different response capacities conferred by the phenotypic plasticity of each species, allowing to ascertain what metabolic pathways are involved in a phenotypic response. Moreover, this facilitates transcriptomics change research (Hirai et al. 2004; Brosché et al. 2005; Fukushima et al. 2009a). Metabolomics can also be coupled to genomic studies for faster determination of the genes involved in adaptive responses (Bino et al. 2004; Oksman-Caldentey and Saito 2005). This approach therefore has great potential to elucidate gene function and to establish data networks. In this way, it advances our knowledge of the development of phenotypic plasticity (Via et al. 1995; Pigliucci 2005) and its evolutionary consequences (Agrawal 2001). In this regard, metabolomics has several advantages compared to more conventional methods of analyses in chemical ecology (extract fractionation, purification and bioassays). Since all compounds are measured at once in only one step, rather than put through iterative purification steps, unstable compounds are more likely to be detected and measured. Metabolomics can also be used as a preliminary screening study of the metabolome response. This does not exclude the simultaneous or subsequent use of target chemical analyses.
Recent rapid improvements in analytical methods and in the ability of computer hardware and software to interpret and visualize large data sets (Gehlenborg et al. 2010) have multiplied the possibilities of rapidly identifying and simultaneously quantifying an increasing number of compounds (e.g., carbohydrates, amino acids and peptides, lipids, phenolics and terpenoids). These advances will enable us not only to take ‘static pictures’ or snapshots of the metabolome, but also to capture and to ‘film’ its dynamic nature. Ecological metabolomics can thus serve as a powerful indication for defining organism lifestyle. All in all, we may now be able to achieve a dynamic, holistic view of the metabolism and health of an organism, a population or an ecosystem, and in this fashion open the door to exciting new insights in ecology.
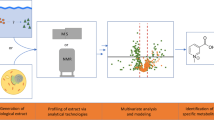
This study reviews the state of the art of ecological applications of metabolomic techniques, giving an overview of the current findings reached by its use in ecophysiological and ecological studies. We also discuss the possible future contribution of metabolomics to progress in ecophysiology and ecology. To achieve such progress, we highlight the need to couple metabolomic studies with other omic studies such as genomics, transcriptomics and proteomics. The aim is to reach an overview of organism response to environmental changes at different time scales and from genotype to phenotype. We also discuss the ecological topics where metabolomics could be more successfully used. Among these topics we highlight ecosystem stoichiometry, plant-herbivore-predator systems, parasitism, climate change and invasive species, among others (Fig. 1).
Ecometabolomics
We use here terminology based on that of Fiehn (2002) and Schripsema (2010), but simplified for metabolomic techniques applied to ecological studies. Briefly, the study type is called metabolomic profiling when the aim is the quantitative analysis of a set of metabolites of a selected number of metabolic pathways or of metabolite types (e.g., polar, semipolar or non-polar) with or without its identification. We propose to call partial ecometabolomic studies (PEM) those metobolomic profiling studies applied to ecophysiological or ecological studies that aim to elucidate the effects of biotic or abiotic factors on a specific whole pathway or intersecting pathways by identifying the metabolite or set of metabolites involved in organism response to environmental changes by using general qualitative and quantitative analytical techniques such as NMR or GC-MS and data-mining statistical analyses. In this way the target studies using more target techniques such as HPLC focused on the study of some limited set of metabolites are not considered ecometabolomic studies.
When it is not required or possible to identify every metabolite, it is often sufficient to rapidly classify samples according to their origin or their ecological or ecophysiological relevance. This process is called metabolic fingerprinting, which now in an ecological context we propose to name ecometabolic fingerprinting. This “holistic” method enables unbiased exploration and examination of sample molecular biochemistry and through suitable interpretation can be used to study plant responses to environmental changes (Gidman et al. 2005, 2006). When the aim is to obtain information about the whole metabolome (the total number of metabolites in one biological system) by identifying and quantifying as many metabolites as possible, we must conduct an analysis approach such as NMR or GC-MS that enables determining and quantifying the maximum number of metabolites. Since such an approach reveals the maximum information about the metabolome of the biological system under study as possible, this approach is called metabolomics. In an ecological context, when the objective is to discern the global metabolomic response of an organism to environmental changes, we propose to call it ecometabolomics. In fact, this latter approach, ecometabolomics, is the most appropriate to comprehend the complete response of an organism to environmental changes.
Analytical techniques
In metabolomic studies it is very important to take into account the pre-analysis treatment. To prevent post-sampling hydrolysis of some compounds, it is important to immediately freeze the sample (most frequently by introducing it immediately into N2 liquid) and thereafter to lyophilize it to completely dry it until the extraction process (see Kim and Verpoorte 2010 for more details on sample preparation). Since all metabolites can provide information about species’ responses to environmental changes, ecometabolomic studies should be designed to detect as many metabolites as possible. As no single solvent allows all metabolites to be extracted, combinations of several different solvents can be used. Kim and Verpoorte have tested different extraction methods for NMR plant metabolomics. The use of a two-phase solvent system, composed of a mixture of chloroform, methanol and water (2:1:1, v/v), has proven to be the most advisable method (Choi et al. 2004). For detailed information about the different extraction properties, see Kim and Verpoorte (2010) and Kaiser et al. (2009).
Currently no single analytical method or combination of methods (i.e., chromatography combined with mass spectroscopy) can detect all metabolites (estimated to be between 100,000 and 200,000 in the plant kingdom) within a given biological sample. Gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance spectrometry (NMR) are the procedures with the best capacity to determine the widest ranging sets of metabolites. GC-MS has proven to be a robust tool for the study of volatile organic compounds (Degen et al. 2004; Ozawa et al. 2008; Llusia et al. 2010), but GC-MS analysis of extracts containing other analytes such as organic acids, sugars, amino acids and steroids is complicated. Many metabolites are non-volatile and must be derivatized prior to GC-MS analysis (Gullberg et al. 2004). In such cases, thermolabile compounds may be lost. Moreover, it is difficult to elucidate the unknown structures of metabolites by using GC-MS alone. LC-MS is of particular importance to study a great number of metabolic pathways at once since plant metabolism embodies a huge range of semi-polar compounds, including many key groups of secondary metabolites, which are better separated and detected by LC-MS (Allwood and Goodacre 2009). Thus, while GC-MS is best suited for compound classes appearing mainly in primary metabolism (frequently after derivation), i.e., amino acids, fatty acids and sugars or volatile compounds, LC-MS is more adequate to determine the overall biochemical richness of plants including several semi-polar groups of secondary metabolites. To gain structural elucidation power, the method of collision-induced dissociation can be used (Jennings 2000). The parent ions providing electrospray ionization (ESI) are isolated and accelerated in mass spectrometry using mass filters, so as to collide with molecules of bath gas, giving rise to the fragment spectrum (MS/MS method). Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR-MS) and ultra-pressure liquid chromatography mass spectrometry (UHPLC-MS) can be used to further increase the number of detectable metabolites. FT-ICR-MS is a very high-resolution technique in that masses can be determined with very high accuracy. This is due to the great sensitivity of this method to separate compounds with different mass-to-charge ratios (m/z) by their different cyclotron frequency in a fixed magnetic field. This method together with previous chromatographic methods has been scarcely used in ecometabolomic studies until now (Hirai et al. 2004; Haesler et al. 2008), but the results are promising. UHPLC-MS constitutes an improvement in the power of separating compounds during the chromatographic phase (high-resolution capacity) with respect to the conventional HPLC-MS method. This is achieved by the application of great pressure to the carrier solution that reduces the dispersion of each chemical compound in the separation column. Moreover, UHPLC permits shortening the time of the separation phase, a fact especially interesting in ecometabolic studies where great number of samples must be processed.
1H-NMR has proven to be an appropriate tool for untarget analyses. It has the advantage that it can be applied to determine polar, semi-polar and non-polar metabolites, and that it produces signals that directly and linearly correlated with compound abundance (Lewis et al. 2007). However, NMR spectroscopy has intrinsic low sensitivity for low concentrations of metabolites and signal overlapping for complex mixtures. This can at times be problematic for structural elucidation of a metabolite at low concentrations. The use of low temperatures to stabilize the detector by modern cryogenically cooled devices can improve the sensitivity up to a factor of five by reducing the thermal noise from the electronics of the NMR spectrometer. Two-dimensional (2D) NMR spectroscopy methods and high-resolution magic angle spinning (Sekiyama et al. 2010) further improve the sensitivity. 2D NMR spectroscopy provides an increased signal dispersion, thus enabling the detection of connectivity between signals and hence helping to identify metabolites. This method includes the total angular momentum (J) resolved method (1H-1H 2D J resolved), correlation spectroscopy (COSY) and total correlation spectroscopy (TOCSY). 1H-1H 2D J resolved yields information on the multiplicity and coupling patterns of resonances, which reduces the degree of spectra complexity but retains all the chemical shifts and the relative intensity of spectral peaks. COSY and TCOSY provide 1H-1H spin-spin coupling connectivities, providing information as to which hydrogens in a molecule are closer in terms of chemical bonds. In the high-resolution magic angle spinning approach, 13C is detected indirectly using the more abundant 1H by using spin-spin interaction 13C-1H. This yields both coordinated 13C-1H-NMR shifts that are useful for identification purposes. Moreover, the stable isotopes 13C, 15N and 31P have been successfully employed in NMR in vivo metabolomic studies in ecophysiological studies (Lundberg and Lundquist 2004; Kikuchi et al. 2004). When for the aim of a study it is important to elucidate chemical structures that probably are unknown, such as for example in plant herbivore relationships, NMR-based metabolomic studies are the most adequate tool (see a recent review on this topic by Leiss et al. 2011). For more detailed information on the advantages and disadvantages of each method, see Summer et al. (2003), Kopka et al. (2004), Moco et al. (2007) and Verpoorte et al. (2008).
Recently, HPLC separation, diode array detection (DAD), MS detection, solid phase extraction (SPE) for enrichment of a metabolite and NMR, namely HPLC-DAD-MS-SPE-NMR (Tang et al. 2009), have been combined, enabling good separation, sensitivity and molecular structure elucidation all at once (Tang et al. 2009). For a further description of data acquisition methods in metabolomics, see Hall (2006), Allwood and Goodacre (2009) and Lindon and Nicholson (2008).
Ecometabolic responses to abiotic factors
Several studies have investigated the responses of some metabolic pathways in organisms to changes in abiotic factors such as climate (temperature and water availability), nutrient availability, salinity or pollution (Tables 1, 2). Changes in the composition of some metabolite groups have been described using analytical target methods in response to changes in several environmental factors, such as drought (Llusia and Peñuelas et al. 1999; Llusia et al. 2008; Peñuelas et al. 2009), temperature (Peñuelas and Llusia 1999; Filella et al. 2007), pollutants (Peñuelas et al. 1999), irradiance (Peñuelas and Llusia 1999) or CO2 (Peñuelas and Llusia 1997). Moreover, several studies have reported that the metabolites produced in response to abiotic or biotic environmental changes further interact with other abiotic and/or biotic ecosystem constituents, e.g., terpene emissions that affect the climatic and atmospheric conditions (Andreae and Crutzen 1997; Kavouras et al. 1998; Peñuelas and Llusia 2003; Peñuelas et al. 2009a; Peñuelas and Staudt 2010). Now ecometabolomic studies provide the possibility to take a step forward in knowledge at the level of global organism responses to environmental changes. Some reports have already begun to explore the possibilities of the use of metabolomic approaches in ecological studies.
Climatic factors
Most work conducted in plants using partial ecometabolomic techniques to investigate metabolic changes under cold stress has involved analyzing the polar metabolites in Arabidopsis sp. grown in controlled conditions (Kaplan et al. 2004, 2007; Cook et al. 2004; Gray and Heath 2005; Davey et al. 2009; Maruyama et al. 2009; Korn et al. 2010), although other plant species have also been studied (Janda et al. 2007) (Table 1). The main responses to low temperatures included increases in metabolites related to amino acid-protein and soluble carbohydrates recognized as cryoprotectors (Carpenter and Crowe 1988). In addition other studies of partial ecometabolomics mainly focusing on lipids have reported an increase in the degree of lipid unsaturation related to cellular membrane adaptation to low temperatures (Cyril et al. 2002; Lindberg et al. 2005). When partial metabolomic studies have been conducted with transcriptomics, it has been observed that the changes in transcript levels of some metabolic processes were not correlated with shifts observed at metabolic levels (Kaplan et al. 2007). This indicates that metabolome shifts are more sensitive than transcriptome changes to detect phenotypic responses to cold stress. Different genotypes of Arabidopsis presented significant differences in the metabolite set that increased under cold acclimation (Kaplan et al. 2004; Davey et al. 2009). The metabolomic technique showed greater sensitivity in detecting genotypic and phenotypic differences to cold response than transcriptomic techniques.
Ecometabolomic studies of polar metabolites in animals in response to cold stress have reported similar results to those observed in plants, e.g., increases in glucose concentration coupled to decreases in glycogen have been observed in Lumbricus rubellus (Bundy et al. 2003) (Table 1). These results further confirm the increase in sugars and amino acids as cryoprotectors under low temperatures (Overgaard et al. 2007). Michaud and Denlinger (2007) conducted an ecometabolomic study using both GC-MS and 1H NMR, including both polar and non-polar metabolites. This gave a clearer picture, showing the enhancement of some metabolic pathways was related to the simultaneous decrease in other metabolic pathways. Apart from sugars, pyruvate and urea also increase at low temperatures. All these metabolites have been related to cell membrane cryoprotection (Story and Storey 1983; Lee 1991).
Contrary to cold stress, warming stress increases the saturation level of fatty acids (Larkindale and Huang 2004), and similarly to cold stress, warming stress increases amino acid and soluble sugar concentrations in plants (Kaplan et al. 2004; Rizhsky et al. 2004; Yamakawa and Hakata 2010), fungi (Pluskal et al. 2010), and the endo- and exometabolome of soil microbes (Coucheney et al. 2008) (Table 1). In animals a decrease in metabolic conditions, i.e., lower ATP and glucose concentrations (Viant et al. 2003), and a rapid increase in concentration of some amino acids with a subsequent decrease (Malmendal et al. 2006) have been linked to an increase in “heat shock” protein synthesis (Feige et al. 1996). Another recent study of Folsomia candida showed a decrease of different amino acid contents in response to heat stress as a result of upstream downregulation of transcription and translation (Waagner et al. 2010). However, the possible immediate increase of other amino acid contents was not measured. An ecometabolomic fingerprinting study of Oncorrhynchus mykiss eggs submitted to warming shock permitted the detection of differences in some groups of metabolites, showing the sensitivity and suitability of fingerprinting the metabolome as a screening method prior to conducting ecometabolome profile studies (Turner et al. 2007). Pluskal et al. (2010) also detected many changes in Schizosaccharomyces pombe secondary metabolism, including decreases in urea cycle intermediates and increases in acetylated compounds.
Some studies have monitored the changes in the concentration of water soluble plant metabolites in response to water availability to study drought effects using partial ecometabolomic techniques in vitro or in the lab (Rizhsky et al. 2004; Pinheiro et al. 2004), greenhouse (Cramer et al. 2007; Charlton et al. 2008; Lugan et al. 2009) and also field experimental conditions (Semel et al. 2007; Mane et al. 2008; Alvarez et al. 2008) (Table 1). An increase in soluble sugars, mainly glucose and sucrose and/or in amino acids or their derivates (citric acid, threonine, homoserine, valine, proline, malate, γ-aminobutyrate) is frequently found under drought conditions, confirming that the reduction in photosynthesis is accompanied by a pronounced mobilization of sugars in soluble form and by a synthesis of soluble amino acids. Both these mechanisms adjust osmotic potential to prevent water losses. These compounds may also protect cellular components such as membranes and enzymes (Shen et al. 1997). A similar observation has been reported by Michaud et al. (2008) in a partial ecometabolomic study of the polar metabolites of the insect Belgica antartida raised in vitro at different moisture levels (Table 1). Unfortunately, significant plant secondary metabolites, such as phenolics, that can minimize the oxidative stress associated with drought (Hura et al. 2007) were not included in such studies. For this reason ecometabolomic studies that aim to study primary but also secondary metabolites at once are necessary to reach a general understanding of plant responses to drought.
The effects of seasonality on the leaf content of some metabolites have been observed in some target studies (Riipi et al. 2004) in mountain birch trees (Betula pubescens). Ecometabolomic studies should allow us to improve our knowledge of global metabolism shifts linked to annual phenological changes in both animals and plants in field conditions.
Nutrient deficiency
Ecometabolomic studies that have aimed to investigate metabolome changes under different scenarios of nutrient availability are scarce (Table 1). N deficiencies in plants have been proven to decrease certain amino acid concentrations, whereas the concentration of several sugars, phosphoesters and secondary metabolites increases (Urbanczyk-Wochniak and Fernie 2005; Bölling and Fiehn 2005) (Table 1). There are also shifts from biosynthesizing some amino acids to synthesizing others (Howarth et al. 2008). These effects were also observed in the earthworm Eisenia veneta (Warne et al. 2001). Partial ecometabolomic studies of polar metabolites found that P stress induced the root accumulation of polyols, which are stress-related metabolites (Hernández et al. 2007). In addition plants modify carbohydrate metabolism in order to reduce P consumption and remove P from many metabolites (glucose 6-P, fructose 6-P, inositol 1-P and glycerol 3-P), thus reducing the levels of organic acids involved in the tricarboxylic acid cycle (TCA) (Huang et al. 2008) (Table 1). Partial ecometabolomic studies in plants have shown that S deficiency tends to increase the concentration of some N-rich metabolites such as certain amino acids and purines (Nikiforova et al. 2005a; Howarth et al. 2008). Rochfort et al. (2009) have observed that changes in both polar and lipophilic metabolite composition in earthworms provides information on the fertility of the soil in which they have been living.
Salt stress
Plant salt stress is increasing globally because of climate change and inappropriate water use by humans and field management (Ellis and Mellor 1995). Current reports using metabolomic techniques have detected the global metabolic shift under salt stress observing the sets of metabolites that more frequently increased, enhancing the plant osmotic potential. Inorganic solutes (Gagneul et al. 2007) and sugars, amino acids and polyols (Kim et al. 2007; Fumagalli et al. 2009; Widodo et al. 2009; Behrends et al. 2010) are the metabolites that most frequently increase under salt stress (Table 1). Some studies have investigated the metabolomic fingerprinting studies of salt-stressed plants (Smith et al. 2003; Johnson et al. 2003) and animals (Bussell et al. 2008), and have been able to identify the molecular groups changing under such stress (Table 1).
Hypoxia
Lack of oxygen is an important ecological trait in some ecosystems such as benthonic communities in intertidal coastal areas. Increases in succinate and valine and decreases in leucine and isoleucine have been observed in 1H NMR partial ecometabolomic studies of polar metabolites in the mussels Mytilus edulis and Mytlus galloprovincialis grown at different hypoxia levels (Hines et al. 2007; Tuffnail et al. 2009) (Table 1). The fish Oryzias latipes has shown increases of soluble phosphates and decreases in ATP and phosphodiesters in response to hypoxia in a PEM of phosphorylated soluble metabolites by 31P NMR (Pincetich et al. 2005).
Global change drivers
Changes in atmospheric composition (CO2, O3, NOx), warming, drought, human-made pollution, trace element pollution and UV radiation can have a strong impact on organism metabolomes. The effects of these abiotic factors on organism metabolites have been currently investigated in some ecometabolomic studies. Metabolites linked to carbohydrate biosynthesis and partitioning, amino acid metabolism, cell wall and hormone biosynthesis pathways were the most affected by [CO2] increases in different genotypes of Arabidopsis thaliana submitted to higher [CO2] (Li et al. 2006). Thus, the responses of the most polar metabolite groups were different depending on the genotypes. However, the use of transcriptome allowed detecting that there were a small number of signature transcripts that appeared as a common response mechanism of all Arabidopsis ecotypes to [CO2] increases irrespective of their underlying genetic diversity and evolutionary adaptation to different habitats (Li et al. 2006). Thus, these results highlight the advantage of using metabolomic with other omic techniques at once to reach a general and integrated overview of the responses of organisms in response to environmental changes.
Increases of phenolic compound contents have been observed in target analysis at high [CO2] exposure (Huttunen et al. 2008) (Table 1). This linkage between high [CO2] environmental concentrations and high C-rich compound concentrations warrants being studied together with the whole organism metabolome. Such rises in C-rich secondary metabolites in plant tissues seem related to the increases in the plant C:N concentration ratio observed as a general plant response to higher [CO2] (Peñuelas et al. 1997; Novotny et al. 2007).
Ecometabolomic fingerprinting using Fourier transformed-infrared spectroscopy FT-IR has been used to investigate patterns of plant metabolome changes due to N deposition. This was done both in an experimentally manipulated N deposition gradient under common garden conditions using Calluna vulgaris and in a natural gradient across the United Kingdom (Gidman et al. 2005, 2006) (Table 2). In both studies FT-IR fingerprinting was able to correlate metabolome changes with N deposition levels. The results showed that the spectra regions corresponding to N–H, C–N, proteins and polysacarid vibrational bands had a positive relationship with higher N-deposition, suggesting an enhancement of N-rich metabolite synthesis and of sugar anabolism. These results highlight the possiblities of the use of this technique as a useful tool for preliminary studies in metabolome research applied to field ecological studies.
Partial ecometabolomic studies allow the detection of the metabolic pathways affected by the pollution produced by different chemotoxic pollutants and also the key metabolites that improve the organism resistance both in plants (Trenkamp et al. 2009; Kluender et al. 2009) and in animals (Warne et al. 2000; Bundy et al. 2001; Viant et al. 2006a, b; Samuelsson et al. 2006; Bon et al. 2006; Jones et al. 2008a; Ekman et al. 2008; Mckelvie et al. 2009; Tuffnail et al. 2009; Hansen et al. 2010) (Table 1). For instance, Bundy et al. (2002) have used ecometabolic fingerprinting NMR studies of the earthworm Eisenia veneta to ascertain initially the metabolites that changed their concentration when the worm was submitted to three different xenobiotics: 4-fluoroaniline, 3,5-difluoroaniline and 2-fluoro-4-methylaniline. In a later step they identified these changing compounds by using HPLC–MS and 13C NMR. This experiment is an example of the utility of metabolic fingerprinting studies to detect the molecular groups which change in the metabolome in response to an abiotic factor as a first step prior to molecular qualitative determination analyses. Using this approach avoids the expense and time consumption that qualitative analyses of the whole metabolome require. Hansen et al. (2010) showed the shifts in metabolism of the marine copepod Calanus finmarchicus in response to diethanolamine (DEA) exposure as a relevant chemical widely used in industrial, agricultural and pharmaceutical applications. The main result of this study was a decrease of choline, taurine, sarcosine and some amino acids. Likewise, the capacity of ecometabolomic fingerprinting to detect metabolomic responses to different herbicide exposures has been recently reported in Lemna minor (Aliferis et al. 2009). In the metabolomic studies of toxicity effects on an organism’s metabolome (polar and non-polar metabolites), those using GC-MS have been able to detect more compounds than those using 1HNMR. In contrast, studies using 1HNMR have greater power to elucidate the compound structure through its great number of sources of qualitative determination (COSY, TOCSY and high-resolution magic angle spinning). Thus, if the aims are also to determine possible novel structures, the use of 1HNMR is advisable. For example, Kluender et al. (2009) using GC-MS detected 283 metabolites but determined only 39, whereas Jones et al. (2008a) using 1HNMR detected and determined 32 analytes in a similar study on chemotoxic pollutant effects on the invertebrate metabolome. Jones et al. (2008a) using both techniques to analyze the same samples detected and determined 32 molecules using 1HNMR and detected 51, but only determined 42 molecules using GC-MS.
A set of recent partial ecometabolomic studies of polar metabolites has also permitted the identification of the metabolites and metabolic pathways affected by certain trace element polluting plants (Bailey et al. 2003; Roessner et al. 2006; Sarry et al. 2006; Le Lay et al. 2006; Sun et al. 2008) and animals (Gibbs et al. 1997; Griffin et al. 2001, 2000; Bundy et al. 2007, 2004; Jones et al. 2008b; Taylor et al. 2009; Guo et al. 2009) (Table 2). These studies have allowed the identification of some metabolites (acetilspermidine, glucose, histidine, l-methylhistidine, mannose) that can be used as biomarkers for Cu, Cd and Zn pollution in various animal species (Bundy et al. 2004, 2007, 2008; Taylor et al. 2009; Guo et al. 2009). In rats, in addition to the changes in amino acid and sugar contents, a further abnormal metabolome composition has been observed as a result of Cd and Cu intake (Jones et al. 2007; Lei et al. 2008), and changes in lipid metabolism under high levels of As have also been observed (Griffin et al. 2000). A tendency to increase the production of AMP, ADP and N-α-methylhistidine, and to generate an imbalance in metabolites related to energetics by reducing ATP levels has been reported in Lumbricus rubellus (Bundy et al. 2007, 2008). In a transcriptomic and ecometabolomic study of Cu toxicity in Lumbricus rubellus, Bundy et al. (2008) observed a decrease in small-molecule metabolites (glucose and mannose) related to an overexpression of transcripts of enzymes involved in oxidative phosphorylation, thus showing that Cu interferes with energy metabolism. In addition such studies have advanced the knowledge about the metabolites involved in phytochelatin mechanisms in plants (Sarry et al. 2006). Pollution by 133Cs induced higher amino acid content in cells of Arabidopsis thaliana, suggesting an induction of the synthesis of abnormal or unwanted proteins under higher levels of 133Cs in the medium. On the other hand, the synthesis increase of enzymes involved in the degradation of short-lived intracellular proteins was detected by proteomic studies (Le Lay et al. 2006). These effects were lower at high levels of K in the growth medium. Altogether these results highlighted the benefit of coupling different omics approaches together with the simultaneous study of different environmental variables such as pollution and nutrient availability levels in the Le Lay et al. (2006) study. Similarly, an increase of some amino acid contents has been observed in Silene cucubalus and Arabidopsis thaliana submitted to Cd pollution (Bailey et al. 2003; Sun et al. 2010) and in Hordeum vulgare submitted to B pollution (Roessner et al. 2006), all supporting the Le Lay et al. (2006) study results.
High levels of UV radiation can be expected in the future if stratospheric O3 levels decrease. This type of radiation is able to damage DNA and other macromolecules (Harm 1980; Jagger 1985). Several target studies have already observed that Quercus ilex and Rhododendrum ferrugineum change their pigment composition and leaf morphology in response to UV radiation in a latitudinal range (Filella and Penuelas 1999) demonstrating that a deep metabolic change can also occur, but these studies do not allow us to reach a global picture of metabolome shift under UV radiation enhancement. Partial ecometabolomic studies carried out exposing Arabidopsis thaliana to high UV radiation showed that flavonoids (Lois 1994), α-glycerophosphates (Broeckling et al. 2005) and phenylpropanoids (Lake et al. 2009) increased their concentrations, thus suggesting that these compounds have a photoprotective role (Table 2). In a partial ecometabolomic study of polar metabolites under light starvation using GC-MS and 13C NMR, a decrease in primary metabolism has been observed in roots of Phaseolus vulgaris (Bathellier et al. 2009). But in this field there is a lack of global ecometabolomic studies aiming to study polar and non-polar metabolites at one time to improve our knowledge of the plant and animal capacity and mechanisms to respond to increasing levels of UV radiation.
Some interesting ecometabolomic studies including polar and non-polar metabolites have already begun to contribute to providing information about the effects of ozone (O3) on the organism metabolome of different taxa. In an ecometabolomic study of rice (Oryza sativa) using electrokinetic chromatography, increases in O3 concentrations were found to enhance γ-aminobutiric acid (GABA), some amino acids and glutathione (Cho et al. 2008) (Table 1). However, when ecometabolomic studies were conducted using GC-MS in the tree Betula pendula as the target species growing in common garden conditions, phenolics and compounds related to the leaf cuticular wax layer were found to be the main metabolites involved in metabolic adaptation to increasing O3 levels (Kontunen-Soppela et al. 2007; Ossipov et al. 2008) (Table 1). These are the first contributions to understanding what metabolic compounds and metabolic pathways are involved in phenotypic responses to O3 pollution, and they highlight that plants can respond to O3 pollution at different metabolic levels, which can vary between species.
Remaining questions
This overview of the studies that have used ecometabolomic techniques to study ecophysiological responses to changes in abiotic variables shows there are several key questions still to be tackled. (1) The abiotic variables studied have been mainly manipulated in controlled conditions. Few studies have been conducted observationally in natural gradients or experimentally in field setups. (2) There is a lack of ecometabolomic studies with polar and non-polar metabolites and also considering the secondary metabolism in plants. (3) There is a lack of information about the response of several taxa or ecotypes, such as vertebrates, trees or shrubs, and there is an insufficient number of comparable studies enabling more general conclusions. (4) Although there are preliminary studies, such as detecting changes in the metabolome in different soils and climatic conditions (Wallenstein et al. 2010), ecometabolomic studies of multifactorial effects are just beginning.
Biotic interactions between two or more species
Plant-fungus
Few studies have applied ecometabolomic techniques to study changes in the plant metabolome during fungal infection, which can be a host-pathogen or a mutualistic relationship. Some partial ecometabolomic studies of polar metabolites have shown that plant defensive responses involve the induction of diverse polar secondary metabolites such as p- and m-coumaric acid, inositol, caffeic acid, indolic derivates, phenylpropanoids and flavonoids, and some non-polar metabolites such as phosphatidyl glycerol, aromatic compounds and fatty acids (Bednarek et al. 2005; Allwood et al. 2006; Strack et al. 2006; Jobic et al. 2007; Cao et al. 2008; Hamzehzarghani et al. 2008; Muth et al. 2009; Abdel-Farid et al. 2009; Lima et al. 2010) and also the decrease of other metabolites such as GABA, fructose and sucrose involved in plant growth and primary metabolism (Jobic et al. 2007; Abdel-Farid et al. 2009) (Table 2). Similarly, higher contents of some amino and organic acids, carbohydrates and mainly of phenolic compounds have been observed to be constitutive defenses in fungus-resistent subspecies of Vitis vinifera when compared with susceptible subspecies of Vitis vinifera (Figueiredo et al. 2008; Ali et al. 2009). These results imply a rerouting of carbon and energy from primary to secondary metabolism and suggest the possible change in the plant nutritional quality for animals feeding with a possible impact on other ecological relationships. The other component of the relationship, the fungus, also changes its metabolism when it infests a plant. For example, Parker et al. (2009) suggested that the fungus Magnaporthe grisacea deploys common metabolic pathways of the host plant species Brachipodium sistachyon to suppress plant defenses and colonize plant tissues. In this vein Jobic et al. (2007) found that glycerol was only produced for the hyphae of the fungus Sclerontinia sclerotiorum when it grew into infected tissues of Helianthus annuus. Glycerol can be synthesized by fungus to enhance fungal growth and to protect fungal cells. Metabolites released from dying plant tissues could stimulate its synthesis. These ecometabolomic studies show not only the metabolites induced in plants by fungal infection (Hamzehzarghani et al. 2005, 2008; Muth et al. 2009; Abdel-Farid et al. 2009; Lima et al. 2010), but also shows that the fungus can use plant photosynthate that thereafter causes hyphal growth, altogether suggesting that the fungus has developed a common metabolic re-program strategy in the plant host (Parker et al. 2009). Another interesting finding reported by four different studies is that fungal infection induces the emission of new volatile organic compounds (VOC) with a composition that varies depending on the species of infecting fungus (Prithiviraj et al. 2004; Vikram et al. 2004; Lui et al. 2005; Moalemiyan et al. 2007) (Table 2). VOC has been shown to have direct and indirect roles in protecting plants against herbivory (Llusia and Peñuelas 2001; Peñuelas and Llusia 2004), as well as in contributing to the plant’s defense strategies against thermal damage (Copolovici et al. 2005; Peñuelas et al. 2005a). Thus, these ecometabolomic studies of plant emissions due to fungus infection suggest a role of defense coordination among plant organs and/or among different individual plants at the community level.
Fungus can also establish symbiotic relationships with plants to enhance the nutrient and water absorption capacity. Akiyama et al. (2005), using HPLC-MS and 1H NMR ecometabolomic studies of root exudates, found that Lotus japonicus exudes sesquiterpene lactones, stimulating the hyphal branching of the symbiotical fungus Giaspora margarita. Cao et al. (2008) studied the symbiotic relationship between Lolium perenne and the endophytic fungus Neotyphodium lolii using mass spectrometry. The ecometabolomic study showed that plants with fungus symbionts contain some fungal compounds such as peramine, mannitol and other metabolites in their metabolome as a result of their relationship with the fungus. Some of these metabolites have been identified as plant-endophyte symbiotic metabolism regulation, but its role is not yet stablished. All hypotheses suggest a possible role in the improvement of the resistance to biotic or abiotic factors such as herbivores or drought (Cao et al. 2008). Thus, the current ecometabolomic studies in plant-fungus symbiotic relationships are promising for a better comprehension of the metabolic mechanisms underlying the symbiosis success in field conditions.
Plant-bacteria and plant-virus
Similar to plant-fungus, the relationship between plants and microbes can be either a host-pathogen relationship or a mutualistic relationship. Shifts in metabolome fingerprinting have been described in host plant-pathogen bacterial relationships (Allwood et al. 2010). Some partial ecometabolomic studies of polar metabolites have shown that bacterial infection induces chemical defensive mechanisms involving an increased synthesis of diverse metabolites such as threonine, sinapoyl-malate, caffeoyl-malate, γ-aminobutic acid, rutin or phenylpropanoids depending on the species (Barsch et al. 2006; Jahangir et al. 2008; López-Gresa et al. 2010) (Table 2).
Pathogen bacteria can change plant metabolism. In this way, studies of polar and non-polar metabolites have reported that some primary plant metabolites increased their concentration under microbe infection, but also that some secondary metabolite pathways were also stimulated by microbe infection, e.g., terpenoid indole alkaloids and phenylpropanoids (Choi et al. 2004) and flavonoids (Cevallos-Cevallos et al. 2009) (Table 2). Ecophysiological studies using metabolomic techniques have also shown that they are able to detect differences in plant metabolism shift depending on the virulence (Simoh et al. 2009) or the nutritional requirements (André et al. 2005) of the microbe strain. Ecometabolomic studies can also help to discern what environmental conditions are more or less favorable for the success of the symbiotic relationships. Barsch et al. (2006) have observed that the host plant Medicago sativa reacts against nitrogen-fixation-deficient bacteroids with a decrease of organic acid synthesis and an early induction of their senescence.
The symbiotic relationships between some plant taxons, such as the Fabaceae, with certain anaerobic bacteria are of great ecological and farming importance. The few metabolic studies available on this topic have observed that plants induced nodule formation by increasing the synthesis and nodule accumulation of particular metabolites such as asparagine, glutamate, putrescine, mannitol, threonic acid or gluconic acid (Desbrosses et al. 2005).
Studies to improve the ecological tools for environmental restoration are also an emerging area. In this field, Narasimhan et al. (2003) in a rhizosphere study using an HPLC-MS partial ecometabolomic analysis of rhizosphere secondary metabolites reported that phenylpropanoid-utilizing microbes are able to enhance soil polychlorinated biphenyl (PCB) depletion. This is useful in soil phytoremediation for a successful selection of rhizosphere microbes that degrade PCBs, which are very toxic organic pollutants.
The repercussions in the metabolome of viral infections in plants in the field have been little studied. Ecometabolomics of polar metabolites suggest that plants respond similarly to bacterial infection, increasing the synthesis of organic acids and terpenoids (Choi et al. 2006; López-Gresa et al. 2010).
Plant-animal
Plant insect interactions are one of the most widely studied topics in ecology. Ecometabolomics offers the possibility of a more in-depth study of the biochemical aspects of this interaction and of its ecological implications. Some partial ecometabolomic studies of polar metabolites have found a great chemical variation in the metabolites used by plants to defend themselves against insect attacks (Widarto et al. 2006; Jones et al. 2006; Poëssel et al. 2006; Jansen et al. 2009; Leiss et al. 2009a, b; Kuzina et al. 2009; Mirnezhad et al. 2010) (Table 2). These studies have demonstrated that some hormones, acyl sugars and mainly different secondary metabolites such as chlorogenic acid, saponins, phenolics, terpenes, alkaloids, terpenes and glucosinolates are the most general plant chemical defenses against insect herbivores. These results further confirm that a great variety of chemicals are used by plants as chemical deterrents and also the different capacities for chemical resistance among different plant species and also among different subspecies of the same species (Mirnezhad et al. 2010). These results also suggest that the consequences at the community level of plant-insect interaction can vary depending on the taxonomy of the plant and insect as a result of the variability of the changes in palatability, stoichiometry and chemical signals. Insect attack not only affects soluble metabolites, but also the molecular composition of volatile emissions from plants (Peñuelas et al. 1995; Ozawa et al. 2000; Gaquerel et al. 2009) (Table 2). Terpenes are induced and emitted in response to internal (genetic and biochemical) and external (ecological) factors, both biotic and abiotic, and play an important role in communication among plants, and in animals have been widely observed (Peñuelas and Llusia 2001; Peñuelas et al. 2005b). Metabolome studies in specialist insects Pieris brassicae feeding on the leaves of Brassica oleracea have shown that insect growth was not affected and that the insect metabolized some flavonoids of B. oleracea (Ferreres et al. 2007) and accumulated certain other flavonoids (Jansen et al. 2009), suggesting a possible defense mechanism against predators. Ecometabolomic studies have also allowed observing changes in hormone concentrations of Pseudotsuga menziesii in response to attack by the insect, Megastigmus spermotrophus (Chiwocha et al. 2007). The conclusion of all these results is that ecometabolomics is a useful tool to discover unexpected bioactive compounds involved in ecological interactions between plants and their herbivores.
Arany et al. (2008) in an ecometabolomic study of polar and non-polar metabolites found that Arabidopsis thaliana shows that aliphatic glucosinolates and total glucosinolates affect the generalist herbivore Spodoptera exigua, but not the specialist herbivore Plutella xylostella. This study demonstrates different strategies of plant defense against generalist and specialist herbivores, although future studies are necessary to advance in this topic. In the research line of plant-herbivore relationships, Yang and Bernards (2007) have studied the metabolome shift after leaf cutting to investigate the metabolic pathways involved in suberification. Some recent studies suggest that ecometabolomic studies can also be useful to study the molecular interaction between mammals and plants (Tucker et al. 2010).
Plants synthesize complex sets of substances that interact with other plants and with animals. Ecometabolomic studies can play a key role in advancing the knowledge of the mechanisms of action of these substances on organisms. An example of this possible role comes from the information provided by several partial ecometabolomic studies that have observed increases in the contents of metabolites in terrestrial plants linked to chemical defensive and/or antistress mechanisms in response to methyl jasmonate (Liang et al. 2006a, b; Hendrawati et al. 2006). Methyl jasmonate has also proved to enhance the monoterpene emission by 20–30% in Quercus ilex leaves (Filella et al. 2006), suggesting that plants change their metabolism not only to enhance a direct defensive response, but also to enhance signaling to attract natural enemies of herbivores. However, we must highlight that in the most studies the animal was an insect, and there is a lack of studies with vertebrate herbivores.
Competition
Peiris et al. (2008), using GC-MS partial ecometabolomics of polar metabolites, have studied the mycelial tissues of three competing fungus species during wood decomposition. They observed that the synthesis of 2-methyl-2,3-dihydroxypropinoic and pyridoxine acids can be involved in defensive mechanisms activated in response to direct contact between the mycelia of the different fungus species studied, thus showing the importance of exudated metabolites as resources for chemical defense in direct competition for space and sources. Recently, Paul et al. (2009) studied the inter-specific competition and allelophatic effects between two diatom species, observing different metabolites in monocultures than those observed in a co-culturing setup. This indicates either transformation or uptake of released metabolites by the competing species. The effects of species richness on plant metabolomes were also studied in five herbs: two tall-growing herbs (Medicago x varia and Knautia arvensis) and three small-growing herbs (Lotus corniculatus, Bellis perennis and Leontodon autumnalis) competing in communities of different species composition and richness (Scherling et al. 2010) (Table 2). They found different metabolome shifts in plant species growing in such different communities. All these results suggest new lines of research in the frame of competition in natural ecosystems by studying the ecometabolomic relationships between competitors.
Other biotic relationships
A functional assessment of antagonistic microbial communities in soil requires in-depth knowledge of the mechanisms involved in these interactions. Metabolome studies provide an adequate tool for this purpose. Haesler et al. (2008) found the metabolite polyketide cycloheximide to be the molecule responsible for the antagonistic effect of the actinobacterial genus Kitasatospora against the oomycetous root pathogen Phytophthora citricola.
Other important biotic relationships such as animal-microbe have been rarely studied by partial ecometabolomic methods (Solanky et al. 2005) (Table 2).
Animal behavior
Animal processes such as migrations, mutualistic associations or reproductive strategies are species-specific traits associated with physiological and metabolic changes. In spite of this, some of these phenomena have not yet been studied using ecometabolomic approaches. Schistocerca gregaria, a desert locust that forms great migratory swarms, has been studied using a 1H NMR partial ecometabolomic analysis of polar metabolites. This study has shown that some metabolites like putrescine are linked to solitary behavior (Lenz et al. 2001), while others such as the alkaloid L-dopa analogue are linked to gregarious behavior (Miller et al. 2008). These studies should provide significant advances in knowledge about and predictions of the future behavior of such populations. This is of great importance for plague management. Another promising study has been conducted in the exudates of the nematode, Caenorhadditis elegans, with 1H NMR and has proved that ascarosides are the substances responsible for mating and sexual reproductive synchronization between males and females of this species (Srinivasan et al. 2008; Pungaliya et al. 2009). However, there is a lack of studies on other taxonomic groups than insects to reach a more global knowledge on this topic.
Interactions between abiotic and biotic factors
Few studies have investigated the effects of abiotic factors on biotic relationships. Among them we highlight ecometabolomic studies of polar metabolites that have investigated the effects of N availability on plant-fungus infection relationships (Rasmussen et al. 2008), showing that N availability affects both plant metabolite concentrations and fungus infection (Table 3). Recently, Larrainzar et al. (2009), studying N2-fixation under drought conditions, observed a decrease in amino acids and sugar concentration and in the N2-fixation activity in roots of Medicago trunculata. Prior to this, Rosenblum et al. (2005) used a 1H NMR partial ecometabolic analysis to study the polar metabolites in red abalone, Haliotis rufenses, during infection by the rickettsia Candidants xenohaliotis californiensis under different levels of food sources. They concluded that food levels and other abiotic factors such as water temperature are responsible for disease development. Likewise the Haliotis spp. metabolome shift response induced by Rickettsia spp. included decreases in amino acids and carbohydrates and increases in taurine, glycine, betaine and homarine similarly at different temperatures (Rosenblum et al. 2006) (Table 3).
Metabolomics coupled with other omics approaches
Metabolomics allows the determination of the phenotype response directly (Fiehn et al. 2000), whereas transcriptomics and proteomics analyze the way genome expression translates to biological response, thus allowing us to detect the genes that are expressed in one determined moment (Colebatch et al. 2004; Fridman and Pichersky 2005; Saito and Matsuda 2010). The simultaneous use of metabolomics with genomics, transcriptomics and/or proteomics provides a global overview from transcription to final metabolic products that allows reaching better knowledge of the regulatory networks of metabolic pathways than the omics studies used without metabolomics analysis. For instance there are many metabolomics pathways regulated at the post-transcriptional level (Kaplan et al. 2007). The integrated omic studies are specially promising for ecological studies since they make possible discerning between genotype dependent and independent response to environmental changes for multiple characters at once (Pena et al. 2010; Wienkoop et al. 2008). This provides an overview of the short-, medium- and long-term responses to environemental changes and their integrative relationships. Metabolomic databases can be combined with data sets from other “omic” technologies (Weckwerth 2008) to enhance data value and permit a system-wide analysis from genome to phenome (just as the genome and proteome signify all of an organism’s genes and proteins, the phenome represents the total sum of its phenotypic traits).
Some past studies have begun to demonstrate these arguments. Metabolomics and transcriptomics can be combined and analyzed mathematically together. In this way, the metabolites and genes regulated by the same mechanisms cluster together (Table 4). For example, integrated experiments in transcriptomic and ecometabolomic studies have been successfully conducted in Arabidopsis at different levels of sulfur supply to elucidate gene-to-gene and metabolite-to-gene networks (Hirai et al. 2004, 2005; Nikiforova et al. 2004, 2005b; Fukushima et al. 2009b). These studies allow the detection of general and specific responses to different nutrient deficiencies and the identification of gene function with the added value of the improvement in the production of useful compounds in plants. Furthermore, some of these studies have provided novel knowledge among metabolic pathways linked to the physiological endpoint involved in plant homeostasis and responses to nutrient stress (Nikiforova et al. 2004, 2005b). Similarly coupling metabolomic with other omic approaches has allowed the improvement of our knowledge of the metabolic process and their regulation in response to other environmental factors such as salinity (Brosché et al. 2005; Gong et al. 2005), drought (Vasquez-Robinet et al. 2008), oxidative stress (Baxter et al. 2007), symbiotic N2 fixation (Hernández et al. 2009; Sanchez et al. 2010), and plant-herbivore (Kant et al. 2004) and plant-microbe (Ward et al. 2010) interactions (Table 4). Similar holistic results can be expected by combining ecometabolomics with proteomics (Weckwerth 2008). For more detailed and specific information, see Macel et al. (2010), who provide detailed information about the possibilities of integration of metabolomics with other omics approaches.
Further applications in ecology
Ecometabolomic studies may provide new perspectives and insights into many ecological topics. In an attempt to highlight the important role that metabolome studies could have in ecology, here we propose the application of metabolome studies to some critical questions currently under debate in ecology (Fig. 1).
Stoichiometry, growth rate and other ecological hypotheses
Organisms are the products of chemical reactions, and their growth depends on the availability of various elements, especially carbon, nitrogen and phosphorus. In this context, ecometabolomic studies also provide an opportunity to make direct advances related to the ecological hypotheses that aim to study ecosystem structure and function from a chemical perspective. One those hypotheses, the growth-rate hypothesis (GRH) (Sterner and Elser 2002), links the relative element content of organisms to their growth rate, the idea being that fast-growing organisms need relatively more P-rich RNA, which is the main component of the protein-producing ribosome, in order to support rapid protein synthesis. Consequently, ecosystem conditions that produce organic matter with low C:P and N:P ratios would be expected to result in higher adaptive growth rates, more efficient energy transfer through a food web and increased biomass of large-bodied animals relative to that of small-bodied organisms (Sterner and Elser 2002). Further assessment of the GRH evidently requires many more studies on the effects of C:N:P ratios on the ratios of different metabolic products such as proteins and RNA, and on growth rates and body sizes in different taxa and ecosystems (Peñuelas and Sardans 2009a). A promising way to couple stoichiometry with phenotypic metabolic expression can now be provided by ecometabolomic studies. Ecometabolomics should thus help to interpret the response of different groups of organisms in allocating resources to growth, storage and defense. It may also provide the elemental and metabolic budgets for different species along gradients from low to fast growth, which would allow a better test of the links between the C:N:P ratio, growth rate and body-size spectrum. Similarly, ecometabolomics can be a promising tool in the frame of metabolic theory of ecology (MTE) (Brown et al. 2004). MTE states that body mass and body temperature together predict per capita rates of metabolism, respiration, growth and resource consumption, and that these rates can be scaled up to the level of populations and communities (West et al. 1999; van der Meer 2006). Ecometabolomics will allow evaluating the change in the metabolism allocation to different general functions (respiration, growth, storage) in response to different environmental situations and changes.
More on biotic relationships
Ecometabolomic studies still need to be used to investigate other important relationships, such as plants-higher herbivores (e.g., mammals) or herbivore-predator. Moreover, most ecometabolomic studies have focused on the metabolic shifts in only one of the members of the relationships. Future ecometabolomic studies should investigate the metabolic shifts in the two species that interact simultaneously in order to have a whole vision of the phenotypic consequences of the biotic relationship. For example, some current studies aimed to study the metabolome shifts of both plants and herbivores (insect) have observed novel and useful information demonstrating the herbivore capacity to use plant metabolites to further defend itself against predators (Jansen et al. 2009). Similar reasoning can be applied to plant-pathogen, plant-symbiont or plant-vertebrate interactions, and in general to all key ecological relationships between two or more organisms.
The possibility of studying the metabolome traits and changes of more complex trophic relationships, such as three or more trophic levels at once, under different environmental circumstances, and if possible in field conditions, remains a future challenge for ecologists. For instance, the study of Harvey et al. (2003) using conventional analytical methods is promising and suggests that the study of complex trophic relationships can take advantage of modern ecometabolomic approaches. These authors, by analyzing water-soluble glucosinolates, studied the complex interactions of four trophic levels, observing that the leaf glucosinolates of Brassica oleraceae and Brassica nigra affect the development of the hyperparasitoid as mediated through the herbivore and its primary parasitoid. B. nigra has a more than 3.5 times higher level of glucosinolates than B. oleracea. Thus, there is a greater constraint in the size and survival of primary and secondary parasitoids reared from herbivores feeding on B. nigra than in those reared from herbivores feeding on B. oleracea. These results demonstrate that herbivore diet can affect the performance of interacting organisms differently across several trophic levels, and suggest that bottom-top web structure and energy fluxes can be mediated by the quality of food sources.
Some studies suggest that symbiotic fungal endophytes control insect host-parasite interaction webs (Omachi et al. 2001; Hartley and Gange 2009). Ecometabolomic studies could characterize the metabolic pathways involved in these complex relationships. Some biotic relationships are mediated by exudated metabolites; the rhizosphere is a case in point. The ecometabolomics of the rhizosphere provides another interesting perspective that also merits future study because the chemical plant and microbial exudates found there have been shown to be a key factor in the regulation of plant-microbe relationships (Micallef et al. 2009; Biedrzycki and Bais 2009) and in absorption processes (Dessureault-Rompre et al. 2007).
Other relevant future applications in the context of multiple trophic relationships involve the analysis of highly significant abiotic gradients such as water availability in terrestrial ecosystems or temperature in aquatic ecosystems. This should provide a global perspective of the phenotype responses that are the most adequate to each environmental change and of the capacity of different species to respond to environmental changes by a plastic metabolome response. In this regard, ecometabolomics may provide insight into species’ stress tolerance, resistance and avoidance, giving information about species’ capability to change their lifestyle and their capacity to respond to environmental changes such as severe drought, direct competition or perturbations. Ecometabolomics provides the possibility of studying different species’ capacity to respond to different environmental factors at short term and to study the effects of those changes in chemical composition onto the trophic webs. Finally, this will increase our knowledge of the mechanisms underlying species composition shifts in ecosystems in response to changes in environmental conditions.
Species competition is an important ecological field where ecometabolomic studies have a promising future because, as mentioned above, only few studies have been conducted, and the results suggest interesting species mechanisms in interspecific competition. Ecometabolomics provides a useful approach to the understanding of the mechanisms underlying competitive relationships. For example, as we have discussed earlier, Peiris et al. (2008), in a ecometabolomic study of the competition among three different fungus species competing during wood decomposition, reported that 2-methyl-2,3-dihydroxypropionic acid and pyridoxine are synthesized by some fungi to inhibit the growth of their direct competitors. Thus, the competitive advantage that could be attributed to simply a greater growth capacity of one species compared to the others is in fact linked, at least partially, to a chemical growth suppression of the competitor species. However, competition is not only an interspecific question. Intraspecific competition is also important in several ecological scenarios, and ecometabolomics is a novel tool to be used to discern which metabolome changes occur in individuals submitted to different levels of intraspecific competition.
Animal behavior is fundamental in the performance of several ecosystems; mutualism, reproductive phenology and several other behavioral phenomena are species characters that affect the performance and structure of whole ecosystems. Still, the mechanisms underlying such animal behavior are as yet not well known—which are the metabolome shifts when behavior changes and which are the implications of organism body composition? Ecometabolomics can contribute considerably in this area.
From individuals to populations and ecosystems
Ecometabolomic applications are not only limited to the ecophysiology of organisms. Some studies upscale the use of metabolomics from individual to population and ecosystem levels. For example, Davey et al. (2008) have shown that metabolite fingerprinting and profiling is sufficiently sensitive to be able to identify the metabolic differences between populations of Arabidopsis petraea. They found two- to fourfold differences in many free amino acid concentrations between different populations. Many free carbohydrate concentrations were also different, while polyhydric alcohol concentrations were not. A principal component analysis of metabolite fingerprints revealed different metabolic phenotypes for each population. At the landscape level, Gidman et al. (2006) have shown that different metabolic fingerprints measured with rapid Fourier transform-infrared spectroscopy in tissue samples of Galium saxatile are correlated with a gradient of N deposition across the entire UK landscape. Ecometabolomics thus allows the investigation of complex ecological systems and provides a rapid and sensitive indicator of ecosystem health. Viant (2007) has gone a step further, using the same argument as in the origin of metabolomics: namely, that the measurement of multiple metabolites (versus one or a small group, such as occurs in classical analytical approaches) can provide a more robust assessment of the metabolic health of an organism, and hence characterizing the health of multiple species will provide a more complete assessment of ecosystem responses to environmental stressors, and even of the nature of the stressor. Ecometabolomic studies may also be used to differenciate between genetically modified and non-modified plants.
Global change
Ecometabolomic studies of the effects of environmental gradients or of manipulation experiments in the field on various species should help to assess the impacts of the different components of global change on natural ecosystems, namely climate change, atmospheric composition changes, pollution, invasiveness and loss of biodiversity, among others. Ecometobolomic studies can help to discern species’and communities’ capacity for adaptation to global changes by highlighting the metabolic pathways that are inhibited or stimulated, and by coupling with transcriptomics to determine what genes are involved in adaptation-evolution mechanisms. Moreover, the knowledge of chemical body changes that can affect the performance of trophic chains aids in understanding shifts in ecosystem structure. The effects of these global changes can only be understood by using natural environmental gradients or field manipulation experiments that allow studying the responses of species and biotic relationships, such as predation, herbivorism or parasitism, in conditions that closely resemble actual environments.
Ecometabolomic studies of organisms growing while subject to different levels of pollutants are especially important to understand organism responses to these pollutants. This is particularly true since the mechanism of action of toxins is frequently based on chemical interactions in the cells that affect metabolic expression. Moreover, ecometabolomic studies can help to discover the metabolic pathways of these chemotoxic compounds. In this vein, studies on the hyperaccumulation of trace elements in plants to discover genotypes that accumulate large amounts of certain trace elements are another example where ecometabolomic studies could be useful. Hyperaccumulator plants are an important tool in phytoremediation and soil restoration strategies, and the use of ecometabolomic studies could facilitate understanding the mechanisms of phenotypic expressions that allow plants to become hyperaccumulators.
Invasiveness is another current ecological problem of increasing global importance. When an ecosystem is invaded by one or more invasive species, some studies have reported changes in ecosystem or body composition stoichiometry (Hughes and Uowolo 2006) and in some groups of metabolites (Llusia et al. 2010; Sardans et al. 2010; Peñuelas et al. 2011). Ecometabolomic studies can help to determine what metabolic pathways and target metabolites are involved in alien success and in the resistance capacity of native species. One example of the possibilities of using ecometabolomic studies in this field is in the advance in the knowledge of the suitability of the increased competitive ability hypothesis (EICA). EICA proposes that invasive plants may still experience attack by local generalist herbivores (Müller-Schärer et al. 2004), but not by specialist herbivores. In this way, selection may favor a reduction in the expression of chemical defenses, which are effective against specialist herbivores, but metabolically demanding, and an increase in the concentrations of less costly qualitative defenses, which may be more toxic to generalist herbivores (Joshi and Vrieling 2005; Stastny et al. 2005; Peñuelas et al. 2010). Using ecometabolomic studies of native and alien plants and of generalist and specialist herbivores conducted in the field and with an accurate use of methodologies can clarify this debate by showing the plant and herbivore metabolome changes and the differences in metabolic adaptation of herbivores to plant sources. Moreover, ecometabolomics will also allow the comparison of the metabolomes of alien plant populations, in their novel habitat, without their specialist herbivores, with the metabolomes of populations of the same species growing in their natural endemic habitat with both their generalist and specialist herbivores.
The loss of biodiversity at a global scale warrants further studies to discern its causes and its consequences. In some cases, the causes are obvious and direct: loss of habitat surface or habitat fragmentation, overexploitation of the resources or direct hunting. But in other cases, the causes are still controversial. For example, decreases in the biodiversity of pollinators in Europe and in other parts of the world are a current hot topic. As far as we know, there are no ecometabolomic studies investigating plant-pollinator metabolomes. Knowledge of changes in the metabolome in the field under climatic or pollutant stress gradients, for example, of pesticides, could be very revealing in the study of the causes and the mechanisms responsible for the decrease in pollinators in some areas of the world.
Challenges
A serious challenge for ecometabolomic studies is to satisfy the need to disentangle the biologically relevant functions and response shifts under environmental changes, which implies determining and quantifying the maximum number of metabolites as possible. The exact number of metabolites remains a mystery, even in the case of microorganisms with simple and well-understood metabolisms. Typical non-plant eukaryotic organisms are estimated to contain from 4,000 to 20,000 metabolites (Fernie et al. 2004), and the plant kingdom produces 100,000–200,000 different metabolites (Fiehn et al. 2001), although the actual number present in any individual plant species is still unknown. Furthermore, the metabolome changes continuously, an additional challenge that is accentuated when measuring the metabolomes of several individuals from a free-living population, which will necessarily include considerable metabolic variation. There will be high levels of variation in metabolite concentrations between individuals, owing to differences in individual genetics, gender, age, organs, health status, and spatial and temporal environmental changes. Simple issues such as the time since the animal last ate or the plant last received sunlight may also be determinant. The treatment of the large temporal and individual variability found in metabolomes, which may tend to mask the sources of variation that are of interest for ecologists, can be successfully approached by trying to ‘film’ temporal changes in metabolite levels and their turnover rates (Peñuelas and Sardans 2009b) instead of merely taking ‘snapshots’ of metabolite levels, and by multiplying the number of individuals sampled. The continuous development of new advances in in vivo NMR spectroscopy and imaging, proton-transfer-reaction mass spectrometry or isotope labeling, and in the treatment of large data sets in bioinformatics will be of great assistance in this line of work (Gehlenborg et al. 2010).
Another challenge to face up to is the risk that the overwhelming ‘-omics-type’ information reaches a field that is conceptually not properly prepared, thereby leading to the loss of an opportunity to advance ecological knowledge. Certain explanatory principles accounting for the complexity of living organisms and their populations and ecosystems, as well as of their responses to the environment, are still lacking. Ecometabolomics will thus need to focus on conceptual advancement and functional trait discovery, and not just on technological development, if it is to shed light on the fundamental system-biological mechanisms at work on scales ranging from the individual to the ecosystem. Certainly, the use of multitrophic interactions by ecometabolomics is a complex task requiring the coupling of field studies to metabolomic studies of the organisms involved in the study. This requires interdisciplinary approaches to relate the changes observed in fied measurements (growth, density, reproduction, predation, etc.) with those observed in organisms’ metabolomes.
Ecometabolomic techniques have proven to have enough sensitivity for ecological studies of the metabolome response of diverse genotypes under different environmental conditions. However, the lack of past experiments in the field focused on the effects of several factors on the organism metabolome limits at present the findings of metabolome studies from an ecological perspective. On the other hand, the experimental designs of future studies should aim to also disentangle the causes of the metabolome shifts. For example, Robinson et al. (2007) observed a strong relation between the anatomical and physiological changes and the metabolic profile changes in Pseudosuga menziesii growing in different localities with different climate and soil traits. Although the study design did not allow a quantitative separation of the different environmental factors, it had the sensitivity to detect the environmental differences in soil type and climate.
A great drawback to dealing with all these challenges comes from the fact that ecometabolomic techniques imply the use of sophisticated and expensive equipment (GC-MS, HPLC-MC, HNMR), which are not always available in the laboratories where ecologists work. However, the present rapid improvements in analytical methods and in the ability of computer hardware and software to interpret large data sets multiply the possibilities of rapidly identifying and quantifying simultaneously more and more compounds with great facility, even for non-specialists in this field. On the other hand, the increase in interdisciplinary research will allow a progressively wider use of these molecular techniques in ecology studies. In the future we envision increasing use of these methodologies in ecological studies. Metabolome studies coupled with transcriptomics and genomics studies, together with statistical analysis improvements and with mathematical modeling (Lindon et al. 2003), should allow a more holistic vision of the organism, population and ecosystem structure and functioning both on a space and time scale, with a better understanding of the consequences of environmental changes from individual to ecosystem level. In a step forward and unlike classical target analytical methods, ecometabolomic studies allow the characterization of the complete metabolome shift and thus help to discern the parts of the genome involved in these relationships. In this way these studies improve our knowledge of ecological consequences throughout the trophic chains, at the adaptation-selection-evolution level.
As mentioned in the analytical techniques section, the different analytical techniques have different capacities to determine different analytical groups (polar-non-polar, volatiles-non-volatiles) and different sensitivities and elucidation powers. On the other hand, the lack of extensive data bases to help in the molecular structure determination is increasingly being solved by the continuous enhancement of available commercial informatic programs and databases (Hall 2006; Allwood et al. 2008). This especially impacts the study of the plant metabolome due to the large number of secondary metabolites. Moreover, the use of several analytical methods to analyze the same organism extracts has been successfully used in some recent studies using 1HNMR and GC-MS (Srinivasan et al. 2008; Jones et al. 2009) and 1HNMR and HPLC-MS (Schroeder et al. 2006; López-Gresa et al. 2010) by thus coupling the greater sensitivity of chromatographic methods with the greater elucidation power of HNMR. In this regard the recent HPLC-DAD-MS-SPE-NMR provides high sensitivity and elucidation power at once (Schlotterbeck and Ceccarelli 2009).
If ecological metabolomics succeeds in overcoming these challenges and uses them as opportunities for advancing knowledge, we can expect to see stimulating new developments and applications in the near future in many areas of ecological sciences, including issues of stress responses, life history variation, population structure, trophic interaction, nutrient cycling and the ecological niche. For example, the temporal and spatial characterization of the responses of individuals, populations and ecosystems to perturbations such as global change and the disentangling of evolutionary aspects of plant and animal communities both offer ecological metabolomics an immediate opportunity as a new and exciting application. In turn, ecology can provide a unique insight and a significant contribution to the study of functional metabolomics by helping to understand the ecological basis for interactions among metabolites.
References
Abdel-Farid IB, Jahangir M, van den Hondel C, Kim HK, Choi YH, Verpoorte R (2009) Fungal infection-induced metabolites in Brassica rapa. Plant Sci 176:608–615
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Ali K, Maltese F, Zyprian E, Rex M, Choi YH, Verpoorte R (2009) NMR metabolic fingerprinting based identification of grapevine metabolites associated with downy mildew resistance. J Agr Food Chem 57:9599–9606
Aliferis KA, Materzok S, Paziotou GN, Chrysayi-Tokousbalides M (2009) Lemna minor L. as a model organism for ecotoxicological studies performing H-1 NMR fingerprinting. Chemosphere 76:967–973
Allwood JW, Goodacre R (2009) An introduction to liquid chromatography-mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochem Analysis 21:33–47
Allwood JW, Ellis DI, Heald JK, Goodacre R, Mur LAJ (2006) Metabolomic approaches reveal that phosphatidic and phosphatidyl glycerol phospholipids are major discriminatory non-polar metabolites in responses by Brachypodium distachyon to challenge by Magnaporthe grisea. Plant J 46:351–368
Allwood JW, Ellis DI, Goodacre R (2008) Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiol Plantarum 132:117–135
Allwood JW, Clarke A, Goodacre R, Mur LAJ (2010) Dual metabolomics: a novel approach to understanding plant-pathogen interactions. Phytochemistry 71:590–597
Alvarez S, Marsh EL, Schroeder SG, Schachtman DP (2008) Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ 31:325–340
André A, Maucourt M, Moing A, Rolin D, Renaudin J (2005) Sugar import and phytopathogenicity of Spiroplasma citri: Glucose and fructose play distinct roles. Mol Plant Microbe Interact 18:33–42
Andreae MO, Crutzen PJ (1997) Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry. Science 276:1052–1058
Arany AM, de Jong TJ, Kim HK, van Dam NM, Choi YH, Verpoorte R, van der Meijden E (2008) Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 18:65–71
Bailey NJC, Oven M, Holmes E, Nicholson JK, Zenk MH (2003) Metabolomic analysis of the consequences of cadmium exposure in Silene cucubalus cell cultures via H-1 NMR spectroscopy and chemometrics. Phytochemistry 62:851–858
Barsch A, Tellstrom V, Patschkowski T, Kuster H, Niehaus K (2006) Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol Plant Microbe Interact 19:998–1013
Bathellier C, Tcherkez G, Mauve C, Bligny R, Gout E, Ghashghaie J (2009) On the resilience of nitrogen assimilation by intact roots under starvation, as revealed by isotopic and metabolomic techniques. Rapid Commun Mass Sp 23:2847–2856
Baxter CJ, Redestig H, Schauer N, Repsilber D, Patil KR, Nielsen J, Selbig J, Liu JL, Fernie AR, Sweetlove LJ (2007) The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol 143:312–325
Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138:1058–1070
Behrends V, Ryall B, Wang XZ, Bundy JG, Williams HD (2010) Metabolic profiling of Pseudomonas aeruginosa demonstrates that the anti-sigma factor MucA modulates osmotic stress tolerance. Mol Biosystems 6:562–569
Biedrzycki ML, Bais HP (2009) Root secretions: from genes and molecules to microbial associations. J Exp Bot 60:1533–1534
Bino RJ, Hall RD, Fiehn O, Kopka J, Saito K, Draper J, Nikolau BJ, Mendes P, Roessner-Tunali U, Beale MH, Trethewey RN, Lange BM, Wurtele ES, Sumner LW (2004) Potential of metabolomics as a functional genomics tool. Trends Plant Sci 9:418–425
Bölling C, Fiehn O (2005) Metabolite profiling of Chlamydomonas reinhardtii under nutrient deprivation. Plant Physiol 139:1995–2005
Bon D, Gilard V, Massou S, Peres G, Malet-Martino M, Martino R, Desmoulin F (2006) In vivo P-31 and H-1 HR-MAS NMR spectroscopy analysis of the unstarved Aporrectodea caliginosa (Lumbricidae). Biol Fert Soils 43:191–198
Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW (2005) Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56:323–336
Brosché M, Vinocur B, Alatalo ER, Lamminmäki A, Teichmann T, Ottow EA, Djilianov D, Afif D, Bogeat-Triboulot MB, Altman A, Polle A, Dreyer E, Rudd S, Lars P, Auvinen P, Kangasjärvi J (2005) Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biol 6:R:101
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Bundy JG, Osborn D, Weeks JM, Lindon JC, Nicholson JK (2001) An NMR-based metabonomic approach to the investigation of coelomic fluid biochemistry in earthworms under toxic stress. Febs Lett 500:31–35
Bundy JG, Lenz EM, Bailey NJ, Gavaghan CL, Svendsen C, Spurgeon D, Hankard PK, Osborn D, Weeks JA, Trauger SA (2002) Metabonomic assessment of toxicity of 4-fluoroaniline, 3,5-difluoroaniline and 2-fluoro-4-methylaniline to the earthworm Eisenia veneta (Rosa): identification of new endogenous biomarkers. Environ Toxicol Chem 21:1966–1972
Bundy JG, Ramlov H, Holmstrup M (2003) Multivariate metabolic profiling using H-1 nuclear magnetic resonance spectroscopy of freeze-tolerant and freeze-intolerant earthworms exposed to frost. Cryoletters 24:347–358
Bundy JG, Spurgeon DJ, Svendsen C, Hankard PK, Weeks JM, Osborn D, Lindon JC, Nicholson JK (2004) Environmental metabonomics: applying combination biomarker analysis in earthworms at a metal contaminated site. Ecotoxicology 13:797–806
Bundy JG, Keun HC, Sidhu JK, Spurgeon DJ, Svendsen C, Kille P, Morgan AJ (2007) Metabolic profile biomarkers of metal contamination in a sentinel terrestrial species are applicable across multiple sites. Environ Sci Technol 41:4458–4464
Bundy JG, Sidhu JK, Rana F, Spurgeon DJ, Svendsen C, Wren JF, Stürzenbaum SR, Morgan AJ, Kille P (2008) ‘Systems toxicology’ approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol 6:25
Bussell JA, Gidman EA, Causton DR, Gwynn-Jones D, Malham SK, Jones MLM, Reynolds B, Seed R (2008) Changes in the immune response and metabolic fingerprint of the mussel, Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress. J Exp Mar Biol Ecol 358:78–85
Cao M, Koulman A, Johnson LJ, Lane GA, Rasmussen S (2008) Advanced data-mining strategies for the analysis of direct-infusion ion trap mass spectrometry data from the association of perennial ryegrass with its endophytic fungus, Neotyphodium lolii. Plant Physiol 146:1501–1514
Carpenter JF, Crowe JH (1988) The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244–255
Cevallos–Cevallos JM, Rouseff R, Reyes-De-Corcuera J (2009) Untargeted metabolite analysis of healthy and Huanglongbing-infected orange leaves by CE-DAD. Electrophoresis 30:1240–1247
Charlton AJ, Donarski JA, Harrison M, Jones SA, Godward J, Oehlschlager S, Arques JL, Ambrose M, Chinoy C, Mullineaux PM, Domoney C (2008) Responses of the pea (Pisum sativum L.) leaf metabolome to drought stress assessed by nuclear magnetic resonance spectroscopy. Metabolomics 4:312–327
Chiwocha S, Rouault G, Abrams S, von Aderkas P (2007) Parasitism of seed of Douglas fir (Pseudotsuga menziesii) by the seed chalcid, Megastigmus spermotrophus, and its influence on seed hormone physiology. Sex Plant Reprod 20:19–25
Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, Tamogami S, Satoh K, Kikuchi S, Higashi T, Kimura S, Saji H, Tanaka Y, Iwahashi H, Masuo Y, Rakwal R (2008) Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J Proteome Res 7:2980–2998
Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JTJ, Brzin J, Zel J, Verpoorte R (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using H-1-NMR spectroscopy and multivariate data analysis. Plant Physiol 135:2398–2410
Choi YH, Kim HK, Linthorst HJM, Hollander JG, Lefeber AWM, Erkelens C, Nuzillard JM, Verpoorte R (2006) NMR metabolomics to revisit the tobacco mosaic virus infection in Nicotiana tabacum leaves. J Nat Prod 69:742–748
Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, Kopka J, Udvardi MK (2004) Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J 39:487–512
Cook D, Fowler S, Fiehn O, Thomashow MF (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA 101:15243–15248
Copolovici LO, Filella I, Llusià J, Niinemets U, Peñuelas J (2005) The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiol 139:485–496
Coucheney E, Daniell TJ, Chenu C, Nunan N (2008) Gas chromatographic metabolic profiling: a sensitive tool for functional microbial ecology. J Microbiol Meth 75:491–500
Cramer GR, Ergül A, Grimplet J, Tillett RL, Tattersall EAR, Bohlman MC, Vincent D, Sonderegger J, Evans J, Osborne C, Quilici D, Schlauch KA, Schooley DA, Cushman JC (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomic 7:111–134
Cyril J, Powell GL, Duncan RR, Baird WV (2002) Changes in membrane polar lipid fatty acids of Seashore paspalum in response to low temperature exposure. Crop Scie 42:2031–2037
Davey MP, Burrell MM, Woodward FI, Quick WP (2008) Population-specific metabolic phenotypes of Arabidopsis lyrata ssp petraea. New Phytol 177:380–388
Davey MP, Woodward FI, Quick WP (2009) Intraspecfic variation in cold-temperature metabolic phenotypes of Arabidopsis lyrata ssp petraea. Metabolomics 5:138–149
Degen T, Dillmann C, Marion-Poll F, Turlings TCJ (2004) High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 135:1928–1938
Depuydt S, Trenkamp S, Fernie AR, Elftieh S, Renou JP, Vuylsteke M, Holsters M, Vereecke D (2009) An integrated genomics approach to define niche establishment by Rhodococcus fascians. Plant Physiol 149:1366–1386
Desbrosses GG, Kopka J, Udvardi MK (2005) Lotus japonicus metabolic profiling. Development of gas chromatography-mass spectrometry resources for the study of plant-microbe interactions. Plant Physiol 137:1302–1318
Dessureault-Rompre J, Nowack B, Schulin R, Luster J (2007) Spatial and temporal variation in organic acid anion exudation and nutrient anion uptake in the rhizosphere of Lupinus albus L. Plant Soil 301:123–134
Ekman DR, Teng Q, Villeneuve DL, Kahl MD, Jensen KM, Durhan EJ, Ankley GT, Collette TW (2008) Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17 alpha-ethynylestradiol with metabolite profiling. Environ Sci Technol 42:4188–4194
Ellis S, Mellor A (1995) Soils and environment. Routledge, London
Feige U, Morimoto RI, Yahara I, Polla BS (1996) Stress-inducible cellular responses. Birkhauser Verlag, Basel
Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L (2004) Metabolite profiling: from diagnostics to systems biology. Nat Rev Mol Cell Bio 5:763–769
Ferreres F, Sousa C, Valentao P, Pereira JA, Seabra RM, Andrade PB (2007) Tronchuda cabbage flavonolds uptake by Pieris brassicae. Phytochemistry 68:361–367
Fiehn O (2002) Metabolomics––the link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L (2000) Metabolite profiling for plant functional genomics. Nature Biotechnol 18:1157–1161
Fiehn O, Kloska S, Altmann T (2001) Integrated studies on plant biology using multiparallel techniques. Curr Opin Biotech 12:82–86
Figueiredo A, Fortes AM, Ferreira S, Sebastiana M, Choi YH, Sousa L, Acioli-Santos B, Pessoa F, Verpoorte R, Pais MS (2008) Transcriptional and metabolic profiling of grape (Vitis vinifera L.) leaves unravel possible innate resistance against pathogenic fungi. J Exp Bot 59:3371–3381
Filella I, Penuelas J (1999) Altitudinal differences in UV absorbance, UV reflectance and related morphological traits of Quercus ilex and Rhododendron ferrugineum in the Mediterranean region. Plant Ecol 145:157–165
Filella I, Penuelas J, Llusia J (2006) Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. New Phytol 169:135–144
Filella I, Wilkinson MJ, Llusia J, Hewitt CN, Penuelas J (2007) Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol Plantarum 130:58–66
Foito A, Byrne SL, Shepherd T, Stewart D, Barth S (2009) Transcriptional and metabolic profiles of Lolium perenne L. genotypes in response to a PEG-induced water stress. Plant Biotechnol J 7:719–732
Fridman E, Pichersky E (2005) Metabolomics, genomics, proteomics, and the identification of enzymes and their substrates and products. Curr Opinn Plant Biol 8:242–248
Fukushima A, Kusano M, Nakamichi N, Kobayashi M, Hayashi N, Sakakibara H, Mizuno T, Saito K (2009a) Impact of clock-associated Arabidopsis pseudo-response regulators in metabolic coordination. Proc Natl Acad Sci USA 106:7251–7256
Fukushima A, Kusano M, Redestig H, Arita M, Saito K (2009b) Integrated omics approaches in plant systems biology. Curr Opin Chem Biol 13:532–538
Fumagalli E, Baldoni E, Abbruscato P, Piffanelli P, Genga A, Lamanna R, Consonni R (2009) NMR techniques coupled with multivariate statistical analysis: tools to analyse Oryza sativa metabolic content under stress conditions. J Agron Crop Sci 195:77–88
Gagneul D, Ainouche A, Duhaze C, Lugan R, Larher FR, Bouchereau A (2007) A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol 144:1598–1611
Gaquerel E, Weinhold A, Baldwin IT (2009) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphigidae) and its natural host Nicotiana attenuata. VIII. An unbiased GCxGC-ToFMS analysis of the plant’s elicited volatile emissions. Plant Physiol 149:1408–1423
Gehlenborg N, O’Donoghue SI, Baliga NS, Goesmann A, Hibbs MA, Kitano H, Kohlbacher O, Neuweger H, Schneider R, Tenenbaum D, Gavin AC (2010) Visualization of omics data for systems biology. Nature Methods 7:S56–S68
Gibb JOT, Svendsen C, Weeks JM, Nicholson JK (1997) H-1 NMR spectroscopic investigations of tissue metabolite biomarker response to Cu(II) exposure in terrestrial invertebrates: identification of free histidine as a novel biomarker of exposure to copper in earthworms. Biomarkers 2:295–302
Gidman EA, Goodacre R, Emmett B, Wilson DB, Carroll JA, Caporn SJM, Cresswell N, Gwynn-Jones D (2005) Metabolic fingerprinting for bio-indication of nitrogen responses in Calluna vulgaris heath communities. Metabolomics 1:279–285
Gidman EA, Stevens CJ, Goodacre R, Broadhurst D, Emmett B, Gwynn-Jones D (2006) Using metabolic fingerprinting of plants for evaluating nitrogen deposition impacts on the landscape level. Global Change Biol 12:1460–1465
Gong QQ, Li PH, Ma SS, Rupassara SI, Bohnert HJ (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J 44:826–839
Gray GR, Heath D (2005) A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic fingerprinting. Physiol Plantarum 124:236–248
Griffin JL, Walker LA, Troke J, Osborn D, Shore RF, Nicholson JK (2000) The initial pathogenesis of cadmium induced renal toxicity. Febs Lett 478:147–150
Griffin JL, Walker L, Shore RF, Nicholson JK (2001) High-resolution magic angle spinning H-1-NMR spectroscopy studies on the renal biochemistry in the bank vole (Clethrionomys glareolus) and the effects of arsenic (As3+) toxicity. Xenobiotica 31:377–385
Gullberg J, Jonsson P, Nordström A, Sjöström M, Moritz T (2004) Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal Biochem 331:283–295
Guo Q, Sidhu JK, Ebbels TMD, Rana F, Spurgeon DJ, Svendsen C, Sturzenbaum SR, Kille P, Morgan AJ, Bundy JG (2009) Validation of metabolomics for toxic mechanism of action screening with the earthworm Lumbricus rubellus. Metabolomics 5:72–83
Haesler F, Hagn A, Frommberger M, Hertkorn N, Schmitt-Kopplin P, Munch JC, Schloter M (2008) In vitro antagonism of an actinobacterial Kitasatospora isolate against the plant pathogen Phytophthora citricola as elucidated with ultrahigh resolution mass spectrometry. J Microbiol Meth 75:188–195
Hall RD (2006) Plant metabolomics: from holistic hope, to hype, to hot topic. New Phytol 169:453–468
Hamzehzarghani H, Kushalappa AC, Dion Y, Rioux S, Comeau A, Yaylayan V, Marshall WD, Mather DE (2005) Metabolic profiling and factor analysis to discriminate quantitative resistance in wheat cultivars against fusarium head blight. Physiol Mol Plant Pathol 66:119–133
Hamzehzarghani H, Paranidharan V, Abu-Nada Y, Kushalappa AC, Dion Y, Rioux S, Comeau A, Yaylayan V, Marshall WD (2008) Metabolite profiling coupled with statistical analyses for potential high-throughput screening of quantitative resistance to fusarium head blight in wheat. Can J Plant Pathol (Revue Canadienne De Phytopathologie) 30:24–36
Hansen BH, Altin D, Booth A, Vang SH, Frenzel M, Sorheim KR, Brakstad OG, Storseth TR (2010) Molecular effects of diethanolamine exposure on Calanus finmarchicus (Crustacea: Copepoda). Aquat Toxicol 99:212–222
Harm W (1980) Biological effects of ultraviolet radiation. Cambridge University Press, New York
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342
Harvey JA, van Dam NM, Gols R (2003) Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J Anim Ecol 72:520–531
Hendrawati O, Yao QQ, Kim HK, Linthorst HJM, Erkelens C, Lefeber AWM, Choi YH, Verpoorte R (2006) Metabolic differentiation of Arabidopsis treated with methyl jasmonate using nuclear magnetic resonance spectroscopy. Plant Sci 170:1118–1124
Hernández G, Ramírez M, Valdés-López O, Tesfaye M, Graham MA, Czechowski T, Schlereth A, Wandrey M, Erban A, Cheung F, Wu HC, Lara M, Town CD, Kopka J, Udvardi MK, Vance CP (2007) Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol 144:752–767
Hernández G, Valdés-Lépez O, Ramírez M, Goffard N, Weiller G, Aparicio-Fabre R, Fuentes SI, Erban A, Kopka J, Udvardi MK, Vance CP (2009) Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol 151:1221–1238
Hines A, Oladiran GS, Bignell JP, Stentiford GD, Viant MR (2007) Direct sampling of organisms from the field and knowledge of their phenotype: key recommendations for environmental metabolomics. Environ Sci Technol 41:3375–3381
Hirai MY, Yano M, Goodenowe DB, Kanaya S, Kimura T, Awazuhara M, Arita M, Fujiwara T, Saito K (2004) Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc Natl Acad Sci USA 101:10205–10210
Hirai MY, Klein M, Fujikawa Y, Yano M, Goodenowe DB, Yamazaki Y, Kanaya S, Nakamura Y, Kitayama M, Suzuki H, Sakurai N, Shibata D, Tokuhisa J, Reichelt M, Gershenzon J, Papenbrock J, Saito K (2005) Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J Biol Chem 280:25590–25595
Howarth JR, Parmar S, Jones J, Shepherd CE, Corol DI, Galster AM, Hawkins ND, Miller SJ, Baker JM, Verrier PJ, Ward JL, Beale MH, Barraclough PB, Hawkesford MJ (2008) Co-ordinated expression of amino acid metabolism in response to N and S deficiency during wheat grain filling. J Exp Bot 59:3675–3689
Huang CY, Roessner U, Eickmeier I, Genc Y, Callahan DL, Shirley N, Langridge P, Bacic A (2008) Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol 49:691–703
Hughes RF, Uowolo A (2006) Impacts of Falcataria moluccana invasion on decomposition in Hawaiian lowland wet forests: the importance of stand-level controls. Ecosystems 9:977–991
Hura T, Grzesiak S, Hura K, Thiemt E, Tokarz K, Wedzony M (2007) Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: accumulation of ferulic acid correlates with drought tolerance. Ann Bot London 100:767–775
Huttunen L, Niemela P, Julkunen-Tiitto R, Heiska S, Tegelberg R, Rousi M, Kellomaki S (2008) Does defoliation induce chemical and morphological defenses in the leaves of silver birch seedlings under changing climate? Chemoecology 18:85–98
Jagger J (1985) Solar-UV actions on living cells. Praefer Publishers, New York
Jahangir M, Kim HK, Choi YH, Verpoorte R (2008) Metabolomic response of Brassica rapa submitted to pre-harvest bacterial contamination. Food Chem 107:362–368
Janda T, Szalai G, Leskó K, Yordanova R, Apostol S, Popova LP (2007) Factors contributing to enhanced freezing tolerance in wheat during frost hardening in the light. Phytochemistry 68:1674–1682
Jansen JJ, Allwood JW, Marsden-Edwards E, van der Putten WH, Goodacre R, van Dam NM (2009) Metabolomic analysis of the interaction between plants and herbivores. Metabolomics 5:150–161
Jennings KR (2000) The changing impact of the collision-induced decomposition of ions on mass spectrometry. Int J Mass Spectrom 200:479–493
Jobic C, Boisson AM, Gout E, Rascle C, Fèvre M, Cotton P, Bligny R (2007) Metabolic processes and carbon nutrient exchanges between host and pathogen sustain the disease development during sunflower infection by Sclerotinia sclerotiorum. Planta 226:251–265
Johnson HE, Broadhurst D, Goodacre R, Smith AR (2003) Metabolic fingerprinting of salt-stressed tomatoes. Phytochemistry 62:919–928
Jones AD, Hanley JC Jr, Stagliano MC, Appel H, Schultz JC (2006) Effect of insect herbivory and Pseudomonas syringae infection on secondary metabolite profiles in Arabidopsis thaliana leaves. In: Ward JL, Beale MH (eds) Proceedings from the 4th international plant metabolomics conference, pp 286. Metabolomics 2:269–334
Jones OAH, Walker LA, Nicholson JK, Shore RF, Griffin JL (2007) Cellular acidosis in rodents exposed to cadmium is caused by adaptation of the tissue rather than an early effect of toxicity. Comp Biochem Phys D 2:316–321
Jones OAH, Dondero F, Viarengo A, Griffin JL (2008a) Metabolic profiling of Mytilus galloprovincialis and its potential applications for pollution assessment. Mar Ecol Prog Ser 369:169–179
Jones OAH, Spurgeon DJ, Svendsen C, Griffin JL (2008b) A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere 71:601–609
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Kaiser KA, Barding GA, Larive CK (2009) A comparison of metabolite extraction strategies for H-1-NMR-based metabolic profiling using mature leaf tissue from the model plant Arabidopsis thaliana. Mag Reson Chem 47:S147–S156
Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135:483–495
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136:4159–4168
Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J 50:967–981
Kaplan F, Badri DV, Zachariah C, Ajredini R, Sandoval FJ, Roje S, Levine LH, Zhang FL, Robinette SL, Alborn HT, Zhao W, Stadler M, Nimalendran R, Dossey AT, Bruschweiler R, Vivanco JM, Edison AS (2009) Bacterial attraction and quorum aensing inhibition in Caenorhabditis elegans exudates. J Chem Ecol 35:878–892
Kavouras IG, Mihalopoulos N, Stephanou EG (1998) Formation of atmospheric particles from organic acids produced by forests. Nature 395:683–686
Keon J, Antoniw J, Carzaniga R, Deller S, Ward JL, Baker JM, Beale MH, Hammond-Kosack K, Rudd JJ (2007) Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death (PCD) of its susceptible wheat host. Mol Plant Microbe Interact 20:178–193
Kikuchi J, Shinozaki K, Hirayama T (2004) Stable isotope labeling of Arabidopsis thaliana for an NMR-based metabolomics approach. Plant Cell Physiol 45:1099–1104
Kim HK, Verpoorte R (2010) Sample preparation for plant metabolomics. Phytochem Anal 21:4–13
Kim JK, Bamba T, Harada K, Fukusaki E, Kobayashi A (2007) Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J Exp Bot 58:415–424
Kluender C, Sans-Piché F, Riedl J, Altenburger R, Härtig C, Laue G, Schmitt-Jansen M (2009) A metabolomics approach to assessing phytotoxic effects on the green alga Scenedesmus vacuolatus. Metabolomics 5:59–71
Kontunen-Soppela S, Ossipov V, Ossipova S, Oksanen E (2007) Shift in birch leaf metabolome and carbon allocation during long-term open-field ozone exposure. Global Change Biol 13:1053–1067
Kopka J, Fernie A, Weckwerth W, Gibon Y, Stitt M (2004) Metabolite profiling in plant biology: platforms and destinations. Genome Biol 5:109
Korn M, Gartner T, Erban A, Kopka J, Selbig J, Hincha DK (2010) Predicting Arabidopsis freezing tolerance and heterosis in freezing tolerance from metabolite composition. Mol Plant 3:224–235
Kuzina V, Ekstrom CT, Andersen SB, Nielsen JK, Olsen CE, Bak S (2009) Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol 151:1977–1990
Lake JA, Field KJ, Davey MP, Beerling DJ, Lomax BH (2009) Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant Cell Environ 32:1377–1389
Larkindale J, Huang BR (2004) Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environ Exp Bot 51:57–67
Larrainzar E, Wienkoop S, Scherling C, Kempa S, Ladrera R, Arrese-Igor C, Weckwerth W, Gonzalez EM (2009) Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Mol Plant Microbe Interact 22:1565–1576
Le Lay P, Isaure MP, Sarry JE, Kuhn L, Fayard B, Le Bail JL, Bastien O, Garin J, Roby C, Bourguignon-J (2006) Metabolomic, proteomic and biophysical analyses of Arabidopsis thaliana cells exposed to a caesium stress. Influence of potassium supply. Biochimie 88:1533–1547
Lee RE (1991) Principles of insect low temperature tolerance. In: Lee RE, Denlinger DL (eds) Insects at low temperatures. Champman & Hall, New York, pp 17–46
Lei RH, Wu CQ, Yang BH, Ma HZ, Shi C, Wang QJ, Wang QX, Yuan Y, Liao MY (2008) Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicol Appl Pharm 232:292–301
Leiss KA, Choi YH, Abdel-Farid IB, Verpoorte R, Klinkhamer PGL (2009a) NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J Chem Ecol 35:219–229
Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PGL (2009b) Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol 150:1567–1575
Leiss KA, Choi YH, Verpoorte R, Klinkhamer PGL (2011) An overview of NMR metabolomic to identify secondary plant compounds involved in host plant resistance. Phytochem Rev 10:205–216
Lenz EM, Hägele BF, Wilson ID, Simpson SJ (2001) High resolution H-1 NMR spectroscopic studies of the composition of the haemolymph of crowd- and solitary-reared nymphs of the desert locust, Schistocerca gregaria. Insect Biochem Molec 32:51–56
Lewis IA, Schommer SC, Hodis B, Robb KA, Tonelli M, Westler WM, Sussman MR JLM (2007) Method for determining molar concentrations of metabolites in complex solutions from two-dimensional 1H–13C NMR spectra. Anal Chem 79:9385–9390
Li PH, Sioson A, Mane SP, Ulanov A, Grothaus G, Heath LS, Murali TM, Bohnert HJ, Grene R (2006) Response diversity of Arabidopsis thaliana ecotypes in elevated [CO2] in the field. Plant Mol Biol 62:593–609
Liang YS, Choi YH, Kim HK, Linthorst HJM, Verpoorte R (2006a) Metabolomic analysis of methyl jasmonate treated Brassica rapa leaves by 2-dimensional NMR spectroscopy. Phytochemistry 67:2503–2511
Liang YS, Kim HK, Lefeber AWM, Erkelens C, Choi YH, Verpoorte R (2006b) Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. J Chromatogr A 1112:148–155
Lima MRM, Felgueiras ML, Graca G, Rodrigues JEA, Barros A, Gil AM, Dias ACP (2010) NMR metabolomics of esca disease-affected Vitis vinifera cv. Alvarinho leaves. J Exp Bot 61:4033–4042
Lindberg S, Banas A, Stymne S (2005) Effects of different cultivation temperatures on plasma membrane ATPase activity and lipid composition of sugar beet roots. Plant Physiol Bioch 43:261–268
Lindon JC, Nicholson JK (2008) Spectroscopic and statistical techniques for information recovery in metabonomics and metabolomics. Annual Review of Anal Chem 1:45–69
Lindon JC, Nicholson JK, Holmes E, Antti H, Bollard ME, Keun H, Beckonert O, Ebbels TM, Reilly MD, Robertson D, Stevens GJ, Luke P, Breau AP, Cantor GH, Bible RH, Niederhauser U, Senn H, Schlotterbeck G, Sidelmann UG, Laursen SM, Tymiak A, Car BD, Lehman-McKeeman L, Colet JM, Loukaci A, Thomas C (2003) Contemporary issues in toxicology––the role of metabonomics in toxicology and its evaluation by the COMET project. Toxicol Appl Pharm 187:137–146
Llusia J, Peñuelas J (1999) Pinus halepensis and Quercus ilex terpene emission as affected by temperature and humidity. Biol Plantarum 42:317–320
Llusia J, Peñuelas J (2001) Emission of volatile organic compounds by apple trees in response to spider mite attack and attraction of predatory mites. Exp Appl Acarology 25(1):65–77
Llusia J, Peñuelas J, Alessio GA, Estiarte M (2008) Contrasting species-specific, compound-specific, seasonal, and interannual responses of foliar isoprenoid emissions to experimental drought in a mediterranean shrubland. Int J Plant Sci 169:637–645
Llusia J, Peñuelas J, Sardans J, Owen SM, Niinemets U (2010) Measurement of volatile terpene emissions in 70 dominant vascular plant species in Hawaii: aliens emit more than natives. Global Ecol Biogeogr 19:863–874
Lois R (1994) Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis-thaliana L.1. Mechanisms of UV-resistance in Arabidopsis. Planta 194:498–503
López-Gresa MP, Maltese F, Bellés JM, Conejero V, Kim HK, Choi YH, Verpoorte R (2010) Metabolic response of tomato leaves upon different plant-pathogen interactions. Phytochem Anal 21:89–94
Lowe RGT, Lord M, Rybak K, Trengove RD, Oliver RP, Solomon PS (2008) A metabolomic approach to dissecting osmotic stress in the wheat pathogen Stagonospora nodorum. Fungal Genet Biol 45:1479–1486
Lugan R, Niogret MF, Kervazo L, Larher FR, Kopka J, Bouchereau A (2009) Metabolome and water status phenotyping of Arabidopsis under abiotic stress cues reveals new insight into ESK1 function. Plant Cell Environ 32:95–108
Lui L, Vikram A, Hamzehzarghani H, AC K (2005) Discrimination of three fungal diseases of potato tubers based on volatile metabolic profiles developed using GC/MS. Potato Res 48:85–96
Lundberg P, Lundquist PO (2004) Primary metabolism in N-2-fixing Alnus incana-Frankia symbiotic root nodules studied with N-15 and P-31 nuclear magnetic resonance spectroscopy. Planta 219:661–672
Macel M, van Dam NM, Keurentjes JJB (2010) Metabolomics: the chemistry between ecology and genetics. Mol Ecol Resour 10:583–593
Malmendal A, Overgaard J, Bundy JG, Sorensen JG, Nielsen NC, Loeschcke V, Holmstrup M (2006) Metabolomic profiling of heat stress: hardening and recovery of homeostasis in Drosophila. Am J Physiol Reg I 291:R205–R212
Mane SP, Robinet CV, Ulanov A, Schafleitner R, Tincopa L, Gaudin A, Nomberto G, Alvarado C, Solis C, Bolivar LA, Blas R, Ortega O, Solis J, Panta A, Rivera C, Samolski I, Carbajulca DH, Bonierbale M, Pati A, Heath LS, Bohnert HJ, Grene R (2008) Molecular and physiological adaptation to prolonged drought stress in the leaves of two Andean potato genotypes. Funct Plant Biol 35:669–688
Maruyama K, Takeda M, Kidokoro S, Yamada K, Sakuma Y, Urano K, Fujita M, Yoshiwara K, Matsukura S, Morishita Y, Sasaki R, Suzuki H, Saito K, Shibata D, Shinozaki K, Yamaguchi-Shinozaki K (2009) Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol 150:1972–1980
McGarvery GB, Pocs R (2006) Metabolite profiling as a tool for investigation of the metabolic response of soybean infection by Phytophthora soja. In: Ward JL, Beale MH (eds) Proceedings from the 4th international plant metabolomics conference, pp 328. Metabolomics 2:269–334
McKelvie JR, Yuk J, Xu YP, Simpson AJ, Simpson MJ (2009) H-1 NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure. Metabolomics 5:84–94
Micallef SA, Shiaris MP, Colón-Carmona A (2009) Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J Exp Bot 60:1729–1742
Michaud MR, Denlinger DL (2007) Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J Comp Physol B 177:753–763
Michaud MR, Benoit JB, López-Martínez G, Elnitsky MA, Lee RE, Denlinger DL (2008) Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing and desiccation in the Antarctic midge, Belgica antarctica. J Insect Physiol 54:645–655
Miller GA, Islam MS, Claridge TDW, Dodgson T, Simpson SJ (2008) Swarm formation in the desert locust Schistocerca gregaria: isolation and NMR analysis of the primary maternal gregarizing agent. J Exp Biol 211:370–376
Mirnezhad M, Romero-González RR, Leiss KA, Choi YH, Verpoorte R, Klinkhamer PGL (2010) Metabolomic analysis of host plant resistance to thrips in wild and cultivated tomatoes. Phytochem Anal 21:110–117
Moalemiyan M, Vikram A, Kushalappa AC (2007) Detection and discrimination of two fungal diseases of mango (cv. Keitt) fruits based on volatile metabolite profiles using GC/MS. Postharvest Biol Tec 45:117–125
Moco S, Bino RJ, De Vos RCH, Vervoort J (2007) Metabolomics technologies and metabolite identification. Trends Anal Chem 26:855–866
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Muth D, Kachlicki P, Krajewski P, Przystalski M, Stobiecki M (2009) Differential metabolic response of narrow leafed lupine (Lupinus angustifolius) leaves to infection with Colletotrichum lupini. Metabolomics 5:354–362
Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132:146–153
Nikiforova VJ, Gakière B, Kempa S, Adamik M, Willmitzer L, Hesse H, Hoefgen R (2004) Towards dissecting nutrient metabolism in plants: a systems biology case study on sulphur metabolism. J Exp Bot 55:1861–1870
Nikiforova VJ, Daub CO, Hesse H, Willmitzer L, Hoefgen R (2005a) Integrative gene-metabolite network with implemented causality deciphers informational fluxes of sulphur stress response. J Exp Bot 56:1887–1896
Nikiforova VJ, Kopka J, Tolstikov V, Fiehn O, Hopkins L, Hawkesford MJ, Hesse H, Hoefgen R (2005b) Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol 138:304–318
Novotny AM, Schade JD, Hobbie SE, Kay AD, Kyle M, Reich PB, Elser JJ (2007) Stoichiometric response of nitrogen-fixing and non-fixing dicots to manipulations of CO2, nitrogen, and diversity. Oecologia 151:687–696
Oksman-Caldentey KM, Saito K (2005) Integrating genomics and metabolomics for engineering plant metabolic pathways. Curr Opin Biotech 16:174–179
Omacini M, Chaneton EJ, Ghersa CM, Müller CB (2001) Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature 409:78–81
Ossipov V, Ossipova S, Bykov V, Oksanen E, Koricheva J, Haukioja E (2008) Application of metabolomics to genotype and phenotype discrimination of birch trees grown in a long-term open-field experiment. Metabolomics 4:39–51
Overgaard J, Malmendal A, Sorensen JG, Bundy JG, Loeschcke V, Nielsen NC, Holmstrup M (2007) Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J Insect Physiol 53:1218–1232
Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41:391–398
Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J (2008) Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl 129:189–199
Paranidharan V, Abu-Nada Y, Hamzehzarghani H, Kushalappa AC, Mamer O, Dion Y, Rioux S, Comeau A, Choiniere L (2008) Resistance-related metabolites in wheat against Fusarium graminearum and the virulence factor deoxynivalenol (DON). Botany 86:1168–1179
Parker D, Beckmann M, Zubair H, Enot DP, Caracuel-Rios Z, Overy DP, Snowdon S, Talbot NJ, Draper J (2009) Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J 59:723–737
Paul C, Barofsky A, Vidoudez C, Pohnert G (2009) Diatom exudates influence metabolism and cell growth of co-cultured diatom species. Mar Ecol Prog Ser 389:61–70
Peiris D, Dunn WB, Brown M, Kell DB, Roy I, Hedger JN (2008) Metabolite profiles of interacting mycelial fronts differ for pairings of the wood decay basidiomycete fungus, Stereum hirsutum with its competitors Coprinus micaceus and Coprinus disseminatus. Metabolomics 4:52–62
Pena A, Teeling H, Huerta-Cepas J, Santos F, Yarza P, Brito-Echeverria J, Lucio M, Schmitt-Kopplin P, Meseguer I, Schenowitz C, Dossat C, Barbe V, Dopazo J, Rossello-Mora R, Schuler M, Glockner FO, Amann R, Gabaldon T, Anton J (2010) Fine-scale evolution: genomic, phenotypic and ecological differentiation in two coexisting Salinibacter ruber strains. Isme J 4:882–895
Peñuelas J, Llusia J (1997) Effects of carbon dioxide, water supply, and seasonality on terpene content and emission by Rosmarinus officinalis. J Chem Ecol 23:979–993
Peñuelas J, Llusia J (1999) Short-term responses of terpene emission rates to experimental changes of PFD in Pinus halepensis and Quercus ilex in summer field conditions. Environ Exp Bot 42:61–68
Peñuelas J, Llusia J (2001) The complexity of factors driving volatile organic compound emissions by plants. Biol Plantarum 44:481–487
Peñuelas J, Llusia J (2003) BVOCs: plant defense against climate warming? Trends Plant Sci 8:105–109
Peñuelas J, Llusia J (2004) Plant VOC emissions: making use of the unavoidable. Trends Ecol Evol 19:402–404
Peñuelas J, Sardans J (2009a) Ecological metabolomics. Chem Ecol 25:305–309
Peñuelas J, Sardans J (2009b) Elementary factors. Nature 460:803–804
Peñuelas J, Staudt M (2010) BVOCs and global change. Trend Plant Sci 15:133–144
Peñuelas J, Llusià J, Estiarte M (1995) Terpenoids––a plant language. Trends Ecol Evol 10:289
Peñuelas J, Idso SB, Ribas A, Kimball BA (1997) Effects of long-term atmospheric CO2 enrichment on the mineral concentration of Citrus aurantium leaves. New Phytol 135:439–444
Peñuelas J, Llusià J, Gimeno BS (1999) Effects of ozone concentrations on biogenic volatile organic compounds emission in the Mediterranean region. Environ Pollut 105:17–23
Peñuelas J, Filella I, Stefanescu C, Llusia J (2005a) Caterpillars of Euphydryas aurinia (Lepidoptera:Nymphalidae) feeding on Succisa pratensis leaves induce large foliar emissions of methanol. New Phytol 167:851–857
Peñuelas J, Llusia J, Asensio D, Munné-Bosch S (2005b) Linking isoprene with plant thermotolerance, antioxidants and monoterpene emissions. Plant Cell Environ 28:278–286
Peñuelas J, Filella I, Seco R, Llusia J (2009a) Increase in isoprene and monoterpene emissions after re-watering of droughted Quercus ilex seedlings. Biol Plantarum 53:351–354
Peñuelas J, Rutishauser T, Filella I (2009b) Phenology feedbacks on climate change. Science 324:887–888
Peñuelas J, Sardans J, Llusià J, Owen SM, Silva J, Niinemets U (2010) Higher allocation to low cost chemical defenses in invasive species of Hawaii. J Chem Ecol 36:1255–1270
Peñuelas J, Sardans J, Llusia J, Owen SM, Niinemets U (2011) Lower P contents and more widespread terpene presence in old Bornean than in young Hawaiian tropical plant species guilds. Ecosphere 2:1–19
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486
Pincetich CA, Viant MR, Hinton DE, Tjeerdema RS (2005) Metabolic changes in Japanese medaka (Oryzias latipes) during embryogenesis and hypoxia as determined by in vivo P-31 NMR. Comp Biochem Phys C 140:103–113
Pinheiro C, Passarinho JA, Ricardo CP (2004) Effect of drought and rewatering on the metabolism of Lupinus albus organs. J Plant Physiol 161:1203–1210
Pluskal T, Nakamura T, Villar-Briones A, Yanagida M (2010) Metabolic profiling of the fission yeast S. pombe: quantification of compounds under different temperatures and genetic perturbation. Mol Biosyst 6:182–198
Poëssel JL, Sauge MH, Corre MN, Renaud C, Gaudillère M, Maucourt M, Deborde C, Dufour C, Loonis M, Lacroze JP, Pascal T, Moing A (2006) Metabolic profiling of shoot apices infested by the peach-potato aphid. In: Ward JL, Beale MH (eds) Proceedings from the 4th international plant metabolomics conference, pp 296. Metabolomics 2:269–334
Prithiviraj B, Vikram A, Kushalappa AC, Yaylayan V (2004) Volatile metabolite profiling for the discrimination of onion bulbs infected by Erwinia carotovora ssp carotovora, Fusarium oxysporum and Botrytis allii. Eur J Plant Pathol 110:371–377
Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC (2009) A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci USA 106:7708–7713
Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol 146:1440–1453
Riipi M, Haukioja E, Lempa K, Ossipov V, Ossipova S, Pihlaja K (2004) Ranking of individual mountain birch trees in terms of leaf chemistry: seasonal and annual variation. Chemoecology 14:31–43
Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Robinson AR, Ukrainetz NK, Kang KY, Mansfield SD (2007) Metabolite profiling of Douglas-fir (Pseudotsuga menziesii) field trials reveals strong environmental and weak genetic variation. New Phytol 174:762–773
Rochfort SJ, Ezernieks V, Yen AL (2009) NMR-based metabolomics using earthworms as potential indicators for soil health. Metabolomics 5:95–107
Roessner U, Patterson JH, Forbes MG, Fincher GB, Langridge P, Bacic A (2006) An investigation of boron toxicity in barley using metabolomics. Plant Physiol 142:1087–1101
Rosenblum ES, Viant MR, Braid BM, Moore JD, Friedman CS, Tjeerdema RS (2005) Characterizing the metabolic actions of natural stresses in the California red abalone, Haliotis rufescens using H-1 NMR metabolomics. Metabolomics 1:199–209
Rosenblum ES, Tjeerdema RS, Viant MR (2006) Effects of temperature on host-pathogen-drug interactions in red abalone, Haliotis rufescens, determined by H-1 NMR metabolomics. Environ Sci Technol 40:7077–7084
Saito K, Matsuda F (2010) Metabolomics for functional genomics. systems biology, and biotechnology. Annu Rev Plant Biol 61:463–489
Samuelsson LM, Forlin L, Karlsson G, Adolfsson-Eric M, Larsson DGJ (2006) Using NMR metabolomics to identify responses of an environmental estrogen in blood plasma of fish. Aquat Toxicol 78:341–349
Sanchez DH, Szymanski J, Erban A, Udvardi MK, Kopka J (2010) Mining for robust transcriptional and metabolic responses to long-term salt stress: a case study on the model legume Lotus japonicus. Plant Cell Environ 33:468–480
Sardans J, Llusia J, Niinemets U, Owen S, Peñuelas J (2010) Foliar mono- and sesquiterpene contents in relation to leaf economic spectrum in native and alien species in Oahu (Hawai’i). J Chem Ecol 36:210–226
Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, Jourdain A, Bastien O, Fievet JB, Vailhen D, Amekraz B, Moulin C, Ezan E, Garin J, Bourguignon-J (2006) The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 6:2180–2198
Scherling C, Roscher C, Giavalisco P, Schulze ED, Weckwerth W (2010) Metabolomics unravel contrasting effects of biodiversity on the performance of individual plant species. Plos One 5:e12569
Schlotterbeck G, Ceccarelli SM (2009) LC-SPE-NMR-MS: a total analysis system for bioanalysis. Bioanalysis 1:549–559
Schripsema J (2010) Application of NMR in plant metabolomics: techniques, problems and prospects. Phytochem Analysis 21:14–21
Schroeder FC, del Campo ML, Grant JB, Weibel DB, Smedley SR, Bolton KL, Meinwald J, Eisner T (2006) Pinoresinol: a lignol of plant origin serving for defense in a caterpillar. Proc Natl Acad Sci USA 103:15497–15501
Sekiyama Y, Chikayama E, Kikuchi J (2010) Profiling polar and semipolar plant metabolites throughout extraction processes using a combined solution-state and high-resolution magic angle spinning NMR approach. Anal Chem 82:1643–1652
Semel Y, Schauer N, Roessner U, Zamir D, Fernie AR (2007) Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype. Metabolomics 3:289–295
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Simoh S, Quintana N, Kim HK, Choi YH, Verpoorte R (2009) Metabolic changes in Agrobacterium tumefaciens-infected Brassica rapa. J Plant Physiol 166:1005–1014
Smith AR, Johnson HE, Hall M (2003) Metabolic fingerprinting of salt-stresse tomatoes. Bulgarian J Plant Physiol Special Issue:153–163
Solanky KS, Burton IW, MacKinnon-SL, Walter JA, Dacanay A (2005) Metabolic changes in Atlantic salmon exposed to Aeromonas salmonicida detected by H-1-nuclear magnetic resonance spectroscopy of plasma. Dis Aquat Organ 65:107–114
Solomon PS, Tan KC, Oliver RP (2005) Mannitol 1-phosphate metabolism is required for sporulation in planta of the wheat pathogen Stagonospora nodorum. Mol Plant Microbe Interact 18:110–115
Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, Malik RU, Edison AS, Sternberg PW, Schroeder FC (2008) A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454:1115–U1146
Stastny M, Schaffner U, Elle E (2005) Do vigour of introduced populations and escape from specialist herbivores contribute to invasiveness? J Ecol 93:27–37
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Story JM, Storey KB (1983) Regulation of cryoprotectant metabolism in the overwintering gall fly larva, Eurosta-solidaginis––temperature control of glycerol and sorbitol levels. J Comp Physiol 149:495–502
Strack D, Ammer C, Schliemann W (2006) Metabolic profiling of arbuscular mycorrhizal roots of Medicago truncatula. In: Ward JL, Beale MH (eds) Proceedings from the 4th international plant metabolomics conference, 276. Metabolomics 2:269–334
Sumner LW, Mendes P, Dixon RA (2003) Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry 62:817–836
Sun XM, Zhang JX, Zhang HJ, Ni YW, Zhang Q, Chen JP, Guan YF (2010) The responses of Arabidopsis thaliana to cadmium exposure explored via metabolite profiling. Chemosphere 78:840–845
Tang HR, Xiao CN, Wang YL (2009) Important roles of the hyphenated HPLC-DAD-MS-SPE-NMR technique in metabonomics. Mag Reson Chem 47:S157–S162
Taylor NS, Weber RJM, Southam AD, Payne TG, Hrydziuszko O, Arvanitis TN, Viant MR (2009) A new approach to toxicity testing in Daphnia magna: application of high throughput FT-ICR mass spectrometry metabolomics. Metabolomics 5:44–58
Trenkamp S, Eckes P, Busch M, Fernie AR (2009) Temporally resolved GC-MS-based metabolic profiling of herbicide treated plants treated reveals that changes in polar primary metabolites alone can distinguish herbicides of differing mode of action. Metabolomics 5:277–291
Tucker DJ, Wallis IR, Bolton JM, Marsh KJ, Rosser AA, Brereton IM, Nicolle D, Foley WJ (2010) A metabolomic approach to identifying chemical mediators of mammal-plant interactions. J Chem Ecol 36:727–735
Tuffnail W, Mills GA, Cary P, Greenwood R (2009) An environmental H-1 NMR metabolomic study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia and starvation. Metabolomics 5:33–43
Turner MA, Viant MR, Teh SJ, Johnson ML (2007) Developmental rates, structural asymmetry, and metabolic fingerprints of steelhead trout (Oncorhynchus mykiss) eggs incubated at two temperatures. Fish Physio Biochem 33:59–72
Urbanczyk-Wochniak E, Fernie AR (2005) Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J Exp Bot 56:309–321
van der Meer J (2006) Metabolic theories in ecology. Trends Ecol Evol 21:136–140
Vasquez-Robinet C, Mane SP, Ulanov AV, Watkinson JI, Stromberg VK, De Koeyer D, Schafleitner R, Willmot DB, Bonierbale M, Bohnert HJ, Grene R (2008) Physiological and molecular adaptations to drought in Andean potato genotypes. J Exp Bot 59:2109–2123
Verpoorte R, Choi YH, Mustafa NR, Kim HK (2008) Metabolomics: back to basics. Phytochem Rev 7:525–537
Via S, Gomulkiewicz R, Dejong G, Scheiner SM, Schlichting CD, Vantienderen PH (1995) Adaptive phenotypic plasticity––consensus and controversy. Trends Ecol Evol 10:212–217
Viant MR (2007) Metabolomics of aquatic organisms: the new ‘omics’ on the block. Mar Ecol Prog Ser 332:301–306
Viant MR, Werner I, Rosenblum ES, Gantner AS, Tjeerdema RS, Johnson ML (2003) Correlation between heat-shock protein induction and reduced metabolic condition in juvenile steelhead trout (Oncorhynchus mykiss) chronically exposed to elevated temperature. Fish Physio Biochem 29:159–171
Viant MR, Pincetich CA, Eerderna RST (2006a) Metabolic effects of dinoseb, diazinon-and esfenvalerate in eyed eggs and alevins of Chinook salmon (Oncorhynchus tshawytscha) determined by H-1 NMR metabolomics. Aquat Toxicol 77:359–371
Viant MR, Pincetich CA, Hinton DE, Tjeerdema RS (2006b) Toxic actions of dinoseb in medaka (Oryzias latipes) embryos as determined by in vivo P-31 NMR, HPLC-UV and H-1 NMR metabolomics. Aquat Toxicol 76:329–342
Vikram A, Prithiviraj B, Hamzehzarghani H, Kushalappa A (2004) Volatile metabolite profiling to discriminate diseases of McIntosh apple inoculated with fungal pathogens. J Sci Food Agr 84:1333–1340
Waagner D, Heckmann LH, Malmendal A, Nielsen NC, Holmstrup M, Bayley M (2010) Hsp70 expression and metabolite composition in response to short-term thermal changes in Folsomia candida (Collembola). Comp Biochem Phys A 157:177–183
Wallenstein MD, Hess AM, Lewis MR, Steltzerae H, Ayres E (2010) Decomposition of aspen leaf litter results in unique metabolomes when decomposed under different tree species. Soil Biol Biochem 42:484–490
Ward JL, Forcat S, Beckmann M, Bennett M, Miller SJ, Baker JM, Hawkins ND, Vermeer CP, Lu CA, Lin WC, Truman WM, Beale MH, Draper J, Mansfield JW, Grant M (2010) The metabolic transition during disease following infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Plant J 63:443–457
Warne MA, Lenz EM, Osborn D, Weeks JM, Nicholson JK (2000) An NMR-based metabonomic investigation of the toxic effects of 3-trifluoromethyl-aniline on the earthworm Eisenia veneta. Biomarkers 5:56–72
Warne MA, Lenz EM, Osborn D, Weeks JM, Nicholson JK (2001) Comparative biochemistry and short-term starvation effects on the earthworms Eisenia veneta and Lumbricus terrestris studied by H-1 NMR spectroscopy and pattern recognition. Soil Biol Biochem 33:1171–1180
Weckwerth W (2008) Integration of metabolomics and proteomics in Mol Plant Physiology––coping with the complexity by data-dimensionality reduction. Physiol Plantarum 132:176–189
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679
Widarto HT, Van der Meijden E, Lefeber AWM, Erkelens C, Kim HK, Choi YH, Verpoorte R (2006) Metabolomic differentiation of Brassica rapa following herbivory by different insect instars using two-dimensional nuclear magnetic resonance spectroscopy. J Chem Ecol 32:2417–2428
Widodo PattersonJH, Newbigin E, Tester M, Bacic A, Roessner U (2009) Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J Exp Bot 60:4089–4103
Wienkoop S, Morgenthal K, Wolschin F, Scholz M, Selbig J, Weckwerth W (2008) Integration of metabolomic and proteomic phenotypes. Mol Cell Proteomics 7:1725–1736
Williams TD, Wu HF, Santos EM, Ball J, Katsiadaki I, Brown MM, Baker P, Ortega F, Falciani F, Craft JA, Tyler CR, Chipman JK, Viant MR (2009) Hepatic transcriptomic and metabolomic responses in the stickleback (Gasterosteus aculeatus) exposed to environmentally relevant concentrations of dibenzanthracene. Environ Sci Technol 43:6341–6348
Yamakawa H, Hakata M (2010) Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol 51:795–809
Yang WL, Bernards MA (2007) Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics 3:147–159
Acknowledgments
This research was supported by the Spanish Government project CGL2006-04025/BOS, CGC2010-17172 and Consolider-Ingenio Montes (CSD2008-00040), by the Catalan Government project SGR 2009-458, and by the European project NEU NITROEUROPE GOCE017841.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sardans, J., Peñuelas, J. & Rivas-Ubach, A. Ecological metabolomics: overview of current developments and future challenges. Chemoecology 21, 191–225 (2011). https://doi.org/10.1007/s00049-011-0083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-011-0083-5