Abstract
Glucosinolates (GSs) are part of a two-component defence system, characteristic for the Brassicales, including the model species Arabidopsis thaliana (L.) Heynh. The defence activity of GSs is associated with different side chain structures. The AOP genes are central in side-chain modification. AOP2 mediates formation of alkenyl GS from a methylsulfinyl precursor, whereas AOP3 catalyzes production of hydroxy-alkyl GSs from the same precursor. Although several studies have assessed the role of GSs in plant defence, the function of specific aliphatic GSs in plant defence is still not clarified. Structural different GSs may influence insect herbivores differentially. We created a set of plant lines derived of a cross between two A. thaliana accessions, Gie-0 × Sap-0, which dominantly accumulate either 3-methylsulfinylpropyl GS or 3-hydroxypropyl GS. The generalist Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) and the crucifer-specialist Pieris brassicae (L.) (Lepidoptera: Pieridae) were used as model insects, to study effects of individual aliphatic GSs on lepidopteran herbivores with a different feeding specialization. However, the experiments revealed that weight gain of S. exigua and P. brassicae third and fourth-larval instars was similar on both chemotypes. But leaf consumption of the generalist was higher on 3-methylsulfinylpropyl-producing lines with low GS levels (23.2 μmol g−1) than on 3-hydroxypropyl-producing lines that contained a more than twofold higher amount of GSs (60 μmol g−1). In contrast, no differential effects of non-hydroxylated and hydroxylated GSs were found on the specialist P. brassicae. Our study indicates that there is no simple relationship between GS content and insect responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, the glucosinolate (GS)–myrosinase system has become an important model system to explore many aspects of insect–plant interactions. Even more questions have arisen from these studies as the complexity of this defence system became evident (Textor and Gershenzon 2009). One aspect remaining to be explored is the ecological relevance of individual GSs within a plant defence strategy against insects with a different feeding specialization. GSs comprise a highly variable class of secondary metabolites in the order Brassicales (Kliebenstein et al. 2001b; Newton et al. 2009) which offers an excellent model system to study GS-mediated plant insect interactions.
About 120 identified GSs are predominantly found in the order Brassicales, including important crop plants like Brassica napus and various Brassica oleraceae cultivars (Fahey et al. 2001). GSs are the largest class of secondary metabolites in Arabidopsis thaliana (L.) Heynh., with about 40 different chemical structures identified (Brown et al. 2003). These sulfur-containing molecules are among the most studied natural products in recent years due to their natural variation and interesting chemistry and biology. Another aspect is their potential use as cancer-preventing agents, biopesticides and biofumigants in agriculture (Talalay and Fahey 2001; Bainard et al. 2009; Gimsing and Kirkegaard 2009). GSs are amino acid-derived secondary metabolites with a common core structure comprising β-thioglucose, an N-hydroxyimino-sulfate moiety, and a variable aglycone side-chain. According to their precursor amino acid they are grouped into different classes: aliphatic GSs derived from Ala, Met, Leu, Ile, or Val, aromatic GSs from Phe or Tyr, and indole GSs from Trp. GSs are part of a two-component system that is activated after tissue damage, e.g. by either chewing insects or mechanical wounding. In intact plants, both components, GSs and myrosinases, are stored separately (Kelly et al. 1998). Cleavage of the glucose moiety of the GS-core structure by endogenous myrosinases generates a different profile of biologically active compounds, e.g. nitriles and isothiocyanates that provide defence against lepidopteran herbivores, bacteria, and aphids (Borek et al. 1998; Kim and Jander 2007; Aires et al. 2009).
GSs often act as deterrents or repellents against generalist feeders, but they are also used as feeding cues and oviposition stimulants by specialist insects (Hopkins et al. 2009). Although GS may provide resistance against herbivorous insects, most of their defensive properties are intensified upon formation of their hydrolysis products (Blau et al. 1978). The formation of the hydrolysis products depends on the chemical structure of the aglycone side chain and the presence of protein factors such as epithiospecifer proteins (ESPs), nitrile-specifier proteins (NSPs), the related thiocyanate forming protein (TFP), and the Epithospecifier-Modifier1(ESM1) (Bones and Rossiter 2006; Wittstock and Burow 2010).
An important determining factor of the final biological activity of GSs is the side chain structure (van Leur et al. 2008). Especially the side-chain of aliphatic methionine-derived GSs is highly variable in Brassica plants and A. thaliana accessions, which is attributed to only a small set of polymorphic loci (GS-ELONG, GS-OX, GS-AOP) generating modular alteration of the aliphatic GS profile (Kliebenstein et al. 2001a, b; Kliebenstein 2009). Side-chain elongation of certain aliphatic amino acid precursors is the starting point in biosynthesis of aliphatic GSs. The second step is biosynthesis of the GS core structure, followed by further transformations of variable side chains in a last step (Halkier and Gershenzon 2006).
Contrary to indolyl GSs, mostly methionine-derived aliphatic GSs undergo a series of modifications and can be highly variable in type and concentrations among Brassicaceae species, and within or between populations of one plant species, (Halkier and Gershenzon 2006). The GS-ELONG locus encodes a set of methylthioalkylmalate synthases that control the carbon chain length of the final GS structure (Kroymann et al. 2003; Benderoth et al. 2006). The GS-OX locus provides enzymes that catalyze the conversion of a methylthioalkyl GS to its methylsulfinylalkyl structure (Hansen et al. 2007), for example the oxygenation of 3-methylthiobutyl to 3-methylsulfinypropyl GS (3MSOP). Studies on the GS-AOP locus with AOP genes and GS levels in A. thaliana ecotypes revealed that different ecotypes naturally produce either hydroxyalkyl (e.g. 3-hydroxypropyl, 3OHP) or alkenyl (e.g. 2-propenyl) GS with corresponding production of either AOP2 or AOP3 functional enzymes (Kliebenstein et al. 2001b; Wentzell et al. 2007). The AOP gene family evolved through tandem gene duplication and encodes for 2-oxoglutarate-dependent dioxygenases. The dioxygenase encoded by AOP2 mediates the formation of alkenyl GSs from a methylsulfinyl precursor, whereas AOP3 catalyzes the production of hydroxyalkyl GSs from the same precursor. In accessions of A. thaliana accumulating methylsulfinyl GSs in their rosettes, AOP2 and AOP3 are not expressed or non-functional enzymes are produced (Kliebenstein et al. 2001a) One example is Columbia, an ecotype where AOP3 is silent and AOP2 contains a 5 bp deletion leading to a non-functional protein (Kliebenstein et al. 2001b).
It remains to be clarified whether specific compounds in plant secondary metabolite profiles or the total concentration of compounds belonging to the same class, determine resistance against insect herbivores (Poelman et al. 2008). In recent years, several detailed studies demonstrated a differential impact of individual GSs on the performance of generalist and specialist feeders (Li et al. 2000; Gols et al. 2008; Poelman et al. 2009). Van Leur et al. (2008) reported evidence that different chemical structures of phenylalanine-derived GSs are responsible for differential effects of Barbarea vulgaris distinct chemotypes to generalist lepidopteran insects. Although prior research has concentrated on the variation of methionine-derived aliphatic GS and its ecological consequences, it is still not clear how decisive the different methionine-derived GSs are within the plant defence against herbivorous insects and how significant the ecological role of individual aliphatic GSs is (Mewis et al. 2005; Poelman et al. 2008). Our previous studies with various A. thaliana accessions indicated that the variability of AOP genes influences plant resistance against insects (Rohr et al. 2006). We used a cross between the A. thaliana accessions Gie-0 (GS-null, nitrile formation upon hydrolysis) and Sap-0 (GS-OHP, isothiocyanate formation upon hydrolysis) to generate two different chemotypes with three-carbon side chains in a similar genetic background, which produce either 3-methylsulfinylpropyl GS (3MSOP) or 3-hydroxypropyl GS (3OHP). Based on the different chemotype lines, we wanted to determine whether there is a differential effect of specific aliphatic GS and distinct hydrolysis products on the performance of two lepidopteran herbivores with a different feeding specialization. Two model insects were selected for the present study a generalist, the significant agricultural pest Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) and a specialist, the semipolyphagous, GS adapted Pieris brassicae (L.) (Lepidoptera: Pieridae).
Materials and methods
Plant cultivation and crossbreeding
Arabidopsis thaliana (L.) accessions for crossbreeding were originally purchased from the Nottingham Arabidopsis Stock Center (NASC) and the GS profile was checked by HPLC. The 3MSOP producing ecotype Gie-0 (N1193, GS-ELONG 3C, AOP-0 genotype, nitrile formation upon hydrolysis) was chosen for the experiments, since previous bioassays revealed that this ecotype is highly resistant to larvae of the generalist S. exigua and the specialist P. brassicae (Rohr et al. 2006). Male crossing partner was the 3OHP producing ecotype Sap-0 (N1506, GS-ELONG 3C, AOP3 genotype, isothiocyanate formation upon hydrolysis). Sap-0 was most suitable for the larvae of S. exigua and P. brassicae in a previous study (Rohr 2009). Seeds of the two accessions were sown in pots filled with soil containing peat (Werkband e.V, Type T), covered with a dome and stratified for 3 days at 4°C in the dark. 10 days after germination the dome was removed and seedlings were transferred to single pots (7 cm diameter, 6 cm height) filled with the same soil used for germination. Plants were cultivated in a climate chamber at 21 ± 1°C, 60 ± 5% relative humidity, at 200 μmol m−1s−1 light intensity and a 10.5:13.5 (day/night) photoperiod. When the plants started to bolt, male-sterile crossing lines of Gie-0 were obtained by removing all anthers with a pair of tweezers. Pollen of Sap-0 was spread on Gie-0 plants by brushing the surface of the anthers against the stigma. A sufficient number of plants in the F1 with a 3MSOP or a 3OHP GS profile were obtained by 10 parallel cross-breedings of Gie-0 with Sap-0. The F1 hybrids of crosses of Gie-0 with Sap-0 (GxS) were selfed. The GS profiles of five plants from a single propagation were examined in each generation up to F3 by HPLC. It was verified that the GS profile was constant throughout the filial generations. In F3, a single plant was randomly chosen from each line for seed production. F4 progeny was used for insect performance assays. The hydrolysis product profile of GxS lines were analyzed by GC-MS.
Insect rearing
Pieris brassicae (L.) and Spodoptera exigua (Hübner) larvae were obtained from continuous laboratory rearing at the Urban Plant Ecophysiology Department at the Humboldt University. Eggs from P. brassicae were originally obtained from Insect Service GmbH (Berlin), whereas the starting population of S. exigua was kindly supplied by Bayer Crop Science (Monheim, Germany). The rearing procedure is described in detail in Rohr et al. (2006).
Feeding assays
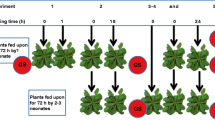
For the feeding assays, 10 lines producing 3MSOP and 10 lines containing 3OHP as the main compound, were used. The experiments were conducted under the same conditions as previously described for plant cultivation. 4-week-old plants of each line received the following treatment: no larva, one larva of S. exigua per plant, and one larva of P. brassicae per plant. The experiment was conducted with ten replications per plant line. Third and fourth instar larvae were used for the bioassays. At the beginning of the experiment, larvae were weighed and transferred to individual plants. They were kept in transparent Mylar cylinder cages (5 cm diameter, 9 cm height) covered with fine mesh gauze on top so that they could move freely on the plant. Control plants also received cages without caterpillars. After 72 h the final weight of larvae was determined and also the plant damage was estimated. The plant diameter was measured and to determine the percentage leaf area removal, damaged plants were grouped into categories according to Stotz et al. 2000. Damage classes are based on the amount of tissue removed on a scale: 0 (0–5%), 1 (6–13%), 2 (14–23%), 3 (24–37%), 4 (38–55%), 5 (56–77%), and 6 (78–100%). Whole rosettes were harvested, immediately flash frozen in liquid nitrogen and stored at −80°C for later analysis. Plants for GS analysis were harvested in pairs (four replicate pairs), and two plants per treatment were harvested separately for molecular biological studies. To determine a differential effect of the corresponding breakdown products of 3MSOP and 3 OHP on larvae of the generalist S. exigua and the crucifer-specialist P. brassicae, the bioassay data were related to hydrolysis products. For this purpose, the average weight gain of larvae on 3MSOP isothiocyanate and nitrile-producing line as well as on 3OHP isothiocyanate and nitrile-producing lines was calculated.
Glucosinolate analysis
Twenty milligrams of lyophilized plant material was extracted with 750 μl of 70% (v/v) boiling methanol for 5 min to destroy the remaining myrosinase activity. 60 μl of 1 mM 4-hydroxybenzyl GS (sinalbin, purified from Sinapsis alba seeds as potassium salt) was added as an internal standard. Re-extraction was carried out twice with 500 μl of 70% boiling methanol. The samples were centrifuged at 4,500g for 5 min to pellet the plant material and supernatants were combined. Samples were dried down to circa 300 μl and filled up to 2 ml with Millipore water. Extracts were transferred to 9 cm Poly-Prep® Chromatography columns (Biorad, CA, USA) filled with 0.5 ml of a 10% (w/v) suspension of DEAE Sephadex A-25 in 2 M acetic acid. Column wash steps are described in Mewis et al. (2005) and GSs in extracts were desulfated overnight with 75 μl of purified aryl sulfatase solution (H-1 from Helix pomatia, Sigma Aldrich) (Graser et al. 2001). Desulfated GSs were eluted with 1 ml of ultrapure water. 40 μl of desulpho GS extracts were analyzed with HPLC using a Dionex P680A instrument equipped with an ASI-100 auto sampler and a PDA-100 photodiode array detector. GSs were separated on a narrow bore column (Acclaim™ 120, 250–2.1, 5 μm, RP18, Dionex) using a gradient program of ultrapure water (A) and 40% (v/v) acetonitrile in ultrapure water (B) at a flow rate of 0.40 ml/min. The run comprised 0.5% B (1 min), 0.5–20% B (7 min), 20% B (2 min), 20–50% B (9 min), 50% B (3 min), 50–99% B (6 min), a 5 min hold at 99% B, 99–0.5% B (3 min), and a 7 min final hold at 0.5% B. The eluent was monitored by photodiode array detection between 190 and 360 nm. Desulfo-GS peaks were identified at 229 nm using retention time and UV spectra. To calculate molar concentration of individual GS, relative response factors (Buchner 1987; Brown et al. 2003) were used to correct for absorbance difference between the reference standard (4-hydroxybenzyl-GS, response factor 0.51) and other compounds. GSs were determined in four replications per treatment and plant line of crosses.
Analysis of glucosinolate hydrolysis products
The extraction method for analyzing GS hydrolysis products used to characterize the different ecotype lines is described in Rohr et al. (2009). Hydrolysis products were analyzed on an Agilent 6890 series gas chromatograph (Agilent Technologies, Waldbronn, Germany) with a DB5 column (J and W Scientific, 30 m, 0.25 mm I.D., and 0.25 μm film). A 1 μl sample was split-less injected at 200°C. A temperature program of 35°C for 3 min, a 10°C/min ramp to 230°C and a cool down with a total analysis time of 37 min was used with Helium as the carrier gas. The column was coupled to an Agilent 5973N quadrupole mass detector for product identification by comparing achieved MS spectra to those of authentic standards and MS libraries. Five plants for each line (replication) were analyzed for their GS hydrolysis products.
Gene expression analysis by RT-PCR
Total RNA was extracted from 100 mg of tissue from control plants of lines of ecotype crosses using the RNeasy Mini Plant Kit (Qiagen GmbH, Hilden, Germany) including the on-column DNase digestion step. RNA was spectrophotometrically quantified at 260 nm (Nanodrop ND1000, Technology Inc., USA) and quality was checked using the A 260/A 280 ratio calculation to be between 1.9 and 2.1. For conversion to cDNA, by Moloney murine leukemia virus (M-MLV) reverse transcriptase, 2 μg of total RNA was used according to the manufacturer’s protocol (Promega, USA).
The PCR reaction was optimized to ensure that the reaction was in the linear range. The semi-quantitative reaction was performed in a total volume of 20 μl, containing 1× PCR buffer (Promega), 0.2 mM dNTP’s, 2.1 mM MgCl2, 0.5 μmol of the forward and reverse primer, 1 unit of Taq DNA polymerase (Promega) and 0.3 μl of cDNA. The following PCR program was performed using an Applied Biosystems gradient cycler: 2 min 96°C, 30 cycles of 15 s at 94°C, 30 s 54°C, and 20 s at 72°C, followed by a final 5 min at 72°C. Actin8 was used as a reference gene and was designed to be intron spanning for possible detection of genomic DNA contamination. Primers listed in Table 1 were designed to amplify AOP3 genes and the reference gene.
To quantify the expression profiles, band intensities were measured by gel electrophoresis using a 1.5% agarose gel containing SYBR® Safe DNA gel stain (Invitrogen) and visualized on a Biostep transilluminator. Phoretix Total Lab Quant software was used to measure band intensities and calculate the amount of PCR product using the low mass ladder (Invitrogen). Expression of AOP3 genes was normalized relative to that of Actin8 by dividing the AOP3 band intensities by the Actin8 intensities. Actin8 was used as a control for sample-to-sample variation in reverse transcription and PCR conditions.
Statistics
Statistical differences in GS levels among the chemically different lines of crosses were analyzed by ANOVA. As the data for larval weight gain and the leaf area were not normally distributed, performances of larvae in experiments were subjected to a Kruskall–Wallis ANOVA Statistical analyses were performed using Sigma Plot (data analysis software system), version 11.0.
Results
Chemical traits of ecotype lines
By crossing Sap-0 (functional AOP3) into an AOP0 background (Gie-0 ecotype) we obtained 50% plants containing 3OHP GS in the filial generation. We selected 10 lines producing the aliphatic GS 3MSOP (Fig. 1a) and 10 lines accumulating the hydroxylated form 3OHP (Fig. 1b) in F3. Lines of crosses did not differ from the parent accessions with regard to overall plant morphology (e.g. growth habit, leaf morphology, growth rate, etc.). But analysis of GS levels revealed considerable quantitative variation between the two chemically different types of lines. The GxS-OHP lines showed a higher total GS content than the GxS-MSOP lines (ANOVA P < 0.001; GxS-MSOP: n = 40, GxS-OHP: n = 40). The constitutive GS content in the GxS-OHP lines with 60.6 μmol per g−1 dry weight was more than twofold higher than in the GxS-MSOP lines with 23.3 μmol per g−1 dry weight. Originally, plants of parent accessions Gie-0 and Sap-0 had similar high GS levels.
Mean concentrations of individual GSs in control plants of 4-week-old A. thaliana. F4 progeny of cross Gie-0 × Sap-0 (GxS) depicted as bar graphs: a lines producing 3MSOP (3-methylsulfinylpropyl GS) and b lines producing 3OHP (3-hydroxypropyl GS). The error bars represent ± standard error of total GS contents in individual lines
Both GxS-OHP and GxS-MSOP lines were characterized by the corresponding predominantly produced aliphatic GS and three other minor aliphatic GSs: 7-methylsulfinylheptyl GS, 7-methylthioheptyl GS, and 8-methylthiooctyl GS (Fig. 1a, b). In addition, aliphatic 4-methylsulfinylbutyl GS and 4-methylthiobutyl GS were detected in these lines. The indole GSs comprised one-third of the total GS amount in the GxS-MSOP lines, but only one-sixth of the total GS amount in the GxS-OHP lines. The main indole GS of the lines were indol-3-ylmethyl GS followed by 1-methoxy-indol-3-ylmethyl GS and 4-methoxy-indol-3-ylmethyl GS.
Some studies describe that particularly the predominant break-down products of GSs, the isothiocyanates, have toxic and growth inhibition effects on insect herbivores (e.g. Borek et al. 1997). Therefore, we analyzed and characterized the different GxS lines according to their predominant hydrolysis product and subsequently related to obtained results to the feeding studies. The GxS-MSOP lines mainly produced either 3-methylsulfinylpropyl isothiocyanate or 3-methylsulfinylpropyl cyanide as the predominant hydrolysis product. The lines GxS-MSOP3, GxS-MSOP5, GxS-MSOP8, and GxS-MSOP9 were classified as nitrile producing lines. In lines GxS-MSOP1, GxS-MSOP7, and GxS-MSOP10, the predominant hydrolysis products were isothiocyanates, whereas lines GxS-MSOP2, GxS-MSOP4, and GxS-MSOP6 contained a mixture of both types of hydrolysis products. The GxS-OHP lines principally contained either 3-hydroxypropyl isothiocyanate or 3-hydroxypropyl cyanide as hydrolysis products. The lines GxS-OHP1, GxS-OHP2, GxS-OHP5, GxS-OHP7, and GxS-OHP10 were characterized as nitrile producing lines, whereas lines GxS-OHP6, GxS-OHP8, and GxS-OHP9 produced predominantly isothiocyanates. Two lines, GxS-OHP3 and GxS-OHP4, contained a mixture of both types of hydrolysis products. These lines were not included in the insect performance assays.
Characterization of ecotype lines by gene expression analysis of AOP3
We used semi-quantitative RT-PCR to measure transcript levels of AOP3 genes in different lines of ecotype crosses to (1) characterize the lines and (2) see whether transcript levels are related to high GS levels in GxS-OHP lines. The GxS-MSOP lines showed no expression of AOP2 or AOP3 (data not presented). All these lines have inherited the AOP null allele from the Gie-0 parent, leading to non-expression of AOP2 and AOP3. The GxS-OHP lines did show expression of AOP3 (Fig. 2). They inherited the functional GS-OHP reaction from the Sap-0 parent; but in comparison to the control gene Actin8 AOP3 expression was very low (Fig. 2). AOP3 transcript levels in the different lines were between 0.04 and 0.47 and were not related to GS levels. For example, the GxS-OHP4 line showed the lowest expression of AOP3 with the highest mean GS levels in control plants. The parent ecotype Sap-0 was characterized by high expression of AOP3 and a comparable high GS content.
Insect performance on GxS-OHP and GxS-MSOP lines
The performance of two caterpillar species was assessed on the chemically distinct lines of ecotype crosses. Percentage larval weight gain of the generalist S. exigua was equivalent on GxS-MSOP and GxS-OHP lines (Kruskall–Wallis ANOVA P = 0.749) (Fig. 3) Similar results were obtained for larvae of the specialist P. brassicae (Kruskall–Wallis ANOVA P = 0.105) (Fig. 3). In addition, leaf area consumption of S. exigua and P. brassicae larvae was measured on the GxS-MSOP and GxS-OHP lines. S. exigua larvae caused more damage to GxS-MSOP lines than to GxS-OHP lines (Fig. 4, Kruskall–Wallis ANOVA P < 0.007). Leaf damage due to P. brassicae feeding was not different between GxS-MSOP and GxS-OHP lines (Fig. 4, Kruskall–Wallis ANOVA P = 0.1).
The larval performance data of the two insect species were also assigned to the respective hydrolysis products of GxS lines. There was no difference in larval growth for the larvae of the generalist on the isothiocyanate and nitrile producing GxS-MSOP lines (Kruskall–Wallis ANOVA, H = 0.437, df = 2, P = 0.804) (Table 2). Also no significant difference in larval growth on different hydrolysis product producing GxS-MSOP lines was observed (Kruskall–Wallis ANOVA, H = 2.055, df = 2, P = 0.358) for the specialist P. brassicae (Table 2). Again for both insects, the generalist (Kruskall–Wallis ANOVA, H = 1.366, df = 2, P = 0.505) and specialist (Table 2) (Kruskall–Wallis ANOVA, H = 1.043, df = 2, P = 0.594) no difference in larval growth was found on the different nitrile and isothiocyanate producing GxS-OHP lines. When relating the data of leaf consumption to the nitrile and isothiocyanate-producing GxS-MSOP or GxS-OHP lines, there was also no difference in herbivory on nitrile and isothiocyanate-accumulating lines.
Discussion
We hypothesized, that A. thaliana lines of crosses between Gie-0 and Sap-0 (GxS) accumulating either 3MSOP or 3OHP are affecting the performance of a generalist and a specialist lepidopteran differentially. In general, generalist herbivores are more negatively affected by GSs. Typically they are influenced by high levels of these secondary compounds with a poor larval performance (Kroymann et al. 2003; Arany et al. 2008). In the present study, the generalist S. exigua performed equally well on GxS-MSOP lines with general low GS levels as well as on GxS-OHP lines with general high GS levels. But the leaf consumption of larvae was higher on GxS-MSOP lines with low GS levels as on GxS-OHP lines that had a more than two-fold higher amount of GSs. This might indicate that GxS-MSOP lines are less suitable as host plants for the generalist insect, because the caterpillars fed more on the MSOP lines, but did not increase more weight than on OHP lines. Contrary, the specialist P. brassicae performed equally well on both chemotypes and the leaf consumption of larvae was likewise equal. This corresponds to results of other studies, which were not able to determine effects of GSs on specialist herbivores. (Olsson and Jonasson 1994; Arany et al. 2008). A literature survey by Nielsen et al. 2001 about plants containing different GS levels and their exposure to crucifer specialists revealed that there is no simple relationship between GS content and insect responses. In most cases, no effects on the specialist were found.
Although our first experiments conducted with the same lines of crosses with divergent GS amounts (Rohr et al. 2009) resulted in a lower larval weight gain of the generalist S. exigua and the specialist P. brassicae on GxS-MSOP lines compared to GxS-OHP lines, the caterpillar weight gain was similar on the different chemotype lines in the present experiments. The dissimilar results may be explained by a different caterpillar age used in the bioassays. In the initial experiments, younger larvae (second instar) were used than in the following experiments. It is possible that the observed lower caterpillar performance on 3MSOP lines compared to 3OHP lines may not be reproducible with older larvae as indicated by this study. Furthermore, it cannot be excluded that other compounds than GSs may have reduced the growth of younger caterpillars. High neonate toxicity and poor larval growth of P. brassicae was observed on Brassicales species, such as Tropaeolum majus L. and Armoracia rusticana P. Gartn., B. Mey and Scherb (Metspalu et al. 2003), whereupon chlorogenic acid has been identified as major active compound (Renwick 2001). Other recent experiments under controlled conditions as well as field studies with B. oleracea, the specialist P. rapae, and the generalist Mamestra brassicae (L.) revealed that the two insect species were negatively affected by high concentrations of the C3 aliphatic GS 3MSOP (Poelman et al. 2009).
In our study, it does not appear that the type of GS, the non-hydroxylated or hydroxylated form of the aliphatic C3 GSs, substantially influenced the growth of the studied insects. But apparently, introduction of AOP3 in Gie-0 resulted in higher total constitutive GS levels in the GxS-OHP lines (Fig.1b). In addition, GxS lines with similar total GS levels may be required to draw any clear conclusion, as indicated by the determined higher leaf consumption of S. exigua on GxS-MSOP lines compared to GxS-OHP lines with no corresponding higher weight increases.
In addition to GSs itself, toxic effects to herbivorous insects, especially generalists, are mainly attributed to their degradation products, particularly to isothiocyanates (Blau et al. 1978; Seo and Tang 1982). On the other hand, less is known about the role of nitriles in plant–insect interactions. Studies with larvae of the generalist Trichoplusia ni (Hübner) revealed a preference for A. thaliana plants producing nitriles as hydrolysis products, whereas isothiocyanates appeared to be rather repellent (Lambrix et al. 2001; Zhang et al. 2006). In the present study with different A. thaliana GxS lines, classified as isothiocyanate or nitrile producing, derived from the same precursor, either 3MSOP or 3OHP GS, we found no differential effect of the different types of hydrolysis products on larval performance of S. exigua and P. brassicae caterpillars. Corresponding to our results, no pronounced effects of GS hydrolysis products on the specialist species P. brassicae would be expected, because specialist insects developed detoxification mechanisms, like it is known for Pieris species, which converts isothiocyanates to nitriles (Wittstock et al. 2004). Experiments by Mumm et al. (2008) with nitrile-producing 35S:ESP plants and isothiocyanate-producing wild type Col-0 displayed that P. rapae caterpillars did not discriminate between the two genotypes in choice tests. Moreover, P. rapae performed equally well on the two genotypes in force-feeding experiments. In contrast, the generalist Spodoptera littoralis Boisduval showed a better performance on the 35S:ESP nitrile-producing lines, than on isothiocyanate producing wild type Col-0 (Burow et al. 2006).
Gene expression analysis of AOP genes revealed that the expression of AOP3 in GxS-OHP lines was very low in relation to the amount of 3OHP present in the corresponding lines. To date there are no studies describing the introduction of AOP3 in another MSOP producing A. thaliana ecotype with resulting higher aliphatic GS accumulation, only the ecotypes Col-0, Cvi, and Sha have been intensively studied for their regulatory networks. Highest levels of GSs accumulated in alkenyl GS producing accessions, which require the presence of AOP2 (Burow et al. 2010). Studies with Cvi and Sha suggest that the highest amounts of aliphatic GSs are attributed to a feedback amplification loop at the transcriptional level between AOP2 and the transcription factor MYB76 (Wentzell et al. 2007; Wentzell and Kliebenstein 2008; Burow et al. 2010), creating a regulatory network for GS regulation. This raises the question of whether regulatory factors or a regulatory network involving AOP3 might be responsible for the high GS concentrations in GxS-OHP lines. Already the parental accession of GxS-OHP lines, Sap-0, had the highest amount of 3OHP in comparison to other accessions accumulating the same aliphatic GSs. GxS-OHP producing lines showed a low expression of AOP3, which was not related to the actual GS phenotype, indicating that even a low enzyme activity of AOP3 is sufficient to generate a 3OHP phenotype.
Further investigation, however, is needed to elucidate the exact role of 3MSOP and 3OHP GS as well as their corresponding hydrolysis products in plant defence against miscellaneous herbivorous insects. In addition, the effects of AOP2 and AOP3 on expression of GS biosynthesis genes and transcription factors in A. thaliana should be included in further studies to explain the given effect of AOP genes on GS accumulation.
References
Aires A, Mota VR, Saavedra MJ, Monteiro AA, Simões M, Rosa EA, Bennett RN (2009) Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J Appl Microbiol 106(6):2096–2105
Arany AM, de Jong TJ, Kim HK, van Dam NM, Choi YH, Verpoorte R, van der Meijden E (2008) Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 18:65–71
Bainard LD, Brown PD, Upadhyaya MK (2009) Inhibitory effect of tall hedge mustard (Sisymbrium loeselii) allelochemicals on rangeland plants and arbuscular mycorrhizal fungi. Weed Sci 57:386–393
Benderoth M, Textor S, Windsor AJ, Mittchel-Olds T, Gershenzon J, Kroymann J (2006) Positive selection driving diversification in plant secondary metabolism. Proc Natl Acad Sci USA 103(24):9118–9123
Blau PA, Feeny P, Contardo L, Robson DS (1978) Allylglucosinolate and herbivorous caterpillars: a contrast in toxicity and tolerance. Science 200:1296–1298
Bones AM, Rossiter JT (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 67:1053–1067
Borek V, Elberson LR, Mc Caffrey JP, Morra MJ (1997) Toxicity of rapeseed meal and methyl isothiocyanate to larvae of the black vine weevil (Coleoptera: Curculionidae). J Econ Entomol 90:109–112
Borek V, Elberson LR, Mc Caffrey JP, Morra MJ (1998) Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil eggs. J Agric Food Chem 90:109–112
Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62:471–481
Buchner R (1987) Approach to determination of HPLC response factors for glucosinolates, pp 50–58. In: Wathelet JP (ed) Glucosinolates in rapeseed: analytical aspects. Martinus Nijhoff Publishers, Dordrecht
Burow M, Müller R, Gershenzon J, Wittstock U (2006) Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol 32:2333–2349
Burow M, Halkier BA, Kliebenstein DJ (2010) Regulatory networks of glucosinolates shape Arabidopsis thaliana fitness. Curr Opin Plant Biol 13:348–353
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51
Gimsing AL, Kirkegaard JA (2009) Glucosinolates and biofumigation: fate of glucosinolates and their hydrolysis products in soil. Phytochem Rev 8(1):299–310
Gols R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA (2008) Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild strains of cabbage, Brassica oleracea. J Chem Ecol 34:132–143
Graser G, Oldham NJ, Brown PD, Temp U, Gershenzon J (2001) The biosynthesis of benzoic acid glucosinolate esters in Arabidopsis thaliana. Phytochemistry 57:23–32
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Hansen BJ, Kliebenstein DJ, Halkier BA (2007) Identification of a flavin-monooxygenase as the S-oxigenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J 50(5):902–910
Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83
Kelly PJ, Bones A, Rossiter JT (1998) Subcellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta 206:370–377
Kim JH, Jander G (2007) Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J 49:1008–1019
Kliebenstein DJ, Lambrix V, Reichelt M, Gershenzon J, Mitchell-Olds T (2001a) Gene duplication and the diversification of secondary metabolism: side chain modification of glucosinolates in Arabidopsis thaliana. Plant Cell 13:681–693
Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, Mitchell-Olds T (2001b) Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol 126:811–825
Kliebenstein DJ (2009) A quantitative genetics and ecological model system: understanding the aliphatic glucosinolate biosynthetic network via QTLs. Phytochem Rev 8:243–254
Kroymann J, Donnerhacke S, Schnabelrauch D, Mitchell-Olds T (2003) Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc Natl Acad Sci USA 100:14587–14592
Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J (2001) The Arabidopsis epithiospecifer protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusiani herbivory. Plant Cell 13:2793–2807
Li Q, Eigenbrode SD, Stringam GR, Thiagarajah MR (2000) Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J Chem Ecol 26:2401–2419
Metspalu L, Hiiesaar K, Jõudu J, Kuusik A (2003) Influence of food on the growth, development and hibernation of large white butterfly (Pieris brassicae). Agron Res 1:85–92
Mewis I, Appel HM, Hom A, Raina R, Schultz JC (2005) Major signaling pathways modulate Arabidopsis thaliana (L.) glucosinolate accumulation and response to both phloem feeding and chewing insects. Plant Physiol 138(2):1149–1162
Mithen R, Raybould AF, Giamoustaris A (1995) Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant–herbivore interactions. Heredity 75:472–484
Mumm R, Burow M, Bukovinskine’Kiss G, Kazantzidou E, Wittstock U, Dicke M, Gershenzon J (2008) Formation of simple nitriles upon glucosinolate hydrolysis affects direct and indirect defense against the specialist herbivore, Pieris rapae. J Chem Ecol 34(10):1311–1321
Newton EL, Bullock JM, Hodgson DJ (2009) Glucosinolate polymorphism in wild cabbage (Brassica oleracea) influences the structure of herbivore communities. Oecologia 160:63–76
Nielsen JK, Hansen MD, Agerbirk N, Petersen BL, Halkier BA (2001) Responses of the flea beetles Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile. Chemoecology 11:75–83
Olsson K, Jonasson T (1994) Leaf feeding by caterpillars on white cabbage cultivars with different 2-propenyl glucosinolate (sinigrin) content. J Appl Ent 118:197–202
Poelman EH, Galiart RJFH, Raaijmakers CE, van Loon JJA, van Dam NM (2008) Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol Exp Appl 127:218–228
Poelman EH, van Dam NM, van Loon JJA, Vet LEM, Dicke M (2009) Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores. Ecology 90(7):1863–1877
Renwick JAA (2001) Variable diets and changing taste in plant–insect relationship. J Chem Ecol 27(6):1063–1076
Rohr F, Ulrichs C, Mucha-Pelzer T, Mewis I (2006) Variability of aliphatic glucosinolates in Arabidopsis and their influence on insect resistance. Commun Agric Appl Biol Sci 71(2 Pt B):507–515
Rohr F (2009) Variabilität aliphatischer Glucosinolate in Arabidopsis thaliana–Ökotypen und deren Einfluß auf die Wirtspflanzeneignung von zwei folivoren Insektenarten, vol 4, p 90. In: Ulrichs C, Büttner C (eds) Berliner ökophysiologische und phytomedizinische Schriften. Der Andere Verlag, Tönning
Rohr F, Ulrichs C, Mewis I (2009) Variability of aliphatic glucosinolates in Arabidopsis thaliana L.—impact on glucosinolate profile and insect resistance. J Appl Bot Food Qual 82:131–135
Seo ST, Tang CS (1982) Hawaiian fruit-flies (Diptera, Tephritidae)—toxicity of benzylisothiocyanate against eggs or 1st instars of three species. J Econ Entomol 75:1132–1135
Stotz HU, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Bauke A, Mitchell-Olds T (2000) Induced plant defense responses against chewing insects ethylene signalling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol 124:1007–1017
Talalay P, Fahey JW (2001) Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr 11(Suppl):3027S–3033S
Textor S, Gershenzon J (2009) Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochem Rev 8:149–170
van Leur H, Vet LEM, van der Putten WH, van Dam N (2008) Barbarea vulgaris phenotypes differentially affect performance and preference of two different species of Lepidopteran herbivores. J Chem Ecol 34(2):121–131
Wentzell A, Rowe CH, Hansen BG, Ticconi C, Halkier BA, Kliebenstein DJ (2007) Linking metabolic QTLs with Network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet 3(9):1687–1701
Wentzell AM, Kliebenstein DJ (2008) Genotype, age, tissue, and environment regulate the structural outcome of glucosinolate activation. Plant Physiol 147:415–428
Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel J (2004) Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci USA 101(14):4859–4864
Wittstock U, Burow M (2010) Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. In: Last R, Chang C, Jander G, Kliebenstein DJ, McClung R, Millar H, Torii K, Wagner D (eds) The Arabidopsis book. doi:10.1199/tab.0134 (http://www.aspb.org/publications/arabidopsis/)
Zhang ZY, Ober JA, Kliebenstein DJ (2006) The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell 18:1524–1536
Acknowledgments
The authors thank Dr. Michael Reichelt and Dr. Jonathan Gershenzon (Max Planck Institute for Chemical Ecology Jena, Germany) for their collaborative help in analyzing the glucosinolate hydrolysis products. This work was funded by the DFG (Deutsche Forschungsgemeinschaft, GZ ME 2095/4-1 and /4-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohr, F., Ulrichs, C., Schreiner, M. et al. Impact of hydroxylated and non-hydroxylated aliphatic glucosinolates in Arabidopsis thaliana crosses on plant resistance against a generalist and a specialist herbivore. Chemoecology 21, 171–180 (2011). https://doi.org/10.1007/s00049-011-0082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-011-0082-6