Abstract
In bumblebees all species of the subgenus Psithyrus are social parasites in the nests of their Bombus hosts. In the bumblebee B. terrestris we investigated how colony size influences survival rates of nest entering females of the social parasite Psithyrus vestalis. Furthermore, we studied whether the host worker’s dominance status and age are reflected in its individual scent and whether Psithyrus females use volatiles to selectively kill host workers. The survival rate of Psithyrus vestalis females drops from 100%, when entering colonies with five workers, to 0% for colonies containing 50 host workers. Older host workers, born before the nest invasion, were selectively killed when Psithyrus females entered the nest. In contrast, all workers born after the nest invasion survived. The host workers’ dominance status and age are reflected by their individual odours: newly emerged workers produced a significantly lower total amount of secretions than 4-day-old workers. In chemical analyses of female groups we identified saturated and unsaturated hydrocarbons, aldehydes, and unsaturated wax-type esters of fatty acids. In a discriminant function analysis different worker groups were mainly separated by their bouquets of hydrocarbons. Killed workers release significantly more scent and of a different chemical composition, than survivors. Survivors alter scent production and increase it beyond the level of the killed workers within 1 day of the invasion. The Psithyrus female clearly maintains reproductive dominance utilizing these differences in the odour bouquets as criteria for killing workers that compete for reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonies of primitive eusocial bumblebees (genus Bombus) are frequently invaded by females of the social parasitic cuckoo bumblebees (subgenus Psithyrus) (van Honk et al. 1981a; Fisher 1987a, b). The most striking difference between the parasites and their hosts, besides a thicker exoskeleton (Fisher and Sampson 1992), is the lack of a pollen collecting apparatus on the posterior tibia of the parasitic females. This makes them totally dependent on the workers of the host colony to rear their own offspring (Fisher 1985).
Psithyrus females rise from hibernation and search for host colonies a few weeks after the beginning of their hosts’ annual colonial life cycle, when the Bombus host queens have already established colonies with a worker force. After entering a host colony the parasite female is attacked by host workers that defend their nest against enemies—recognized through foreign scent—by hissing (Kirchner and Röschard 1999) and, of course, by stinging. Upon intrusion, the parasite tries to adopt (Fisher 1984b; Dronnet et al. 2005) or imitate (Zimma et al. 2004; Sramkova et al. in preparation) the existing nest odour or use repellents (Zimma et al. 2003) to reduce the attacks launched by the inhabiting workers.
Several nest selection strategies of the Psithyrus parasites can be distinguished: some species specialize in one host and invade only nests of this very species, while others favour a multi-host strategy (for a listing see Pouvreau 1973) resulting in more invasion options at the cost of reduced chances of succeeding (Fisher 1983, 1985). Furthermore, the parasite female takes into account the nest size and has to weigh potential reproductive success versus the chance of being killed upon invasion, both increasing in relation to colony size (Fisher 1984b, 1987b).
The Psithyrus female may judge the nest size and host species only using information available from outside the nest, such as entering and leaving frequencies of foragers, morphology of entering and leaving individuals (Cederberg 1983), and, of course, the odour of the colony or individuals (Wcislo 1986). This suggests that there is a species-specific and a colony-specific component to the odour (Fisher et al. 1993; Dronnet et al. 2005). It was proven that Psithyrus ashtoni recognize their host species by olfactory cues (Fisher 1983, 1985), and Psithyrus rupestris use the trail pheromone laid by their Bombus lapidarius host workers to find the entrance to a nest (Cederberg 1983).
Females of some Psithyrus species kill the host queen after a successful invasion (Sladen 1912; van Honk et al. 1981a), while other species (e.g. Psithyrus sylvestris and B. pratorum) choose coexistence and suppress reproduction of the queen (Küpper and Schwamberger 1995; Dronnet et al. 2005). In both cases, the colony scent composition changes, and workers are subjected to an observed behaviour of being physically pressed down and touched all over the back as well as on the sides (so-called mauling, primarily claimed to be observed in rather primitive species with smaller colony sizes) (Free et al. 1969).
In bumblebees at a certain stage of the colony development, the so-called competition point, the queen’s dominance diminishes, and dominant workers start to reproduce and lay eggs (Duchateau and Velthuis 1988; Küpper 1996). It is, thus, essential for the reproductive success of the Psithyrus female to control these workers as long as possible, which becomes increasingly difficult for larger numbers. On the other hand, more workers supply more Psithyrus offspring with nutrition. Therefore, upon and after her invasion the parasite female tries to kill as few workers as possible and should select the ones with the highest likelihood of trying to reproduce thus posing an opposition and a threat to her own success as such workers eat all non-self-laid eggs (Fisher 1987a, b). Individual odour could reveal to the parasite which dominant workers pose the greatest threat to her own reproductive success. In B. terrestris, there are fertility signals in egg-laying females (Sramkova et al. 2008) that could be used by the parasite female to identify dominant egg-laying workers.
The aim of our study was to investigate how colony size influences survival rates of B. terrestris workers and Psithyrus vestalis social parasite females during nest invasion. Furthermore, we investigated whether the host worker’s dominance status and its age are reflected in its individual scent and whether nest selection by Psithyrus and individual killing criteria could be based on olfactory recognition. Such a correlation between age and scent production has already been found in other social insects, such as Lasioglossum malachurum, where female sex pheromone production (consisting of n-alkanes, n-alkenes, and isopentenyl esters of unsaturated fatty acids) significantly decreases with age and after mating, and males clearly determine the female attractiveness based on the produced compounds (Ayasse et al. 1993, 1995, 1999). Furthermore, in honey bees the “blank slate” hypothesis states that newly emerged individuals do not release volatiles at all and easily adopt the odour of a surrounding colony (Breed et al. 2004). In the following we will use the term Psithyrus for the parasites and Bombus for the hosts to more clearly distinguish hosts and parasites.
The following questions are addressed:
Is the survival rate of nest entering Psithyrus females correlated to the size of the invaded colony?
Is there an age-dependent change of the chemical signature in B. terrestris workers?
Does the chemical signature of the Bombus host worker change as a result of a parasite invasion?
What factors determine the survival of workers in a parasitized colony?
Materials and methods
Rearing bumblebees
In February/March 2002 and 2004, nest searching B. terrestris queens were collected at various locations in the surroundings of Bonn, Germany, and used for founding B. terrestris colonies in the laboratory. Psithyrus vestalis females were collected 4-6 weeks later in the same region. Each single female was transferred into a wooden nest box and reared in a dark room at 26–28°C and 70% humidity. Each nest box was connected by a plastic tube to a second identical wooden food box where the females were provided ad libitum with a 50% sugar solution of API-Invert® (72,7%; Südzucker AG, Germany; 1 g citric acid and 3 g potassium sorbate were added per litre). Fresh pollen obtained from Koppert Biological Systems (The Netherlands) was placed directly onto the comb of the nest. Daily observations of the colonies were made under red light. The initiation of the competition point was registered by observing the behaviour of egg-laying workers.
Sample collection
We marked all workers individually according to their age with numbered plastic tags glued to their dorsal thorax (‘Opalithplättchen”, Christian Graze, Weinstatt-Endersbach, Germany). Headspace samples of single workers (method described in Ayasse et al. 1995) were collected from all workers of four different colonies of B. terrestris. All headspace samples were obtained by placing individual females in pre-cleaned 20 ml glass vials for 30 min in a dark room at room temperature. After removal of the females, the vials were closed with a screw cap and stored at −20°C for 40 min in the freezer. The inner surface of the vials was rinsed immediately afterwards with 1.9 ml of pentane (Merck, Uvasol) in order to collect condensed substances emitted by the females. The samples were stored in the freezer until analysis. We collected headspace samples of newly emerged workers, 1-, 2-, and 3–4 days old workers from queenright colonies that were not invested by Psithyrus females.
Shortly afterwards, single Psithyrus vestalis females were put into the food boxes connected to the four host colonies so that a parasite female could slowly adapt to the potential host nest before entering it. Usually she entered the host nest within a few hours and killed several of the attacking host workers. In two of the four cases, the Psithyrus female was not successful in taking over the colony. These two colonies were excluded from further analyses. One day after the invasion, headspace samples were collected from the surviving workers of the two successfully invaded colonies. Workers prior to invasion could thus be divided after nest invasion by the Psithyrus female into survivors and culled workers killed by the parasite.
Intrusion bioassays
Bioassays on nest invasion success were conducted with colonies containing five (n = 9), twenty (n = 9), and fifty workers (n = 4). In rare cases, one or two callow workers were taken out of a colony to adjust the colony size to the exact number of workers. The parasitic female was introduced into a colony’s food box and could decide freely if and when to enter the potential host colony through the plastic tube. Only Psithyrus females that entered the colony were included in the sample. The invasion was considered a success when the parasite built two egg cells and laid eggs within them.
Chemical analysis
Before the chemical analyses, the samples were concentrated to 50 μl in a water bath and 1 μg n-undecane was added as an internal standard to each sample. For chemical analysis 1 μl of the sample was injected splitless into a HP 5890 Series II gas chromatograph equipped with a DB-5 capillary column (30 m × 0.25 mm i.d. J&W Scientific, Folsom, CA, USA) and a flame ionization detector (FID), using hydrogen as the carrier gas (constant flow, 2.0 ml/min), operating at 50°C for 1 min, after which the split valve was opened and the temperature increased by 10°C/min up to 310°C. Structure elucidation of individual compounds was performed with a HP 6890 gas chromatograph (Hewlett Packard, Series, Palo Alto, CA) connected to a mass selective detector (GC/MS, Agilent Quadrupol 5972). The temperature program was the same as described above. Helium was used as the carrier gas (1.5 ml/min constant flow). Based on our previous work (Sramkova et al. 2008), structure assignments were carried out by comparison of mass spectra and retention times of natural products with corresponding data of synthetic reference samples, with the NIST database, and a database of the Institute of Experimental Ecology. Peak identities between different runs were confirmed by GC/MS.
Statistical analysis
Excluding peaks below 0.5% of the total bouquet and compounds not separable on the DB5 column, relative amounts of 37 compounds were used for a principal components analysis (PCA) followed by discriminant function analyses (DFA) (Backhaus et al. 1987; Norusis 1993a, b) using the SPSS 13.0 statistical system. All DFAs were performed with 10 principal components (PCs) with an Eigenvalue >1. The standardized discriminant function coefficients and the component loadings were used to assess the importance of individual compounds. A compound was considered to have a high component loading when the loading was above 0.5. The total amounts of compounds of different worker groups were compared with Mann–Whitney U tests and a Benjamini and Hochberg correction (Benjamini and Hochberg 1995). A Pearson correlation analysis was performed with the total amounts of scent in worker groups of different age.
Results
Survival rate of nest-entering Psithyrus females and host workers
The size of the host colony and thus the number of attacking workers largely determined the survival rate of the intruding Psithyrus females. In host colonies consisting of only five workers, 100% of the parasitic females successfully invaded the host nest. Two out of three Psithyrus females successfully invaded colonies of around 20 workers. All parasite females were killed while entering colonies with over 50 workers (Fig. 1).
Furthermore, our investigation showed that a Psithyrus vestalis female is clearly able to recognize the age and physiological status of a host worker. Nearly no individuals born after the invasion of the colony were killed by the parasite, whereas among the workers born before introduction the percentage of killed workers increased with age (Fig. 2). About 50% of the workers killed by the parasite female were more than 2-days old.
Chemical analyses
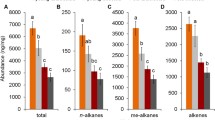
Chemical analyses were used to determine whether the age of a worker is expressed by the amount or composition of odour substances. In total, 37 substances contributed at least 0.5% of the total scent production. Twenty three of these 37 compounds could be identified by GC/MS analyses or coinjection (Fig. 3). We found saturated and unsaturated hydrocarbons with a chain length between C21 and C31, octacosenal, triacontenal, and 3 unsaturated wax-type esters which are already described in the literature (Tengö et al. 1991; Hefetz et al. 1996; Ayasse et al. 1999; Sramkova et al. 2008).
Quantitative differences—absolute amounts In queenright unparasitized host colonies the mean total amount of secretions increased in workers in the first 4 days after emergence (Fig. 4, newly emerged: 6.85 ± 1.23 SE, N = 9; 1 day old: 11.33 ± 4.1 SE, N = 6; 2 days old: 25.17 ± 6.0 SE, N = 12; three or more days old: 38.79 ± 4.84 SE, N = 15). Pair wise U tests revealed that the daily increases are not significant. However, a Pearson correlation analysis (P = 0.012) shows that age and scent production are significantly and positively correlated; the newly emerged workers produced significantly less scent than workers that were 4 days old (Fig. 4).
However, the mean total amounts of scent secreted by workers killed during the invasion (22.69 ± 3.24 SE, N = 11) differed significantly from that of the survivors (8.56 ± 1.39 SE, N = 11) (Fig. 4). Surviving workers, on average two to 3 days old showed a highly significant increase in the total amount of scent within the first day after the intrusion of the parasitic females (U test, P < 0.05, Fig. 4). The total amount of secretions they produced (45.16 ± 6.18 SE, N = 15) was significantly greater than the total amount of odour the killed workers produced, though those were even older when they died.
Qualitative differences—relative amounts a canonical DFA was performed with ten PCs with an Eigenvalue > 1 explaining 81.95% of the total variance to test for differences in odour bouquets between colonies. There was a significant colony specific difference (DFA: discriminant function 1: χ2 = 59.81, df = 10, P = 0.00017). The standardized discriminant function coefficients and the components loadings in a PCA revealed that 6 unsaturated hydrocarbons, (Z)-12-nonacosene, (Z)-11-nonacosene, 3 hentriacontene isomers, hentriacontadiene, 2 alkanes, hentriacontane, heneicosane, and 2 so far unidentified compounds are most important for the discrimination of workers from different colonies.
Therefore, in a further step both colonies were regarded separately. Because of a small number of surviving workers we were only able to perform a further DFA with one of the two colonies. We found significant differences between worker groups within that colony (DFA: discriminant function 1: χ2 = 37.689, df = 20, P < 0.01; discriminant function 2: χ2 = 12.622, df = 9, P = 0.18). Workers that were killed by the Psithyrus within 1 day of nest invasion females contained odour bouquets significantly different from those of survivors that altered the composition of volatiles after nest invasion (Fig. 5). The standardized discriminant function coefficients and the components loadings in a PCA showed that worker groups were mainly separated by 9 unsaturated hydrocarbons, 4 hentriacontene isomers, 2 hentriacontadiene isomers, (Z)-12-nonacosene, (Z)-11-nonacosene, and tricosene, 2 alkanes, heneicosane and hentriacontane, octacosenal, and 6 not yet identified compounds.
Comparison of the odour bouquets of headspace samples of B. terrestris workers grouped by survival (group 1: culled workers; group 2: survivors) of the Psithyrus vestalis invasion and by the time interval passed thereafter. A PCA was performed with the relative proportions of 37 compounds. We used ten principle components, explaining 81.95% of total variance to perform a DFA. All investigated groups of females differed significantly
Discussion
Survival of parasite females and host workers
The timing of entry into a host nest is quite crucial for a Psithyrus female (Sladen 1912). If the host colony is in an advanced developmental stage and contains a large number of older workers the parasite female may be successfully repulsed or killed by the defenders (summarized in Benton 2006). However, if the host colony is invaded too early, only a few host workers are available to rear the brood of the parasitic bee. Our results clearly show that the survival rate of a Psithyrus female is dependent on the size of the colony: while in small colonies consisting of five workers all parasite females survived, 30% were killed in colonies of 20 workers, and all Psithyrus females were killed in colonies having 50 workers, which matches intrusion success rates observed in several combinations of hosts and parasites, such as B. affinis/B. terricola (Fisher 1987b) and Psithyrus ashtoni/B. affinis (Fisher 1984b).
Other investigations additionally showed that the survival rate of parasite females may vary from species to species and depend from various circumstances (Sladen 1912; Fisher 1984b, 1987a, b), notably, whether the second hatch of workers emerged (Benton 2006), and on the level of energy reserves to be defended (Cartar and Dill 1991). Therefore, a good strategy for a Psithyrus vestalis female should be to select colonies with about 10–15 workers depending on the circumstances.
We found a strong negative correlation between the survival rate of the host workers and their age. A parasite female should preferably kill the older workers that directly compete with her for reproduction, since according to other studies older workers are more likely to possess developed ovaries and are among the first egg-layers (Van Honk et al. 1981b; Duchateau 1989), while younger workers born more than 3 days after the competition point have no chance of reproducing (Van Doorn and Heringa 1986). After being accepted into a host colony the Psithyrus female has to control worker reproduction in order to maximize her reproductive success. In a former study, Frehn and Schwammberger (2001) showed that P. vestalis females are only able to control reproduction in small host colonies consisting of mostly young workers. Furthermore, the parasite females were able to recognize the physiological state of Bombus workers and had more frequent contact with the egg-laying workers than with the non-egg-laying workers.
Do Psithyrus females use olfactory recognition signals to select their victims?
In social insects, age-dependent changes in cuticular hydrocarbons and lipids have been reported in several studies (Ayasse et al. 1995; Dahbi et al. 1998; Breed et al. 2004; Sramkova et al. 2008). In bumblebees a positive correlation between worker age, dominance status, and fertility was shown in former investigations (Van Honk et al. 1981b; Duchateau 1989). Queens and dominant workers seem to recognize and were found to be more aggressive toward workers with developed ovaries (Van Doorn and Heringa 1986; Duchateau 1989; Röseler et al. 1990).
Olfactory recognition signals have been frequently identified in social insects (Monin 2006) and amongst them also dominance-group specific odour signals providing information about the reproductive state of the emitter in B. hypnorum (Ayasse et al. 1995) and B. terrestris, which is thought to act as a fertility signal providing information about the reproductive state of females to other individuals within the colony (Sramkova et al. 2008). Our results indicate that nest invading Psithyrus females may use these volatile signals to differentiate between potential rivals for reproduction and harmless, helpful workers.
In our investigations freshly emerged individuals showed very low amounts of volatiles, and the total amount of which increased within the next 2 days. Therefore, we conclude that the blank slate hypothesis proved for honeybees (Breed et al. 2004) also holds for B. terrestris. Newly emerged adult workers first present a blank slate, absent recognition cues. After a couple of days they either acquire a colony recognition phenotype through social interactions with other workers and an exchange of volatiles as in several ant species (Soroker et al. 1994) or they increase biosynthesis and produce it by themselves.
Besides the differences in total scent production we also demonstrated that culled workers and survivors differed in their odour bouquets. Noticeably, among the compounds mainly responsible for discrimination of odour bouquets we identified saturated and unsaturated hydrocarbons. There is good evidence from wasps, bees, and ants that cuticular hydrocarbons signal an individual’s reproductive activity (Monin 2006). In many species a strong correlation has been found between the reproductive status of an individual and its profile in cuticular hydrocarbons. Therefore, hydrocarbons may be used by nest invading Psithyrus females to recognize potential rivals for reproduction amongst the host workers, which are then killed by the parasite.
Scent variation after invasion of parasite
The increase in the total amount of substances we observed in surviving workers 1 day after a parasite invasion could have several reasons. First, the workers could try to win an arms’ race for dominance against their new oppressor or against fellow workers as observed earlier in B. terrestris (Alaux et al. 2004). Observations in other species of social insects indicate that reproductive workers that are on the way to develop ovaries alter their odour profile distinguishing them from subordinates. In the ponerine ant Harpegnathos saltator, very few mated workers start to lay eggs once the queen becomes senescent, and these workers are characterized by the production of 13,23-dimethylheptatriacontane, a compound that is not present in infertile workers. Furthermore they start to produce cuticular hydrocarbons with an elongated chain length (Liebig et al. 2000).
Secondly, the variation could be the result of scent impregnation of the workers by mauling (Fisher 1988) or head rubbing behaviour as was shown in females of Psithyrus citrinus parasitizing nests of B. impatiens and B. vagans (Fisher 1984a). After a Psithyrus female enters a host colony she is usually attacked by host workers because she is recognized as an intruder. In order to overcome that problem she may acquire the host scent performing chemical camouflage (Sramkova et al., in preparation). The function of the mauling behaviour, during which the Psithyrus female rubs her head on the surface of the workers, is not yet clear. Fisher (1983, 1985) suggested that chemicals are transferred by the Psityhrus female with a function to dominate host workers as stated elsewhere (Benton 2006). Mauling behaviour is not shown by all species of social parasitic bumblebees, but it can be found in P. vestalis (Van Honk et al. 1981a; Frehn and Schwammberger 2001; Fisher 1984b, 1987b vs. Küpper 1996).
A further function of the mauling behaviour could be to mask the colony odour and to achieve corporate identity (Bunk et al. in preparation) instead of only acquiring host odour. Direct physical contact would obviously be the most efficient means of transfer in small colonies, where every worker can be treated in sufficiently short intervals. Correspondingly, mauling is claimed to take place predominantly in rather primitive species of social insects with smaller colony sizes (Fletcher and Ross 1985). The result would be an increase of the total amount of scent in the host workers. All explanations seem likely, and future investigations have to show if workers actively increase the total amount of volatiles or if the Psithyrus female accounts for this phenomenon.
Colony-specific odour cues
In social insects colony-specific recognition cues do have a function in nest- and nest mate recognition (Hölldobler and Michener 1980; Breed 1998; Breed et al. 2003; Dani et al. 2005). Recognition of nestmates is important to maintain co-operation among colony members and to repel non-nestmates, parasites, and robbers. In congurence with other investigations our results indicate that long chain alkanes and alkenes may have a function in nestmate recognition (Tengö et al. 1992; Sick 1993; Sick et al. 1994). Interestingly, in former investigations the same compounds were found on the surface of eggs laid by queens of B. terrestris (Ayasse et al. 1999) and were shown to be responsible for colony-specific differences between eggs.
Conclusions
Our investigation shows that nest invading Psithyrus females have to assess the size of a host colony before they dare to enter it. Inside the host colony while being attacked by the host workers they selectively kill older workers which might compete for reproduction. They obviously recognize dominant workers by the scent of their chemical fertility signal (Sramkova et al. 2008) and kill them to maximize their own reproductive potential. Further studies on dominance behaviour and the chemical composition of cuticular lipids of parasites and hosts—including the use labelled compounds—could reveal whether the increase of scent in workers after a Psithyrus has entered a nest is due to elevated scent production of the workers or caused by transfer of organic compounds by the Psithyrus female.
References
Alaux C, Savarit F, Jaisson P, Hefetz A (2004) Does the queen win it all? Queen–worker conflict over male production in the bumblebee, Bombus terrestris. Naturwissenschaften 91(8):400–403 (4)
Ayasse M, Engels W, Hefetz A, Tengö J, Lübke G, Francke W (1993) Ontogenetic patterns of volatiles identified in Dufour’s gland extracts from queens and workers of the primitively eusocial halictine bee, Lasioglossum malachurum (Hymenoptera: Halictidae). Insect Soc 40:41–58
Ayasse M, Marlovits TC, Tengö J, Taghizadeh T, Francke W (1995) Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L (Hymenoptera, Apidae). Apidologie 26(1):163–180
Ayasse M, Engels W, Lübke G, Francke W (1999) Mating expenditures reduced via female sex pheromone modulation in the primitively eusocial halictine bee, Lasioglossum (Evylaeus) malachurum (Hymenoptera: Haltictidae). Behav Ecol Sociobiol 45:95–106
Backhaus K, Erichson B, Flinke W, Schuchard-Ficher C, Weiber R (1987) Multivariate Analysemethoden. Springer, New York
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57(1):289–300
Benton T (2006) Bumblebees. Collins, London
Breed MD (1998) Recognition pheromones of the honey bee. Bioscience 48(6):463–470
Breed M, Diaz P, Lucero K (2003) Olfactory information processing in honeybee, Apis mellifera, nestmate recognition. Anim Behav 68:921–928
Breed MD, Perry S, Bjostad LB (2004) Testing the blank slate hypothesis: why honey bee colonies accept young bees. Insect Soc 51:12–16
Cartar RV, Dill LM (1991) Costs of energy shortfall for bumble bee colonies: predation, social parasitism, and brood development. Can Entomol 123(2):283–293
Cederberg B (1983) The role of trail pheromones in host selection by Psithyrus rupestris (Hymenoptera, Apidae). Ann Entomol Fenn 49:11–16
Dahbi A, Cerda X, Lenoir A (1998) Ontogeny of colonial hydrocarbon label in callow workers of the ant Cataglyphis iberica. C R Acad Sci Paris 321:395–402
Dani F, Jones G, Corsi S, Beard R, Pradella D, Turilazzi S (2005) Nestmate recogniton cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem Sens 30:1–13
Dronnet S, Simon X, Verhaeghe JC, Rasmont P, Errard C (2005) Bumblebee inquinilism in Bombus (FernaldaePsithyrus) sylvestris (Hymenoptera, Apidae): behavioural and chemical analyses of host–parasite interactions. Apidologie 36:59–70
Duchateau MJ (1989) Agonistic behaviours in colonies of the bumblebee Bombus terrestris. Dissertation, University of Utrecht, 77–98
Duchateau MJ, Velthuis HHW (1988) Development and reproductive strategies in the bumble bee, Bombus terrestris. Behaviour 107:186–207
Fisher RM (1983) Recognition of host nest odour by the bumble bee social parasite Psithyrus ashtoni (Hymenoptera: Apidae). J N Y Entomol Soc 91:503–507
Fisher RM (1984a) Dominance by a bumble bee social parasite (Psithyrus citrinus) over workers of its host (Bombus impatiens). Anim Behav 32(1):304–305
Fisher RM (1984b) Evolution and host specificity: a study of the invasion success of a specialized bumblebee social parasite. Can J Zool 62:1641–1644
Fisher RM (1985) Evolution and host specificity: dichotomous invasion success of Psithyrus citrinus (Hymenoptera: Apidae), a bumblebee social parasite in colonies of its two hosts. Can J Zool 63:977–981
Fisher RM (1987a) Queen-worker conflict and social parasitism in bumble bees (Hymenoptera: Apidae). Anim Behav 35:1026–1036
Fisher RM (1987b) Temporal dynamics of facultative social parasitism in bumble bees (Hymenoptera: Apidae). Anim Behav 35:1628–1636
Fisher RM (1988) Observations on the behaviours of three European cuckoo bumble bee species. Insectes Soc 35(4):341–354
Fisher RM, Sampson BJ (1992) Morphological specializations of the bumble bee social parasite Psithyrus ashtoni (Cressson) (Hymenoptera: Apidae). Can Entomol 124:69–77
Fisher RM, Greenwood DR, Shaw GJ (1993) Host recognition and the study of a chemical basis for attraction by cuckoo bumble bees (Hymenoptera: Apidae). J Chem Ecol 19(4):771–786
Fletcher DJC, Ross KG (1985) Regulation of Reproduction in Eusocial Hymenoptera. Annu Rev Entomol 30:319–343
Free JB, Weinberg I, White A (1969) The egg-eating behaviour of Bombus lapidarius. Behaviour 35(1969):313–317
Frehn E, Schwammberger KH (2001) Social parasitism of Psithyrus vestalis in free-foraging colonies of Bombus terrestris (Hymenoptera: Apidae). Entomol Gener 245:103–105
Hefetz A, Taghizadeh T, Francke W (1996) The exocrinology of the queen bumble bee Bombus terrestris (Hymenoptera: Apidae, Bombini). Z Naturforsch 51c:409–422
Hölldobler B, Michener CD (1980) Mechanisms of identification and discrimination in social hymenoptera. In: Markl H (ed) Evolution of social behaviour hypotheses and empirical tests. Verlag Chemie GmbH, Weinheim, pp 35–58
Kirchner WH, Röschard J (1999) Hissing in bumblebees: an interspecific defense signal. Insectes soc 46:239–243
Küpper G (1996) Psithyrus sylvestris (Lep.) in Völkern von Bombus pratorum (L.) (Hymenoptera: Apidae). Dissertation, Universität Bochum
Küpper G, Schwammberger KH (1995) Social parasitism in bumble bees (Hymenoptera, Apidae): observations of Psithyrus sylvestris in Bombus pratorum nests. Apidologie 26:245–254
Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B (2000) Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? PNAS 97(8):4124–4131
Monin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fenn 43:515–530
Norusis MJ (1993a) SPSS for Windows: base system user’s guide, release 6.0. SPSS, Chicago
Norusis MJ (1993b) SPSS for Windows: professional statistics, release 6.0. SPSS, Chicago
Pouvreau A (1973) Les enemies des bourdons. I. Etude d’une zooce¨ nose: le nid des bourdons. Apidologie 4:103–148
Röseler PF, van Honk CGJ et al (1990) Castes and reproduction in bumblebees. In: Engels W (ed) Social Insects, an evolutionary approach to castes and reproduction. Springer, Berlin, pp 147–166
Sick M (1993) Auffinden und olfaktorisches Erkennen von Wirtsnestern durch Kuckucksbienen (Gattung Sphecodes: Halictidae) und deren verwandtschaftliche Beziehungen zu den Wirtsbienen. Dissertation, Eberhard – Karl Universität Tübingen
Sick M, Ayasse M, Tengö J, Engels W, Lübke G, Francke W (1994) Host–parasite relationships in six species of Sphecodes bees and their halictid hosts: nest intrusion, intranidal behaviour and Dufour‘s gland volatiles (Hymenoptera: Halictidae). J Insect Behav 7(1):101–117
Sladen FWL (1912) The humblebee—its life-history and how to domesticate it. Mac Millan, London
Soroker V, Vienne C, Hefetz A, Nowbahari E (1994) The postpharyngeal gland as a “gestalt” organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81:510–513
Sramkova A, Schulz C, Twele R, Francke W, Ayasse M (2008) Fertility signals in the bumblebee Bombus terrestris (Hymenoptera: Apidae). Naturwissenschaften 95(6):515–522
Tengö J, Hefetz A, Bertsch A, Schmitt U, Lübke G, Francke W (1991) Species specificity and complexity of Dufour’s gland secretion of bumble bees. Comp Biochem Physiol 99B:641–646
Tengö J, Sick M, Ayasse M, Engels W, Svensson B, Lübke G, Francke W (1992) Species specificity of Dofour’s Gland Morphology and Volatile Secretions in Kleptoparasitic Sphecodes Bees (Hymenoptera: Halictidae). Biochem Syst Ecol 20(4):351–362
Van Doorn A, Heringa J (1986) The ontogeny of a dominance hierarchy in colonies of the bumblebee Bombus terrestris. Insectes Soc 33:3–25
Van Honk CG, Röseler PF, Velthuis HHW, Malotaux ME (1981a) The conquest of a Bombus terrestris colony by a Psithyrus vestalis female. Apidologie 12:57–67
Van Honk CG, Velthuis HHW, Röseler PF, Malotaux ME (1981b) The mandibular glands of Bombus terrestris queens as a source of queen pheromones. Ent Exp Appl 28:191–198
Wcislo WT (1986) Host nest discrimination by a cleptoparasitic fly, Metopia campestris (Fallén) (Diptera: Sarcophagidae: Miltogramminae). J Kans Entomol Soc 59:82–88
Zimma BO, Ayasse M, Tengö J, Ibarra F, Schulz C, Francke W (2003) Do social parasitic bumblebees use chemical weapons? (Hymenoptera: Apidae). Comp Physiol A 189:769–775
Zimma B, Ayasse M, Tengö J, Ibarra F, Francke W (2004) The role of semiochemicals in the reproductive biology of parasitic bumblebees. Mitt Dtsch Ges Allg Angew Entomol 14:195–198
Acknowledgments
We wish to thank Dr. Stefan Jarau for his valuable feedback in proofreading of the manuscript. Dr. Robert Hodgkison helped to revise the English. Anna Sramkova would like to thank the Friedrich Naumann Stiftung for financial support. We thank the German Research Foundation (DFG) for financial support (AY 12/2-1). All experiments comply with the current laws of the country in which they were performed: in this case, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sramkova, A., Ayasse, M. Chemical ecology involved in invasion success of the cuckoo bumblebee Psithyrus vestalis and in survival of workers of its host Bombus terrestris . Chemoecology 19, 55–62 (2009). https://doi.org/10.1007/s00049-009-0009-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-009-0009-7