Abstract
Phenolic constituents are the principle bioactive compounds exist in Heavenly Blue (Ipomoea). Little was found in the literature concerning the previous phytochemical and biological studies of Ipomoea tricolor. From leaves of this plant, six compounds were identified: rhoifoloside (1), luteolin-7-O-β-D-glucoside (2), 5,7,4′-trihydroxy-6-methoxyflavone-7-O-β- d -glucoside (3), apigenin (4), 5,7-dihydroxy-3,3′,4′-trimethoxyflavone (5), and 2-hydroxymethylhydroquinone-6-carbaldehyde (6). Their structures were elucidated on the basis of chromatographic, chemical, and spectroscopic methods. All metabolites were reported for the first time in the genus Ipomoea. In vitro and in vivo investigations of the flavones 1, 3, and 5 were assessed as modulators of Alzheimer’s amyloid-beta peptide (Aβ) production. The results indicated that all the three flavones were able to modulate the Aβ concentration both in vitro and in vivo without any cytotoxic effect. A dose-dependent inhibition of Aβ42 secretion was observed. The results showed no inhibition activity of these flavones against cyclooxygenase (COX)-1 and COX-2 up to 500 nM concentration and concomitant reduction in prostaglandin synthesis, indicating that the reduction in Aβ42 levels may be independent of COX activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds have been reported to inhibit the development of neurodegenerative diseases like Alzheimer disease (AD) (Williams and Spencer 2012). Different plant phenolics showed strong inhibition against prolyl endopeptidase, a serine protease, widely distributed in various organs, particularly in the brains of AD patients (Lee et al. 2007; Rizk et al. 2017). These phenolics including flavones have also the potential to prevent the progression of neurodegenerative pathologies and to promote cognitive performance.
Ipomoea ‘Heavenly Blue’ is a twining annual with heart-shaped leaves and funnel-shaped, sky-blue flowers. Several phenolic compounds, namely, phenolic acids, flavonoids, and organic acids have been described in several Ipomoea species (Meira et al. 2012). In addition, Ipomoea species are known for the presence of anthocyanins, are known potent antioxidants (Meira et al. 2012).
The in vitro immune stimulating activity of I. pes-caprae extract was reported in human mononuclear cells (Philippi et al. 2010). I. obscura was reported to ameliorate cyclophosphamide-induced toxicity by modulating the immune system and levels of proinflammatory cytokine and GSH (Hamsa and Kuttan 2010). Nitric oxide (NO) scavenging activity of I. digitata was reported and may regulate pathological conditions caused by excessive generation of NO and its oxidation product, peroxynitrite (ONOO−) (Meira et al. 2012). I. batatas, of 17 Korean native plants, exerted the highest protective effects against the oxidative stress induced by amyloid beta peptide (Aβ). The effectiveness was determined using the assays of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and 2′,7′-dichlorofluorescin diacetate (Kim et al. 2011). In biochemical studies, the level of lipid peroxidation was reduced by the administration of the plant extract and increased catalase activities using the brain tissue of mice. The scavenging ability of trypsin inhibitor, isolated from I. batatas, against NO and ONOO− was reported as well (Huang et al. 2007).
Aβ has been shown to induce the mitochondrial dysfunction, redox imbalance and caspase activation, which results in neuronal cell death. Treatment with antioxidants provided a new therapeutic strategy for AD patients. AD is the most common age-related neurodegenerative disorder characterized pathologically by senile plaque.
The anthocyanins of purple I. batatas are well known strong radical scavengers. Their effect on Aβ toxicity in PC12 cells was reported to reduce the intracellular reactive oxygen species generation, Aβ-induced toxicity and lipid peroxidation dose-dependency (Ye et al. 2010).
Phenolic compounds, such as scopoletin and taraxerol that were isolated from the root of I. digitala, inhibited acetylcholinesterase (AChE) activity. This enzyme is responsible for the metabolic hydrolysis of the neurotransmitter acetylcholine. Polyacylated anthocyanins in flower petals of Heavenly Blue can efficiently screen harmful UV-B induced DNA damage (Mori et al. 2005). This action might be largely due to aromatic acyl residues. Other bioactive metabolities as ergotamine, an ergoline alkaloid, isolated from the plant (Meira et al. 2012; Rosas-Ramírez et al. 1996) showed a vasoconstrictor activity and is useful in the treatment of migraine headaches (Meira et al. 2012).
In literature, little was found concerning the phytochemical and biological studies of Heavenly Blue plant. This, together with the reported importance of Ipomoea species activity (Meira et al. 2012; Mori et al. 2005; Rosas-Ramírez et al. 1996) influenced the authors to study the phenolic profile of Heavenly Blue (I. tricolor) leaves, alongside the in vitro and in vivo investigation of major flavones as modulators of Alzheimer’s Aβ production.
Materials and methods
Plant materials
Leaves of Heavenly Blue; Ipomoea tricolor Cav. (Family: Convolvulaceae) were collected in May 2014 from a private nursery for ornamental plants, El-Qanater, Qalyubia governorate. The identification of the plant was performed by Treas Labib, Herbarium Section, El-Orman Botanical Garden, Giza, Egypt. A voucher specimen is deposited in the herbarium of the National Research Centre, Egypt.
Chemicals and analytical instruments
The NMR spectra were recorded at 300, 400 (1H) and 75, 100 (13C) MHz, respectively, on a Varian Mercury 300 (Palo Alto, CA, USA) and JEOL GX-400 NMR spectrometers, using the DMSO-d 6 , CD3OD or CDCl3 deuterated solvents. The chemical shifts (δ) are reported in parts per million (p.p.m.) and coupling constants (J) in Hz. The UV-analyses of the pure samples were recorded, separately, as MeOH solutions and with different diagnostic UV shift reagents on a Shimadzu UV 240 (P/N 240–58000) instrument. Thin layer chromatography (TLC) was performed on pre-coated silica gel 60 F254 plates (0.2 mm, Merck). Column chromatography was carried out on silica gel Merck 60 (230–400 mesh), Sephadex LH-20 (Pharmacia, Uppsala, Sweden), microcrystalline cellulose (Merck, Darmstadt, Germany), and polyamide S (Fluka, Steinheim, Switzerland). For paper chromatography, Whatman No. 1 paper sheets (Whatman Ltd., Maidstone, England) were used. The spray reagents; R1: Naturstoff reagent, NA/PE (a) 1% diphenyl boryloxyethanolamine in ethanol, (b) 5% polyethylene glycol 400 in methanol, heating the dry chromatogram at 120 °C for 10 min and visualizing under UV light (365 nm) and R2: AlCl3 (1% in ethanol) were used to visualize the compounds. Solvent systems S1 [n-BuOH/HOAc/H2O (4:1:5, v/v/v, top layer)], S2 (15% aqueous HOAc), and CH2Cl2/MeOH (S3, 8:2 and S4, 9:1, v/v)] were used.
Phytochemical study
Extraction and isolation
The air-dried powdered leaves of Heavenly Blue (1.3 kg) were exhaustively extracted with 80% aqueous methanol (3 L, then 3 × 2 L) at room temperature by maceration method. The solvent was removed under reduced pressure to give 69.2 g. The extract was defatted with light petroleum ether (60–80 °C, 3 × 2 L) to afford a viscous brown residue (56.2 g). The defatted residue was dissolved in water, and the water-soluble part was desalted by precipitation with excess methanol to give a dry brown residue (33.7 g). This residue was suspended in deionized water and was subjected to a preliminary fractionation on a polyamide S column (C) using a step-gradient of water/methanol (100:0–0:100 v/v) for elution. The homogeneity of the fractions was tested by comparative paper chromatography (Co-PC), comparative thin layer chromatography (Co-TLC) with the use of suitable solvent systems (S1–S4). Accordingly, the fractions (53 fractions, 100 mL each) were collected into five major collective fractions (I–V). A dark material of fraction I (water, 8.3 g) was found to be of non-phenolic character. Fraction II (10–30% methanol, 4.2 g) was applied on silica gel sub-column with methylene chloride: methanol (3:1) as an eluent, followed by repeated column chromatography (CC) on Sephadex LH-20 with methanol (20%) for elution to afford pure compounds 1 (38 mg) and 2 (16 mg). Fraction III (35 –60% methanol, 5.2 g) was chromatographed on microcrystalline cellulose CC using n-butanol/iso-propanol/water, 4:1:5 v/v/v, organic layer) as an eluent, followed by repeated and separate CC on Sephadex LH-20 C eluted with methanol/water (70:30–100:0 v/v) to give compounds 3 (29 mg) and 4 (13 mg). Fraction IV (70% methanol, 1.9 g) was separated on a Sephadex C twice with methanol as an eluent resulting in compound 5 (25 mg). Fraction V (85% methanol, 0.9 g) was subjected to silica gel column eluted with n-hexane: dichloromethane (1:1) for elution to afford 6 (14 mg). Co-PC using Whatman grade no. 1 filter paper (systems S1 and S2) and co-TLC (systems S3 and S4) were used, with spraying by R1 and R2 spray reagents for visualizing and the detection of the compounds. Complete acid hydrolysis of major compounds 1 and 3 was carried out using 2-NHCl and reflux for 3 h. The reaction mixture was diluted with water and then extracted with ethyl acetate. The aglycones (in the organic phase) and the sugar moieties (in the aqueous phase) were identified by co-TLC and co-PC, respectively. Sugar samples were identified by co-PC with authentic standards in pyridine-EtOAc-AcOH-H2O; 36:36: 7:21, v/v/v/v) for elution and aniline phthalate (0.93 g aniline and 1.66 g phthalic acid dissolved in 100 mL n-butanol saturated with water, and heat to 105 °C) as spray reagent. The spectral data of isolated compounds were illustrated as the following:
Compound (1): yellow amorphous powder; R f: 0.32 (S1) and 0.55 (S2); UV spectral data: λ max, nm, (MeOH): 270, 334, (+NaOMe): 242sh, 272, 300, 390, (+NaOAc): 266, 350, 389sh, (+NaOAc/H3BO3): 269, 336, (+AlCl3): 274, 300, 348sh, 388, (+AlCl3/HCl): 275, 304, 347, 385; 1H NMR (300 MHz, DMSO-d 6): δ p.p.m. 7.90 (2 H, d, J = 8.7 Hz, H-2′/6′), 6.94 (2 H, d, J = 8.7 Hz, H-3′/5′), 6.76 (1 H, d, J = 2 Hz, H-8), 6.34 (1 H, d, J = 2 Hz, H-6), 5.20 (1 H, d, J = 7 Hz, glucose H-1"), 5.12 (1 H, brs, rhamnose H-1‴), 3.74-3.19 (m, rest of sugar protons), 1.16 (3 H, d, J = 6 Hz, CH3-6‴); 13C NMR (75 MHz, DMSO-d 6): δ p.p.m. 182.2 (C-4), 164.4 (C-2), 162.6 (C-7), 161.3 (C-5), 161.2 (C-4′), 157.0 (C-9), 128.6 (C-2′/6′), 121.2 (C-1′), 116.1 (C-3′/5′), 105.5 (C-10), 103.4 (C-3), 100.5 (C-1‴), 99.4 (C-1"), 98.9 (C-6), 94.6 (C-8), 77.3 (C-5"), 77.1 (C-2"), 76.4 (C-3"), 72.0 (C-4‴), 70.6 (C-2‴), 70.5 (C-3‴), 69.7 (C-4"), 68.4 (C-5‴), 60.5 (C-6"), 18.2 (C-6‴).
Compound (2): yellow amorphous powder; R f: 0.15 (S1) and 0.36 (S2); UV spectral data: λ max, nm, (MeOH): 257, 266, 356, (+NaOMe): 270, 406, (+NaOAc): 257, 266, 352, (+NaOAc+H3BO3): 260, 372, (+AlCl3): 274, 294sh, 324sh, 429, (+AlCl3/HCl): 272, 298sh, 356sh, 380; 1H NMR (400 MHz, CD3OD): δ p.p.m 8.47 (1 H, br s, OH-5), 7.89 (2 H, d, J = 8.1 Hz, H-2′/6′), 6.92 (2 H, d, J = 8.1 Hz, H-5′), 6.88 (1 H, d, J = 2.1 Hz, H-8), 6.80 (1 H, s, H-3), 6.46 (1 H, d, J = 2.0 Hz, H-6), 5.06 (1 H, d, J = 6.9 Hz, H-1"), 3.10-3.52 (m, glucosyl protons); 13C NMR (100 MHz, CD3OD): δ p.p.m. 182.0 (C-4), 164.8 (C-2), 163.6 (C-7), 161.0 (C-5), 156.8 (C-4′), 150.2 (C-9), 146.1 (C-3′), 121.4 (C-6′), 119.1 (C-1′), 116.4 (C-5′), 114.0 (C-2′), 105.2 (C-10), 99.8 (C-1"), 99.7 (C-6), 94.6 (C-8), 77.0 (C-5"), 76.2 (C-3"), 73.1 (C-2"), 69.8 (C-4"), 60.9 (C-6").
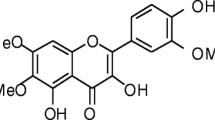
Compound (3): yellow amorphous powder; R f: 0.49 (S1) and 0.32 (S2); UV spectral data: λ max, nm, (MeOH): 222, 274, 335; (+NaOMe): 233, 270, 350, 390; (+NaOAc): 232, 269, 334, 396sh; (+NaOAc/H3BO3): 232, 269, 334; (+AlCl3): 230sh, 282, 302sh, 364; (+AlCl3/HCl): 230sh, 282, 304sh, 354. 1H NMR (300 MHz, DMSO-d 6 ): δ p.p.m. 8.01 (2 H, d, J = 8.4 Hz, H-2′/6′), 7.03 (2 H, d, J = 8.4 Hz, H-3′/5′), 6.82 (1 H, s, H-8), 5.36 (1 H, d, J = 7 Hz, H-1"), 3.78 (3 H, s, OCH3-6), 3.9–3.2 (6 H, m, glucosyl protons); 13C NMR (75 MHz, DMSO-d 6 ) δ p.p.m. 182.6 (C-4), 164.8 (C-2), 162.2 (C-4′), 157.0 (C-7/9), 152.6 (C-5), 133.0 (C-6), 129.4 (C-2′/6′), 121.8 (C-1′), 116.6 (C-3′/5′), 106.1 (C-10), 103.0 (C-3), 101.4 (C-1"), 94.6 (C-8), 78.1 (C-5"), 77.2 (C-3"), 73.6 (C-2"), 70.0 (C-4"), 61.9 (C-6"), 61.4 (OCH3).
Compound (4): yellow amorphous powder; R f: 0.83 (S1) and 0.14 (S2); UV spectral data: λ max, nm, (MeOH): 268, 335, (+NaOMe): 277, 332, 382, (+AlCl3): 276, 300, 346, 381, (+AlCl3/HCl): 275, 299, 340, 382, (+NaOAc): 274, 301, 340, 376, (+NaOAc/H3BO3): 267, 301sh, 337; 1H NMR (400 MHz, DMSO-d 6 ): δ p.p.m. 7.92 (2 H, d, J = 8.4 Hz, H-2′/6′), 6.92 (2 H, d, J = 8.4 Hz, H-3′/5′), 6.72 (1 H, s, H-3), 6.50 (1 H, br s, H-8), 6.22 (1 H, br s, H-6).
Compound (5): yellow amorphous powder; R f: 0.89 (S1) and 0.17 (S2); UV spectral data: λ max, nm, (MeOH): 250, 269, 254; (+NaOMe): 278, 311, 282, (+AlCl3): 273, 277, 300, 355, 399, (+AlCl3/HCl): 264, 276, 302sh, 350, 397, (+NaOAc): 277, 312, 394, (+NaOAc/H3BO3): 256, 270, 353; EI/MS m/z (%): 344 (M+, 100), 329 (M+-15, 22.3), 301 (M- 3xCH2, 51.62), 258 (4.78), 167 (18.90); 1H NMR (300 MHz, CDCl3): δ p.p.m. 12.67 (1 H, s, OH-5), 7.78 (1 H, d, J = 2.0 Hz, H-2′), 7.66 (H, dd, J = 8.4, 2.2 Hz, H-6′), 7.12 (1 H, d, J = 8.4 Hz, H-5′), 6.47 (1 H, d, J = 2.2 Hz, H-8), 6.40 (1 H, d, J = 2.2 Hz, H-6), 3.96, 3.86, 3.84 (3 H, s, OCH3-3,3′,4′).
Compound (6): off-white amorphous powder; R f: 0.91 (S3) and 0.77 (S4); EI/MS m/z (%) 167 ([M-H]+, 67.45), 149 ([M-H2O]+, 36.40), 126 ([M—CH2CO], 16.88); 1H NMR (400 MHz, CDCl3): δ p.p.m. 9.58 (2 H, s, H-7), 7.19 (1 H, d, J = 2.1, H-5), 6.24 (1 H, d, J = 2.1, H-3), 4.74 (1 H, s, H-8); 13C NMR (100 MHz, CDCl3): δ p.p.m. 197.8 (CHO), 160.2 (C-1), 152.8 (C-4), 149.2 (C-2), 130.4 (C-6), 122.1 (C-5), 110.4 (C-3), 58.1 (C-8).
In vitro Alzheimer’s Aβ production modulation
Chemicals
All solvents and chemicals which have been used in this study were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Culture of H4 cells expressing the double Swedish mutation (K595N/M596L) of human APP (APPsw) and exposure to the flavones. H4 cells were cultured in DMEM (high glucose) containing 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine and 200 μg/mL G418. The cells were cultured onto 24 well plates (2 × 105cell per well) and allowed to grow to 80% confluence for 24 h, in 5% CO2/95% air in humidified atmosphere. Different concentrations (0–300 nM/mL) of each purified flavone were added to the cells overnight in a final volume of 0.5 mL. R-flurbiprofen was used as positive control (3–1000 μM). DMSO (0.5%) was used as negative control. At the end of the incubation, 100 μL of each supernatant were used for measuring Aβ (Hawas et al. 2013).
Measurement of Aβ peptides
Each supernatant was treated with a biotinylated mouse monoclonal antibody (4G8, Signet Laboratories Inc., Dedham, MA, USA), specifically recognizing the 17–24 amino acid region of Aβ and two rabbit polyclonal antibodies (C-term 42 and C-term 40, BioSource International, Camarillo, CA, USA), specifically recognizing the C-terminus of Aβ42 and Aβ40, respectively and the Aβ levels in cell supernatants were evaluated in comparison to an Aβ peptide standard. The total Aβ content was calculated as sum of Aβ 40 and Aβ 42 (Hawas et al. 2013).
Inhibition of cyclooxygenase (COX)-1 and COX-2 assay
The inhibition of the cyclooxygenase activity was estimated by measuring prostaglandin E2 (PGE2) production form arachidonic acid using an enzyme-immunoassay as described previously (Hawas et al. 2013).
In vivo Alzheimer’s Aβ production modulation
Young male transgenic mice (Tg2576) expressing the human APP gene with the Swedish double mutation (K670N/M671L) under the transcriptional control of the hamster prion protein promoter (Hsiao et al. 1996) were used for the in vivo studies.
Ethics
The experiment and handling with animals complied with the ethical guidelines of the ethical committee of EEC for the use of laboratory animals. It was approved by the Medical Ethical Committee of the National Research Centre in Egypt (Approval No.: 12 096).
Animal treatment
Three groups of male mice 5–6 months of age, each group composed of six male mice were given by oral gavage vehicle or a solution of each flavone (100 nM per kg per day) or R-flurbiprofen as positive control, once daily for 7 days. On day 7, animals were killed as described previously (Weggen et al. 2001; Glaser et al. 1995; Berechid et al. 2002).
Plasma and brain Aβ separation
In order to measure the baseline plasma and the Aβ42 concentration, one blood sample was collected 24 h before starting treatment. On day 7 of treatment, the mice were killed and blood samples were collected in EDTA-tested tubes. The brains were quickly removed and placed on an ice-cold plate. Cortex and hippocampus were dissected and immediately frozen on dry ice and stored at −80 °C, as well as the plasma for Aβ42 and flavonoid level measurements. Plasma was diluted 1:4 for Aβ42. For measurement of Aβ42, in brain tissues, samples were homogenized in 70% formic acid at 1:10 (w/v), and then centrifuged at 15,000×g for 25 min at 4 °C. The supernatants were collected and neutralized with 1 M Tris, pH 11 at 1:20 (w/v) dilution with 3X protease inhibitor mixtures. Levels of Aβ42 in plasma and in brain homogenate supernatants were measured with commercial ELISA kits (The Genetics Company, Zurich, Switzerland) as described previously (Jeong et al. 2007). Flavone levels in plasma and in brain samples were measured by HPLC as previously described (Gee et al. 1998). All experiments were conducted in triplicate (n = 3). All the values were represented as mean ± SD. IC50 were determined by probit analysis using SPSS software program (SPSS Inc., Chicago, IL); with P values < 0.05 considered statistically significant.
Results and discussion
Phytochemical investigation
Identification of compounds
Heavenly Blue was subjected to fractionation and isolation of phenolic compounds using polyamide followed by a series of silica gel, polyamide, Sephadex LH-20, or cellulose columns. The chromatographic properties of compounds (1–4) (color under UV light, change with ammonia vapor and responses toward NA/PE spray reagent or AlCl3) suggested their flavonoidal character. They showed UV spectra of two major absorption bands in methanol at the range of 257–276 nm (band II) and at 334–350 nm (band I) indicating their flavonoidal nature (Markham 1982). This was further reinforced by their deep purple fluorescence under UV, and changed into bright yellow to greenish–yellow fluorescence (365 nm) upon spraying with NA/PE. They gave bright yellow to yellowish–green upon the exposure to ammonia vapor, and an orange yellow to yellow with exposure to an AlCl3 reagent. Compounds 1–3 were expected to be of flavonoidal nucleus without hydroxyl group at C-3 on the basis of their chromatographic properties and UV spectral analyses in methanol and on addition of the diagnostic shift reagents. These compounds showed the absence of the bathochromic shift in band II on addition of NaOAc, which was consistent with the substitution at 7-OH (Mabry et al. 1970). Compound 4 showed the presence of a shoulder at 332 nm in NaOMe along with a bathochromic shift in band II upon addition of NaOAc to suggest the presence of a free hydroxyl group at C-7. Bathochromic shift of band I on addition of NaOMe with increase in intensity indicated free 4′-OH. Also, the free 5-OH of compounds 1–4 was confirmed by a bathochromic shift in band I in AlCl3/HCl. Compound 3 was suggested to be a 6- or 8-hydroxy flavone (Harborne and Mabry 1982) and it showed a low bathochromic shift (<20 nm) of band I after addition of the previous shift reagent which referred to the MeOH indicates 5-OH with 6-oxygenation. The presence of ortho-dihydroxyl pattern at ring B of compound 2 was confirmed by a bathochromic shift in band I with Na OMe, NaOAc, and AlCl3 partially remained after additional of the acids (Mabry et al. 1970). The UV data of compound 5 with the diagnostic shift reagents was in agreement with the presence of methoxy groups in 3,3′, and 4′ positions. The substituted 4′-OH was confirmed by the diminished peak in band I with NaOMe. Other UV spectral data, with the shift reagents of compounds was in agreement with 7-O-substituted flavones of apigenin nucleus (1 and 3) and luleolin in compound 2.
1H NMR spectra data of compound 1 and 3, exhibited A2X2 spin coupling system for a pair of two equivalent protons at about δ 7.95 (H-2′/6′) and 7.00 (H-3′/5′), respectively indicating 1,4 di-substituted B-ring. Compound 1 showed the presence of a terminal rhamnosyl moiety on OH-2" that was confirmed from the characteristic position of H-1‴ as brs signal at δ 5.12. Compound 1 was a glycosyloxyflavone and it is apigenin derivative having an α-(1 → 2)-l-rhamnopyranosyl)-β-d-glucopyranosyl moiety attached to the 7-OH group (Harborne and Mabry 1982).
Compound 2 showed the anomeric proton of O-glucosyl moiety appeared at δ 5.06 (with J = 6.9 Hz). Refer to the attachment of the glucose moiety to OH-7 (Markham et al. 1978). A γ-pyrone C-ring and 7-O-substituted ring A were followed from H-3 singlet at δ 6.72 (s, H-3) and the downfield location of both H-6 and 8 at δ 6.46 (d, H-6), 6.78 (d, H-8) of compound 2. Compound 2 showed also an ABX system at δ 7.42 (br d, J = 8.1), and 7.44 (br s) assigned to H-6′ and H-2′ and H-5′ with an ortho-doublet at δ 6.84) J = 8.1 Hz), characteristic for 3′,4′-dioxygenated B-ring. Similarly, a singlet at δ 6.70 assigned to H-3 to confirm a flavone identity of the aglycone in 3. 1H NMR spectrum of compound 3 showed the glycosidation at 7-OH as indicated by downfield of H-8 which appeared at δ 6.82 as a singlet where refers to the substitution at C-6. Another singlet signal at δ 3.79 p.p.m. was interpreted for a C-6 due to the absence of H-6 signal. Also, an anomeric doublet was located at δ 5.16 p.p.m. was for H-1" of 7-O-glucosyl moiety. 1H NMR spectral data of compound 4 showed the aromatic protons of the B-ring as two doublets at δ 7.92 and 6.92 p.p.m. with J = 8.4 Hz due to ortho coupling assigned to H-2′/6′ and H-3′/5′, respectively. The aromatic protons of the A-ring revealed as two brs at δ 6.50 and 6.22 due to meta coupling assigned to H-8 and H-6, respectively. H-3 appeared at δ 6.72 as a t signal group (Harborne and Mabry 1982).
13C NMR spectra exhibited the characteristic 13 carbon resonances of an apigenin aglycone in compounds 1, 3, and 4 and 15 carbon resonances, characteristic for a luteolin moiety (Agrawal and Bansal 1989). Compound 1 showed the presence of a 7-O-2-O-α-rhamnosyl-d-glucoside moiety that was followed from its typical twelve C-resonances, including the downfield shifted C-2" and the upfield of C-1" due to the attachment of the rhammnosyl on OH-2". The connection of β-O-glucoside moiety on C-7 of the compound 2 was indicated by the slight upfield shift of C-7 at δ 162.6 (Harborne and Mabry 1982). The O-glucosyl moiety of compound 2 was confirmed from the signals of glucose with C-1" at δ 99.8 p.p.m., and C-6" at 60.83 p.p.m. The methoxylation of compound 3 was confirmed by upfield shift with about −8 p.p.m. of C-7 (δ 157.0 p.p.m.) and C-5 (δ 152.6 p.p.m.) and the O-glycosidation at C-7 was confirmed by the downfield shift of C-8 (δ 94.2 p.p.m.). Compound 4 exhibited a downfield signal at δ 181.67 assignable to C-4. The other downfield signals at δ 164.3, 163.7, and 161.2 were assigned to the hydroxylated carbons (C-7, 5, and 4′). Other remaining C-resonances were assigned by their comparison with the previously reported data of the related structures (Markham et al. 1978; Agrawal and Bansal 1989).
The structures of the known compounds were finally confirmed by comparative chromatography of their acid hydrolysis products against authentic samples. Complete acid hydrolysis (2 N HCl) of the major flavone glycosides (1 and 3) yielded glucose in the aqueous phase and apigenin (aglycone) was detected in the organic phase (Co-PC). Rhamnose was detected with compound 1 by TLC. Final confirmation of the structures of compounds 1–4 was achieved by NMR analysis (Fig. 1). They were identified as rhoifoloside (1), luteolin-7-O-β-d-glucopyranoside (2), 5,7, 4′-trihydroxy-6-methoxyflavone-7-O-β- d -glucoside (3), and apigenin (4).
1H NMR of compound 5 showed a downfield shift of both H-2′ and H-5′ to 7.7 and 7.05 p.p.m. (δ ~ 0.2 p.p.m.), respectively, due to the methoxylation at the 3′ and 4′ position. The appearance of H-8 and H-6 resonances as two meta coupled doublets at their normal δ values 6.47 and 6.40 resulted in a free 5,7-dihydroxy A ring and it is also confirmed the position of the third OCH3 group at C-3. In addition three singlets each integrated to three protons were detected at 3.96 and 3.86, 3.84 to confirm the structure as 5,7-dihydroxy-3,3′,4′-trimethoxy flavone (5). EI-MS spectra of compound 6 showed [M-H]+ at m/z 167 (67.45%) corresponding to the molecular formula C8H8O4. The loss of a molecule of water gave rise to ion peak at m/z 149 (36.40 %) and the loss of one HCHO molecule gave rise to ion peak at m/z 126 (16.88%). It was identified as 2-hydroxymethylhydroquinone-6-carbaldehyde.
The structures and purities of the isolated plant phenolics in this study were elucidated by extensive chromatographic and conventional chemical and spectroscopic methods of analysis (UV, and 1H and 13C NMR) as well as retention time comparison with authentic standard compounds which have been isolated in our previous work (Awad et al. 2014). Reviewing the literatures oligosaccharides, oxylipins, polyacylated anthocyanin and resin glycosides were isolated from Heavenly Blue (Meira et al. 2012; Rosas-Ramírez et al. 1996).
In vitro Alzheimer’s Aβ production modulation
Phytochemicals, especially flavonoids are of current interest because of their important biological and pharmacological properties. This is the first report to address the respective bioactive compounds from the leaves of I. tricolor Cav. Heavenly Blue.
The flavonoids may protect the PC-12 cell from Aβ-induced injury through intracellular calcium influx, inhibition of oxidative damage and mitochondria dysfunction (Abd-Alla et al. 2016). The present study provides that flavones may be a promising approach for the treatment of AD and other oxidative-stress-related neurodegenerative diseases. In AD, Aβ is the main constituent of the accumulated amyloidic plaques in the brain. β-secretase and γ-secretase are two aspartic proteases responsible for degradation of APP into Aβ42 and Aβ38 which results in extracellular Aβ deposits. Aβ42 has been reported to be a major Aβ constituent of amyloid plaques. Until now there is no causal therapy for AD is clinically accessible in spite of the intensive reach in this field. Therefore, in the present study efforts have been exerted in order to investigate the effect of some isolated flavones as a natural source for modulation of Aβ.
In the previously published work, the ability of some flavonoids to react with the biologically relevant reactive nitrogen species, nitric oxide, peroxynitrite, and nitrous acid were investigated in vitro, for the first time (Awad et al. 2014). According to those results, the major isolated flavones; rhoifoloside (1), 5,7, 4′-trihydroxy-6-methoxyflavone-7-O-β-D-glucoside (3), and 5,7-dihydroxy-3,3′,4′-trimethoxyflavone (5) have been selected to study the effect of them to alter APP processing and generation of Aβ, particularly the Aβ42 was investigated. Therefore, H4 human neuroglioma cells expressing the double Swedish mutation (K595N/M596L) of human APP (APPsw) were used and treated with increasing concentrations of these three flavones, and analyzed for Aβ40 and Aβ42 levels in culture medium using ELISA. In addition, the inhibition activity of different concentrations of these three flavones against COX-1 and COX-2 was also investigated. The results showed no inhibition activity of these flavones against COX-1 and COX-2 up to 500 nM concentration and concomitant reduction in prostaglandin synthesis, indicating that the reduction in Aβ42 levels may be independent of COX activity.
In H4 cells, reduction in the Aβ42 supernatant concentration was achieved at different concentrations of each of the three investigated flavones without significant reduction in total Aβ (Aβ40+Aβ42) level. The relative inhibitory activity of these compounds is summarized in Table 1 as IC50 values. A dose-dependent inhibition of Aβ42 secretion was observed (Fig. 2). This finding indicates that the pharmacological action of these three compounds is via the functional inhibition of γ-secretase. The cytotoxicity of these compounds was assessed, in order to insure that Aβ42 reduction is not related to cytotoxicity. No toxicity was detected by both MTT and LDH assay in H4 cells of concentration up to 500 nM.
Under these experimental conditions the relative potencies of the three compounds were in the order: 1 > 3 > 5 > R-flurbiprofen.
Compound 5 is a trimethoxylated flavone (3,3′,4′-trimethoxyflavone). Another polymethoxylated flavone; nobiletin (with 3′,4′ methoxy groups) from citrus peels, was reported to ameliorate learning and memory impairment in olfactory-bulbectomized mice, amyloid precursor protein transgenic mice, NMDA receptor antagonist-treated mice, and senescence-accelerated mouse prone 8 (Nakajima et al. 2015). This polymethoxylated flavone improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of AD (3XTg-AD) that progressively develops amyloid plaques, neurofibrillary tangles, and cognitive impairments.
Previously published works, the radical scavenging activity usually increased with a decrease in glycosylation (Jeong et al. 2007). Luteolin, a well known flavone, showed the most potent anti-AD activity as determined by its inhibition of AChE, butyrylcholinesterase (BChE), and β-site amyloid precursor cleaving enzyme 1 compared with its C-glycosylated derivatives (Choi et al. 2014). The results in this study reveal that the compounds which have O-glucoside showed the best anti-Alzheimer activity in vitro (compounds 1 and 3). These findings could be explained by the fact that a glucose moiety is able to interact with glucose transporters (Gee et al. 1998), with increases in the intracellular uptake and bioavailability of these glucosides (Awad et al. 2014). These activities may be attributed to the presence of free phenolic hydroxyl group in ring B compared to the presence of methoxy groups in compound 5 (Rizk et al. 2017). This result is in line with the literature (Yamada et al. 2015). It has been reported that the differences in radical scavenging activity between polyhydroxylated and polymethoxylated flavonoids may attributed to differences in both hydrophobicity and molecular planarity (Van Acker et al. 1996; Ollila et al. 2002). The methoxy groups introduce unfavorable steric effects and increase lipophilicity and membrane partitioning. This may explain the lower activity of compound 5, which has three methoxy groups that could cause steric hindrance and decrease the molecular planarity.
In vivo Aβ modulation activity
Young male transgenic mice (Tg2576) were used for the in vivo studies in order to study the ability of the three flavones to treat, or at least to modulate AD in vivo. These animals have been reported to show many of the neuropathological features of AD, and to excrete high levels of Aβ in a regionally specific manner. Therefore, we applied the results of the in vitro experiments to in vivo model of Alzheimer.
In these mice after oral gavage of 100 nM per kg per day for 7 days, an excellent alteration in the Aβ42 concentration level in both plasma and brain was achieved for each of the three investigated flavones compared to the positive control (R-flurbiprofen). The relative inhibitory activity of these compounds is summarized in Table 2 as a percentage alteration of Aβ42 in both plasma and brain.
Under these experimental conditions, the relative potencies of the three compounds in plasma were in the order: 5 > 1 > 3 > R-flurbiprofen (positive control). However, the relative potencies of the three compounds in brain were in the order: 5 > 3 > 1 > R-flurbiprofen.
Concerning the concentration of each flavone compound in plasma and brain, the results showed that, the concentrations of the investigated three compounds were much higher than those of R-flurbiprofen (positive control).
The concentrations of the flavones in plasma were in the order:
5 > 3 > 1 > R-flurbiprofen
The concentrations of the flavones in brain were in the order:
5 ≥ 3 > 1 > R-flurbiprofen
Theoretically, in order to have a good neurodegenerative drug candidate, it is essentioal that this drug will be able to cross the blood brain barrier (BBB) after systemic administration (Gilgun-Sherki et al. 2001).
There are two pathways to cross this BBB; aqueous pathway (minor pathway) and lipophilic pathway (major pathway). Therefore, addition of hydrophobic groups (increasing the lipophilicity of a drug) to a molecule may help it to penetrate the brain (Rowland et al. 1992). Glucose derivatives may penetrate the BBB via glucose carrier as well (Bonate 1995).
Table 2 showed that the percentage alteration in the Aβ42 concentration level ranged from 68.79 to 89.98% in the brain. This may be attributing to the amount of the flavones that was able to cross the BBB. According to these data, the three flavones were able to cross BBB in very high concentrations compared to the positive control, R-flurbiprofen, ranging from 2.4 × 1011 to 1.1 × 1012 fold higher than R-flurbiprofen. These results may be attributed to both the higher lipophilicity of all three flavones compared to R-flurbiprofen and/or the presence of glucose as a glycoside in the flavones skeleton of two of these compounds (1 and 3), which may enhance the penetration of these compounds through the BBB via glucose transporters.
Finally, although all of the investigated polyphenols showed high efficiency when used both in vitro and in animal model, they are needed to be examined in small clinical studies, as well as in a large-scale controlled study to check whether the data is conflicting or not. It is also important to determine whether these compounds can be used as prophylactics, in order to slow down the progression of neurodegenerative diseases such as AD in populations that are at high risk, such as the elderly.
Conclusion
The data presented in this study are the first report to address the respective bioactive compounds from the leaves of Heavenly Blue. Six compounds were purified and identified. All metabolites were reported for the first time in the genus Ipomoea. Three major flavones were able to modulate the Aβ concentration both in vitro and in vivo without any cytotoxic effect. In vitro, these activities may be attributed to the presence of free phenolic hydroxyl groups in ring B rather than the presence of methoxy groups. The methoxy groups may introduce unfavorable steric effects and increase lipophilicity and membrane partitioning. However, in vivo, the activities obtained may be attributed to both the higher lipophilicity of all three flavones and/or the presence of glucose as a glycoside in the flavones skeleton, which may enhance the penetration of these compounds through the BBB via glucose transporters. The present study provides that these three flavones may represent a promising approach for the treatment of AD and other oxidative stress-related neurodegenerative diseases.
References
Abd-Alla HI, Shalaby NM, Hamed MA, El-Rigal NS, Al-Ghamdi SN, Bouajila J (2016) Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch Pharm Res 39:10–20
Agrawal PK, Bansal MC (1989) Flavonoid glycosides. In: Agrawal PK (Ed.) studies in organic chemistry 39, 13C-NMR of flavonoids. Elsevier science, New York, pp 283–364
Awad HM, Abd-Alla HI, Mahmoud KH, El-Toumy SA (2014) In vitro anti-nitrosative, antioxidant, and cytotoxicity activities of plant flavonoids: a comparative study. Med Chem Res 23:3298–3307
Berechid BE, Kitzmann M, Foltz M, Roach AH, Seiffert D, Thompson LA, Olson RE, Bernstein A, Donoviel DB, Nye JS (2002) J Biol Chem 277:8154–8165
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403
Bonate PL (1995) Animal models for studying transport across the blood-brain barrier. J Neurosci Methods 56:1–15
Choi JS, Islam MN, Ali MY, Kim YM, Park HJ, Sohn HS, Jung HA (2014) The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch Pharm Res 37:1354–1363
Gee M, DuPont MS, Rhodes MJ, Johnson IT (1998) Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic Biol Med 25:19–25
Gilgun-Sherki Y, Melamed E, Offen D (2001) Oxidative stress induced-neurodegenerative diseases: the need for ntioxidants that penetrate the blood brain barrier. Neuropharmacology 40:959–975
Glaser K, Sung ML, O’Neill K, Belfast M, Hartman D, Carlson R, Kreft A, Kubrak D, Hsiao CL, Weichman B (1995) Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur J Pharmacol 281:107–111
Hamsa TP, Kuttan G (2010) Ipomoea obscura ameliorates cyclophosphamide-induced toxicity by modulating the immune system and levels of proinflammatory cytokine and GSH. Can J Physiol Pharmacol 88:1042–1053
Harborne JB, Mabry TJ (1982) The Flavonoid: Advances in research. Chapman and Hall Ltd, London, pp 1–18
Hawas UW, El-Kassem LT, Awad HM, Taie HA (2013) Anti-Alzheimer, antioxidant activities and flavonol glycosides of Eryngium campestre L. Curr Chem Biol 7:188–195
Hsiao K, Champman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Huang GJ, Sheu MJ, Chen HJ, Chang YS, Lin YH (2007) Inhibition of reactive nitrogen species in vitro and ex vivo by trypsin inhibitor from sweet potato ‘Tainong 57’ storage roots. J Agr Food Chem 55:6000–6006
Jeong JM, Choi CH, Kang SK, Lee IH, Lee JY, Jung H (2007) Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J Pharm Pharm Sci 10:537–546
Kim JK, Choi SJ, Cho HY, Kim YJ, Lim ST, Kim CJ, Kim EK, Kim HK, Peterson S, Shin DH (2011) Ipomoea batatas attenuates amyloid β peptide-induced neurotoxicity in ICR mice. J Med Food 14:304–309
Lee SH, Jun M, Choi JY, Yang EJ, Hur JM, Bae K, Seong YH, Huh TL, Song KS (2007) Plant phenolics as prolyl endopeptidase inhibitors. Arch Pharm Res 30:827–833
Mabry TJ, Markham KR, Thomas MB (1970) The systematic identification of flavonoids.. Springer-Verlag, Berlin, pp 41–164
Markham KR (1982) Techniques of Flavonoid Identification. New York. Academic Press, London, pp 181–207
Markham KR, Ternai B, Stanley R, Geiger H, Mabry TJ (1978) 13C NMR studies of flavonoids. III. Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 34:1389–1397
Meira M, da Silva EP, David JM, David JP (2012) Review of the genus Ipomoea: traditional uses, chemistry and biological activities. Rev Bras Farmacogn 22:682–713
Mori M, Yoshida K, Ishigaki Y, Matsunaga T, Nikaido O, Kameda K, Kondo T (2005) UV-B protective effect of a polyacylated anthocyanin, HBA, in flower petals of the blue morning glory, Ipomoea tricolor cv. Heavenly Blue. Bioorg Med Chem 13:2015–2020
Nakajima A, Aoyama Y, Shin EJ, Nam Y, Kim HC, Nagai T, Yokosuka A, Mimaki Y, Yokoi T, Ohizumi T, Yamada K (2015) Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav Brain Res 289:69–77
Ollila F, Halling K, Vuorela P, Vuorela H, Slotte JP (2002) Characterization of flavonoid-biomembrane interactions. Arch Biochem Biophys 399:103–108
Philippi ME, Duarte BM, Da Silva CV, De Souza MT, Niero R, Cechinel Filho V, Bueno EC (2010) Immunostimulatory activity of Calophyllum brasiliense, Ipomoea pes-caprae and Matayba elaeagnoides demonstrated by human peripheral blood mononuclear cells proliferation. Acta Pol Pharm 67:69–73
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood brain barrier to exogenesis peroxidase. J Cell Biol 34:207–217
Rizk MZ, Abo-elmatty DM, Aly HF, Abd-Alla HI, Saleh SM, Younis EA (2017) Healing potency of citrus, hesperetin and naringenin loaded with silicate nanoparticles on neurotoxicity induced by acrylamide toxic dose. Der Pharma Chem 9:99–108
Rosas-Ramírez D, Escalante-Sánchez E, Pereda-Miranda R, Bah M, Pereda-Miranda R (1996) Detailed FAB-mass spectrometry and high resolution NMR investigations of tricolorins A-E, individual oligosaccharides from the resins of Ipomoea tricolor (Convolvulaceae). Tetrahedron 52:13063–13080
Rowland LP, Fink ME, Rubin LL (1992) Cerebrospinal fluid: blood brain barrier brain oedema and hydrocephalus. In: Kandel ER, Schwartz JH, Jessle T (Eds) Principles of Neural Science.. Elsevier, New York, pp 1050–1060
Schmidt B, Braun HA, Narlawar R (2005) Drug development and PET-diagnostics for Alzheimer’s disease. Current. Med Chem 12:1677–1695
Van Acker SA, De Groot MJ, van den Berg DJ, Tromp MN, den Kelder GD, van der Vijgh WJ, Bast A (1996) A quantum chemical explanation of the antioxidant activity of flavonoid. Chem Res Toxicol 9:1305–1312
Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH (2001) A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414:212–216
Williams RJ, Spencer JP (2012) Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Rad Biol Med 52:35–45
Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M (2015) Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. Adv Exp Med Biol 863:79–94
Ye J, Meng X, Yan C, Wang C (2010) Effect of purple sweet potato anthocyanins on β-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem Res 35:357–365
Acknowledgements
The authors wish to thank the financial support of National Research Centre (NRC) and the Science and Technology Development Fund (STDF), Egypt (Grant No 260).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Awad, H.M., Abd-Alla, H.I., Ibrahim, M.A. et al. Flavones from Heavenly Blue as modulators of Alzheimer’s amyloid-beta peptide (Aβ) production. Med Chem Res 27, 768–776 (2018). https://doi.org/10.1007/s00044-017-2100-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2100-x