Abstract
Hepatocellular carcinoma is a major example for inflammatory-associated cancer. cucurbitacins are natural triterpenoids known for their potent anticancer and anti-inflammatory activities. Recent studies showed that cucurbitacins protect the HepG2 cell lines against carbon tetrachloride-induced toxicity, however the mechanism is unknown. A molecular docking study coupled with in vitro biological assays were conducted to test the hepatoprotective effect of cucurbitacin on the inhibition of potential inflammatory factors. The effect of cucurbitacins on the activation of NF-kB pathway was analyzed using in cell-based NF-kB immunoassay. Enzyme-linked immunosorbent assays revealed the potential of Cuc D and dihydro cucurbitacin D to prevent the production of tumor necrosis factor-alpha and interleukin-6 from HSC-T6 cells. Thus, Cuc D and dihydro cucurbitacin D could have hepatoprotective effects on the activated rat HSC-T6 cells due to inhibition of the production of tumor necrosis factor-alpha and interleukin-6 through NF-kB pathway. In-silico molecular modeling data revealed potential cucurbitacin analogs with higher binding affinity to the hydrophobic pocket of NF-kB and IKKβ compared to standard IKK inhibitor (PS-1145).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucurbitacins (Cucs) are naturally occurring highly oxygenated tetracyclic triterpenes, found in family Cucurbitaceae, characterized by the tetracyclic cucurbitane skeleton named as 19-(10→9β)-abeo-10α-lanost-5-ene (Chen et al. 2005; Adewusi and Afolayan 2010). Cucs have been known for their multiple biological activities, such as anti-inflammatory, hepatoprotective, and anticancer activity against different cancer cell lines (Dhiman et al. 2012; Alghasham 2013; Ahmed et al. 2014; Ahmed et al. 2017). Cucurbitacin E, B, D, and I showed anti-inflammatory activity by inhibiting cyclooxygenase (COX) enzymes with more specific inhibition towards COX-II enzyme (Jayaprakasam et al. 2003). Cucurbitacin B and dihydro cucurbitacin D (DHCD) exhibited anti-inflammatory and immunosuppressive activity by inhibiting the production of tumor necrosis factor-alpha (TNF-α) from lymphocyte and macrophage cells (Berasain et al. 2009). DHCD also inhibited the generation of nitric oxide (NO) and inducible NO synthase from peritoneal macrophages by blocking NF-kB pathway (Park et al. 2004; Escandell et al. 2007; Jia et al. 2015). Bartalis and Halaweish 2011 reported the hepatoprotective activity for Cucs by unknown mechanism. Cucs are known for their inhibitory effect towards both JAK2/STAT3 tyrosine phosphorylation in cancer cells, NF-kB activation and TNF-α production from macrophage and peripheral blood lymphocyte (Chen et al. 2005). Herein, mechanistic investigation for Cucs’ hepatoprotection activity was studied via the inhibition of TNF-α production from liver cells. Also, IKKβ/NF-kB pathway that leads to the production of pro-inflammatory cytokine interleukin-6 (IL-6) and TNF-α was investigated (Karin and Delhase 2000; Maeda et al. 2005; Popa et al. 2007; Nakagawa et al. 2009).
Material and methods
Isolation and extraction of Cucs
The isolation of Cucs was achieved as previously described in the literature with minor modifications (Bartalis and Halaweish 2011). Briefly, Cucurbita andreana gourds were cut into small pieces and homogenized in 70% methanol in deionized water. The homogenate was filtered through cheesecloth, and the insoluble residue was re-homogenized in 70% methanol and re-filtered. The organic solvent was removed under reduced pressure. The aqueous phase was partitioned with hexane then ethyl acetate. The ethyl acetate fraction was concentrated and applied to flash column chromotography for separation with gradient elution of ethyl acetate and hexane solvent mixture. Similar fractions were pooled together and final purification was performed using preparative thin-layer chromatography (TLC). Chemical structure of isolated compounds were elucidated using 1H and 13C NMR using Bruker AVANCE-400 MHz NMR spectrometer. Samples were dissolved in CDCl3 and TMS (δ = 0 ppm) as the internal standard.
Cell viability and cytotoxicity assay
HSC-T6 was received from Professor. S. Friedman (Mount Sinai Hospital, NY). Monolayer cultures of HSC-T6 cells were grown in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum and penicillin-G and incubated at 37 °C and 5% CO2. Cells were seeded in 96-well plates at 30,000 cells per well and incubated for 24 h. Compounds were added to a specified final concentration (100, 20, 4, 0.8, and 0.16 μM) through five-serial dilutions. Finally, the cells were treated with 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in phosphate buffer saline, purchased from Sigma-Aldrich, for 4 h and lysed with 0.01 N HCl containing 10% Sodium dodecyl sulfate (SDS). Plates were read on Spectramax M2 plate reader at 570 nm. All experiments were performed in triplicate.The cell viability was calculated by the following equation:
Viability (%) = 100 × (A drug−A NC)/(A PC−A NC)
where, A drug is the absorbance at 570 nm of cells treated with the drug, A NC is the absorbance for the negative control (NC, no cells), and A PC is the absorbance of positive control (PC, cells with no drug).
Hepatoprotective activity of DHCD and DHCB against carbon tetrachloride-induced hepatotoxicity on HSC-T6 was tested. The MTT assay was conducted as previously described (Bartalis and Halaweish 2011); and carbon tetrachloride (CCl4) in 5% ethanol were used as a hepatotoxic chemical. Cells were seeded in 96-well plates at 30,000 cells per well and incubated for 24 h. Compounds were added in a specified concentration (50, 20, or 12 μM) along with 4.5 × 10−3 M in 5% ethanol CCl4. After 24 h, the cells were treated with 5 mg/ml MTT solution for 4 h and lysed with SDS solution. Plates were read on Spectramax M2 plate reader at 570 nm 4 h after lysing. All experiments were performed in triplicate. The cytotoxicity of tested compounds was calculated by the following equation:
where A drug is the absorbance of cells treated with drugs and toxin, A T is the absorbance of the s cells treated with toxin only and the A untreated is the absorbance of the untreated cells.
TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA)
Cells were seeded in 24-well plates at 200,000 cells per well and incubated for 24 h. HSC-T6 cells were incubated with serum-free media contains 5 μg/ml lipopolysaccharide (LPS) to activate NF-kB, and stimulate the induction of cytokines or LPS for the indicated time periods (24 h). The concentration of TNF-α and IL-6 concentration in the culture supernatant was measured using commercially available ELISA assay (R & D system) according to the manufacture procedure. The percentage inhibition of TNF-α in the samples was calculated using the following formula:
where A 0 is the absorbance of the PC (5 μg/ml LPS) and A 1 is the absorbance of sample containing LPS and drugs.
Phospho-RelA/NF-kB P65 and phospho-IKKB-α (S32/S36) cell-based immunoassay
Hepatic stellate cells (HSC) were seeded in 96-well plates at 30,000 cells/well in the corresponding media. The cells were incubated at 37 °C for 24 h. The cells were treated with the EC50 doses of test compounds and stimulated with 5 μg/ml of LPS for 60 min in case of phospho RelA/NF-kB P65 immunoassay or for 30 and 60 min in case of phospho IKKB-α immunoassay prior to the fixation of the cells using 4% formaldehyde. Followed by addition of permeabilization buffer, then quenching solution, and blocking buffer before the addition of the primary antibody to be incubated overnight. Then, secondary antibody was added followed F1 and F2 substrate. Fluorescence was measured using ELISA plate reader with excitation at 540 nm and emission at 600 nm, and then at excitation at 360 nm and emission at 450 nm.
Molecular modeling simulations
Molecular docking of Cucs into the active site of the IKKβ protein was carried out using Openeye® Molecular Modeling Software Package (http://www.eyesopen.com/oedocking) using OMEGA 2.5.1.4and Fast Rigid Exhaustive Docking (FRED) Receptor, 2.2.5; OpenEye Scientific Software, Santa Fe, NM (USA) (Hawkins et al. 2010; Mcgann 2011). The virtual library of Cucs was energy minimized using MMFF94 force field and followed by multi-conformers generation using OMEGA Application. The receptor PDB file was taken from the Protein Data Bank (PDB) (IKK PDB ID 3BRT) and was prepared using FRED Application to assign grid box based on the co-crystallized ligand dimensions. Snap shots could be visualized and obtained using VIDA application showing the main interaction forces between the analogs and the receptor of interest to execute semi-flexible docking.
Results
Extraction and isolation of cucurbitacin
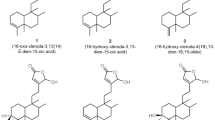
Five cucurbitacin aglycons and one glycoside were isolated from ripe fruits of Cucurbita texana (Cucurbitaceae) using column chromtography, preparative TLC and high performance liquid chromatography. Cucurbitacin D, B, E, DHCD, DHCB, and E glycoside (Fig. 1) were identified by comparison with standard compounds using. The rest of isolated Cucs in this study were characterized using 1H-NMR and13C-NMR to reveal compatible results with the literature (Ahmed et al. 2014; Bartalis and Halaweish 2011).
Cell viability and cytoprotection assay
DHCD and DHCB did not affect the cellular viability of HSC-T6 cells. This indicates that the two compounds were not toxic at doses of ≤100 μM. Based on the literature, the low cytotoxic activity for both of DHCD and DHCB is due to the lack of double bond at the Δ23–24 position in the basic skeleton of Cucs (Bartalis and Halaweish 2011). The cytoprotection activities of DHCD and DHCB against CCl4-induced hepatic toxicity were determined at three concentration levels (at 12.5, 20, and 50% of their IC50 values). The Cytoprotection data showed that DHCD and DHCB provide good protection level (≥ 50%) on cells, as shown in Table 1.
TNF-α and IL-6 ELISA assay
Cucs have shown inhibitory activity towards TNF-α (Park et al. 2004) and in order to investigate the potential mechanisms of cucurbitacin hepatoprotection activity, inhibition effect of Cucs of the pro-inflammatory cytokines upon stimulation with LPS in HSC-T6 cell line was examined. The inhibitory effect of DHCD and DHCB on the production of TNF-α and IL-6 was examined using colorimetric ELISA assays. Cells were pre-incubated with Cucs for 1 h and then stimulated with 5 μg/ml LPS for 24 h. Cell culture medium were collected, and analyzed for both TNF-α and IL-6 using commercially available ELISA. Our data showed that protective dose of cucurbitacin D and DHCD dramatically reduced LPS-induced production of the proinflammatory cytokines TNF-α and IL-6 in activated rat hepatic stellate cells. TNF-α and IL-6 detection from a cultured medium of activated HSC-T6 was performed using ELISA. Cuc D and DHCD inhibited the production of TNF-α by 96.4 and 35.3%, respectively, where release of TNF-α from LPS-stimulated cells after 24 h of incubation was between 20.00 ± 1.48 and 17.00 ± 0.89 pg, respectively, which was significantly higher than the un-stimulated control group. This clearly shows significant decrease in comparison to LPS stimulation after 24 h (P < 0.05), as shown in Fig. 2. On the other hand, Cuc B, DHCB, and Cuc E showed an increase in the LPS-induced production of the proinflammatory cytokines TNF-α in HSC-T6 cell line. The amount of TNF-α released from cells treated with non-toxic dose of DHCB, Cuc B, and Cuc E was 22 ± 0.89, 63 ± 0.75, and 52 ± 3.27 pg, respectively (P < 0.05), as shown in Fig. 2.
The same pattern of the release of IL-6 after 24 h was demonstrated. Cuc D and DHCD inhibited the production of IL-6 by 56.3 and 32.5%, respectively. Dexamethasone inhibited the released of IL-6 by 48.4% (Fig. 3). The release of IL-6 from LPS-stimulated cells after 24 h of incubation with Cuc D and DHCD was between 64 ± 0.98 and 83 ± 2.32 pg, respectively. This was significantly higher than the un-stimulated control group, which was between 27 ± 1.44 and 34 ± 2.00 pg. The amount of IL-6, which was released from the HSC-T6 treated with LPS in the presence of 35 µM of Cuc D and 60 µM of DHCD after 24 h was 28 ± 5.40 and 56 ± 0.22 pg, respectively, and from cells treated with 0.0001 µM dexamethasone and 125.5 µM silibinin was 36 ± 2.25 and 33 ± 1.30 pg, respectively. The amount of IL-6 released from Cuc D treated cells was significantly decreased in comparison with PC and standards (P < 0.05). However, DHCB and Cuc B significantly increases the production of IL-6 from activated HSC compared with control group (P < 0.05). The amount of IL-6 produced from activated HSC-T6 cells treated with non-toxic dose of cucurbitacin DHCB and Cuc B was 192 ± 5.41 and 446 ± 10.70 pg, respectively.
Phospho-RelA/NF-kB P65 cell-based immunoassay
Since activation of NF-kB is an essential step in the transcriptional induction of the pro-inflammatory cytokines TNF-α and IL-6 (Rosenfeld et al. 2000). Cuc B has been previously reported for their significant effect in the transactivation activity of RelA/p65 (Jin et al. 2011). Based on that, Phospho-RelA/NF-kB P65 cell-based immunoassay was performed to determine whether Cucs influence NF-kB activation or not. Previous reports showed that the activation levels of NF-kB in HSCs can be detected within 1 h of LPS stimulation (Mühlbauer et al. 2004; Covert et al. 2005). Cells were treated with Cucs and standards in the presence and the absence of 5 μg/ml LPS for 1 h. Cells were fixed and treated with primary and secondary antibodies. Fluorogenic agents were added to detect phosphorylated RelA (P65) NF-kB subunit. Cuc D showed significant inhibition for the total and phosphorylated RelA/NF-kB P65 compared to the positive stimulation induced by LPS alone (P < 0.05), up to 1.4–1.5-fold decrease in the signaling induced by LPS in case of the phosphorylated RelA/NF-kB P65 on S536 and total RelA/NF-kB P65, respectively compared with only 1.2-fold decrease in phosphorylated RelA/NF-kB P65 on S536 and no effect on total RelA/NF-kB P65 in case of standard IKKβ inhibitor (PS-1145) (P > 0.05). Cuc B also showed 1.2-fold and 1.4-fold decrease of phosphorylated RelA/NF-kB P65 on S536 and total RelA/NF-kB P65, respectively (P < 0.05). No significant effect of other test compounds on the phosphorylation of RelA/NF-kB P65 and total RelA/NF-kB P65 was observed, as shown in Fig. 4.
The effect of Cucs on LPS-induced RelA/NFκB p65 phosphorylation. a Phosphorylated RelA/NF-kB P65. b Total RelA/NF-kB P65 in cells. HSC-T6 cells were cultured in 96-well plates and treated with the indicated amounts of LPS alone (PC) or LPS along with test compounds for 60 min. The NC represents untreated cells. Values represent the mean ± SEM (n = 3)
Phospho-IKKB-α (S32/S36) cell-based immunoassay data
Degradation of IKKB-α protein was reported as an essential step for NF-kB activation, where IKKB-α is a regulatory protein attached to NF-kB subunits to keep them inactive in non-stimulated cells. HSC-T6 cells were treated with Cucs compounds and standards in the presence and the absence of 5 μg/ml LPS for 30 and 60 min. Cells were fixed and treated with primary and secondary antibodies. Fluorogenic agents were added to detect phosphorylated RelA (P65) NF-kB subunit. As shown in Fig. 5, Cuc D showed up to 1.6-fold decrease of phosphorylated IKKB-α (S32/S36) induced by LPS after 60-min treatment compared with 1.7-fold decrease by the standard (PS-1145). Additionally, Cuc B, DHCD and DHCB also showed significant inhibition of phosphorylated IKKB-α (S32/S36) by up to 1.2-fold decrease (P < 0.05). Dexamethasone and silibinin showed only 1.3-fold decrease in the phosphorylation of IKKB-α (S32/S36) induced by LPS compared to PC.
Semi-flexible molecular docking
Molecular docking results showed potential binding affinity of Cuc D and DHCD towards IKK receptor, within the same binding catalytic domain, as shown in Fig. 6. This was based on elucidation of physical properties of consensus score compared to ATP (endogenous substrate) and PS-1145.
Discussion and conclusion
Few in vivo studies revealed the hepatoprotective activity of Cucs against chemically induced hepatotoxicity. Cucs showed hepatoprotective activity by reducing serum liver enzyme levels and liver inflammation. To the best of our knowledge, the hepatoprotective mechanism of Cucs has not previously been investigated. Therefore, we investigated the potential affiliated molecular targets to elucidate hepatoprotection mechanism for Cucs. Herein, we investigated the inhibitory effect of cucurbitacin compounds on the production of TNF-α, IL-6 from hepatic stellate cells using colorimetric ELISA assays. Cuc D and DHCD effectively inhibited TNF-α production by a percentage of 96.4 and 35.3%, respectively and IL-6 production by a percentage of 56.3 and 32.5%, respectively from LPS-induced rate hepatic stellate cells. We also investigated the effect of Cucs on the activation of NF-kB transcription factor using cell-based immunoassay. Cuc D and DHCD inhibited the activation of NF-kB signaling induced by LPS. Thus, these results showed that the inhibition of TNF-α and IL-6 production was caused by blocking of NF-kB activation. NF-kB activation has been demonstrated an important role in promoting inflammatory-associated cancer and hepatocellular carcinoma development. Also, inhibition of IL-6 is potential for liver tumor suppression. Based on this study, Cucs can have the potential to protect liver cells from chemically induced liver toxicity throughout the inhibition of NF-kB pathway in liver cells and as a result prevent the production of TNF-α and IL-6 cytokine. In the present study, we also found that Cuc B, DHCB, and Cuc E did not inhibit the production of TNF-α and IL-6 from hepatic stellate cells; on the contrary, they increased the production of both cytokines. These results can be explained by the fact that low TNF-α amount promotes the activation of NF-kB throughout TNF/TNFR singling and lead to the production of more TNF-α, IL-6 and other pro-inflammatory cytokines. Our cell-based immunoassay data showed that DHCB, CucE and Iso-D did not inhibit phosphorylation of RelA/NF-kB P65. However, those compounds showed significant inhibition of phospho-IKB-α in HSCs (P < 0.05). The present study revealed that Cuc D and DHCD inhibited TNF-α generation from HSCs. Excessive generation of TNF-α by improper regulation of its production could contribute to inflammation. Thus, the inhibitory effect of Cuc D and DHCD on TNF-α production could help in explaining the anti-inflammatory action of Cucs. Also, we investigated the potential binding interaction between Cucs and targeted receptors, IKKβ showing that Cuc D and DHCD bound to the ATP binding pocket, which explain the polypharmacological activity of Cucs. Six Cucs (B, D, DHCD, DHCB, Iso D and E glucoside) were isolated from Cucurbita texana using flash column chromatography. Isolated compounds were characterized by spectroscopic techniques (1H NMR and 13C NMR). The TNF-α and IL-6 ELISA assay showed that cucurbitacin D and DHCD significantly inhibit the LPS-induced TNF-α and IL-6 from liver cells. Cell-based NF-kB immunoassay reveled that Cuc D and DHCD inhibited the phosphorylation RelA/NF-kB P65 in liver cells and the phosphorylation of IKB-α inhibitory protein in liver cells compared with IKKβ inhibitor PS-1145. Based on that, we suggested the potential of Cuc D and DHCD to protect liver cells from chemically induced hepatotoxicity by blocking NF-kB pathway and prevent the generation of proinflammatory cytokines TNF-α and IL-6.
References
Adewusi E, Afolayan A (2010) Review of natural products with Hepatoprotective activity. J Med Plants Res 4:1318–1334
Ahmed MS, El-Senduny F, Taylor J, Halaweish F (2017) Biological screening of cucurbitacin inspired estrone analogs targeting mitogen activated protein kinase (MAPK) pathway. Chem Biol Drug Des, doi:10.1111/cbdd.12963. [Epub ahead of print]
Ahmed MS, Halaweish FT (2014) Cucurbitacins: potential candidates targeting mitogen-activated protein kinase pathway for treatment of melanoma. J Enzyme Inhib Med Chem 29:162–167
Ahmed MS, Kopel L, Halaweish F (2014) Structural optimization and biological screening of a steroidal scaffold possessing cucurbitacin-like functionalities as B-raf inhibitors. ChemMedChem 9:1361–1367
Alghasham A (2013) Cucurbitacins - a promising target for cancer therapy. Int J Med Health Sci 7:77–89
Bartalis J, Halaweish F (2011) In vitro and QSAR studies of cucurbitacins on HepG2 and HSC-T6 liver cell lines. Bioorg Med Chem 19:2757–2766
Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA (2009) Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci 1155:206–221
Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005) Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 22:386–399
Covert M, Leung H, Gaston E, Baltimore D (2005) Achieving stability of lipopolysaccharide-induced NF-kB activation. Science 309:1854–1857
Dhiman K, Gupta A, Sharma D, Gill N, Goyal A (2012) A review on the medicinally important plants of the family cucurbitaceae. Asian J Clin Nutr 4:16–26
Escandell J, Recio M, Máñez S, Giner R, Cerdá-Nicolás M, Ríos J (2007) Cucurbitacin R reduces the inflammation and bone damage associated with adjuvant arthritis in lewis rats by suppression of tumor necrosis factor-alpha in T lymphocytes and macrophages. J Pharmacol Exp Ther 320:581–590
Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT (2010) Conformer generation with OMEGA: algorithm and validation using high quality structures from the protein databank and cambridge structural database. J Chem Inf Model 50:572–584
Jayaprakasam B, Seeram NP, Nair MG (2003) Anticancer and antiinflammatory activities of cucurbitacins from cucurbita andreana. Cancer lett 189:11–16
Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan X, Xu Z, Zhang P (2015) Cucurbitacin E inhibits TNF-α-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol 29:884–890
Jin R, Jin X, Dat N, Lee J (2011) Cucurbitacin B suppresses the transactivation activity of RelA/p65. J Cell Biochem 112:1643–1650
Karin M, Delhase M (2000) The IKB kinase (IKK) and NF-kB : key elements of proinflammatory signalling. Semin Immunol 12:85–98
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121:977–990
Mcgann M (2011) FRED pose prediction and virtual screening accuracy. J Chem Inf Model 5:578–596
Mühlbauer M, Weiss T, Thasler W, Gelbmann W, Schnabl B, Schölmerich J, Hellerbrand C (2004) LPS-mediated NFkappaB activation varies between activated human hepatic stellate cells from different donors. Biochem Biophys Res Commun 325:191–197
Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N, Shiina S, Omata M (2009) Serum IL-6 Levels and the risk for hepatocarcinogenesis in chronic hepatitis c patients: an analysis based on gender differences. Int J Cancer 125:2264–2269
Park C, Lim H, Han K, Baek S, Sohn O, Lee W, Kim Y, Yun H, Baek K (2004) Inhibition of nitric oxide generation by 23, 24- dihydrocucurbitacin D in mouse peritoneal macrophages. J Pharmacol Exp Ther 309:705–710
Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF (2007) The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48:751–762
Rosenfeld M, Prichard L, Shiojiri N, Fausto N (2000) Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol 156:997–1007
Acknowledgements
The authors would like to thank the Libyan ministry of higher education for funding this work, OpenEye® molecular modeling software for supporting an academic license, and Dr. Friedman (Mount. Sinai Hospital, NY) for providing the HSC-T6 cell lines. This work was supported by the Libyan ministry of higher education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Arjaibi, H.M., Ahmed, M.S. & Halaweish, F.T. Mechanistic investigation of hepato-protective potential for cucurbitacins. Med Chem Res 26, 1567–1573 (2017). https://doi.org/10.1007/s00044-017-1872-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1872-3