Abstract
The essential oils obtained by the hydrodistillation from the fresh flowers, leaves, stems, and roots of Ferula communis L., growing in Tunisia were analyzed by GC and GC/MS. Thirty-two components were identified in the oil of flowers with camphor (18.3 %), α-pinene (15.3 %), and β-eudesmol (9.3 %) as the main constituents. Twenty-nine compounds were identified in the oil of stems with β-eudesmol (28.1 %), δ-eudesmol (11.1 %), and α-eudesmol (9.6 %) as the main compounds. Twenty compounds were characterized in the oil of roots with dillapiole (7.9 %), guaiol (7.3 %), and spathulenol (6.8 %). In the oil of leaves, α-eudesmol (25.2 %), β-eudesmol (20.7 %), δ-eudesmol (10.1 %), and caryophyllene oxide (7.2 %) were found as the main constituents. This study was undertaken to evaluate the antioxidant activity using DPPH (2,2′-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), reducing power, and catalase activity. We tested also the antibacterial, cytotoxic, and cholinesterase inhibition properties of the essential oil of different organs of F. communis. The essential oil of the stems showed the highest antioxidant activity (IC50 = 0.03 ± 0.001 mg mL−1), in DPPH assay and the important result of catalase (303.03 µmol H2O2 degraded/min/protein) of F. communis. The antibacterial activity of the oil was determined by micro-well dilution assay. The best results (MIC = 0.156 ± 0.02 mg mL−1) were exhibited by the essential oil of the leaves of F. Communis against Pseudomonas aeruginosa. Besides, the strongest cytotoxic activity against Hela cells was shown with essential oils’ leaves with an inhibition percentage of 79.05 % at the concentration of 500 µg mL−1. However, the best inhibition percentage of A 549 cells was detected for essential oils’ leaves with an inhibition percentage of 54.56 % at 250 µg mL−1. Our finding showed that the essential oil of the flowers was the most active, with 64.623 % of inhibition against butyrylcholinesterase at 10 mg mL−1 from the incubation time of 30 min.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family of Apiaceae contains an extremely wide variety of aromatic plants. Among this rich array of plants yielding essential oils, the genus Ferula communis L. distributed worldwide is very important, including 172 species (Stravroula et al., 2013) that are spread around naturally on the pasture of the Eastern Mediterranean, and goat flocks grazed it with other herbaceous vegetation. The Tunisian flora comprises four species: F. communis, Ferula tingitana, Ferula tunetana (Jabrane et al., 2010a), and Ferula lutea (Znati et al., 2012). F. communis has got green vegetation during April and May and then gets dry. Ferula plants include phytoestrogenic substances that affect reproductive hormones such as progesterone, testosterone, and estrogens (Aragno et al., 1988). The active substances of phytoestrogen are isoflavones and ferutinin (Valle et al., 1987; Appendino et al., 2001). Ferula varieties also include some terpene and terpenoid compounds (http://pubs.acs.org/action/doSearch?action=search&author=Shikishima%2C+Y&qsSearchArea=author Nagatsu et al., 2002).
In eastern Mediterranean mountainous regions of Turkey, some people eat ground F. communis root by mixing it with honey. Goat keepers and other citizens of the region claim that this plant has an aphrodisiac role for both animals and humans (Keskin et al., 2004).
This species possesses several traditional medicinal properties. It is used for skin diseases, rheumatism, cracks in the feet, helminthic disease, pains in the joints, hysteria, and dysentery. It is antispasmodic, vermifugal, and aphrodisiac and can be used against moths or ringworm and as a depilatory. In Morocco F. communis is frequently used to treat various diseases, and orally it is prescribed by practitioners as an anti-helmintic, diuretic, analgesic, for pains in the joints, female sterility, rheumatism, and an emetic. The roots are also used for hair care (Bellakhdar, 1978).
Chemical composition studies of F. communis showed that it contains 4-hydroxycoumarin derivatives, some of which were demonstrated to have hypothrombonic action. 4-Hydroxycoumarin anticoagulants are known (Nicholas and Rettie, 2008), on the other hand, to be used for the treatment and management of thromboembolic disease in humans. In this study we try to evaluate the cholinesterase inhibition properties of the essential oils of F. communis. Alzheimer’s disease is the cause of a major cholinergic deficit, particularly neurotransmitter involved in attention and memory.
One way to increase the activity of the cholinergic system is to avoid the breakdown of acetylcholine by blocking cholinesterase. There are two types of cholinesterase enzyme: acetylcholinesterase (AchE), also known as acetylcholine. The acetylhydrolase is found primarily in the blood and neural synapses and pseudocholinesterase. The second one butyrylcholinesterase (BchE or BuChE), also known as plasma cholinesterase, is found primarily in the liver, and it serves as a model of the predominant treatment used in Alzheimer’s disease. In this frame, plants can be a source of inhibitors of acetylcholinesterase. In fact, from 1981 to 2006, 25 % of new anti-Alzheimer’s molecules were shown to be of natural origin (Newman and Cragg, 2007).
To enhance further our work, we studied the catalase antioxidant activity. Catalase is a metalloenzyme which dismutases hydrogen peroxide to water and oxygen. It is widely distributed in animals, plants, and all aerobic microorganisms. Catalase is an enzyme present in most of the aerobic cells, and it protects them from oxidative stress (inflammatory, tumor, diabetes, cardiovascular diseases, anemia and Wilson’s disease) (Al-Abrash et al., 2000).
Given a great interest in novel natural compounds able to fight against Alzheimer’s, cancer and many other diseases, we undertook the present study as a primary biological investigation of the anticholinesterase activity, cytotoxic, antioxidant, and antibacterial effects of different organs of F. communis L. essential oils.
Materials and methods
Plant material
Ferula communis L. was collected in April 2010 in Grombalia (Tunisia). The identification was performed at the laboratory of Plant Biology and Botanic, the High Institute of Biotechnology of Monastir, the University of Monastir, Tunisia. A voucher specimen (C.M-10) has been deposited in our laboratory.
Extraction of the essential oils
Fresh flowers, leaves, stems, and roots were subjected to hydrodistillation for 4 h using a Clevenger-type apparatus. The obtained essential oils were collected by decantation, dried over sodium sulfate, filtered, and stored at 4 °C until analyzed.
Analytical GC
Gas chromatographic analysis was carried out on a HP-5890 gas chromatograph fitted with a HP-Wax and HP-5 capillary columns (30 m × 0.25 mm, 0.25 μm film thickness). The GC oven temperature was programmed at 60 °C (held for 10 min) and heated to 220 °C at 5 °C/min. The injector and detector temperatures were maintained at 250 °C. Helium was used as carrier gas at a flow rate of 2 mL/min. Volume injected was 0.1 μL of 1 % hexane solution. The identification of the components was performed by comparing their retention times with those of pure authentic samples and by mean of their linear retention indices (L.R.I.) relative to the series of n-hydrocarbons.
Analytical GC–MS
GC/EIMS analysis were performed with a Varian CP-3800 gas chromatograph equipped with a HP-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion-trap mass detector. Analytical conditions are: injector and transfer line temperatures 220 and 240 °C, respectively; the oven’s temperature programmed from 60 to 240 °C at 3 °C/min; carrier gas helium at 1 mL/min; injection of 0.2 μL (10 % hexane solution); split ratio 1:30. The identification of the constituents was based on the comparison of the retention times with those of authentic samples, comparing their linear retention indices relative to the series of n-hydrocarbons, and on computer matching against commercial (NIST 98 and ADAMS) and home-made library mass spectra built up from pure substances and components of known oils and MS literature data (Stenhagen et al., 1974; Massada, 1976; Jennings and Shibamoto, 1980; Davies, 1990; Swigar and Silverstein, 1981; Adams, 2007). Moreover, the molecular weights of all the identified substances were confirmed by GC/CIMS, using MeOH as CI ionizing.
Antioxidant activity
DPPH radical-scavenging assay
The antioxidant activity of the essential oil of different organs of F. communis L. was determined by the slightly modified method of Hatano et al. (1988).
In total, 0.5 mL of each sample concentration was mixed with the same volume of DPPH ethanol solution. After 30-min incubation in the darkness at 25 °C, the absorbance of the sample at 520 nm was read. A mixture of 0.5 mL of DPPH solution and 0.5 mL of ethanol was used as a blank. The decrease in absorption induced by the samples was compared to that of the positive control, BHT (butylated hydroxytoluene). The calculated IC50 values denoted the concentration required to scavenge 50 % of DPPH radicals. The results were expressed in an inhibited percentage versus samples’ concentrations (mg mL−1) at 30 min. All the measurements were performed in triplicate.
ABTS radical-scavenging assay
The radical-scavenging capacity of antioxidant for the ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) radical was determined as described by Re et al. (1999). ABTS was generated by mixing 7 mM of ABTS at pH 7.4 (5 mM NaH2PO4, 5 mM Na2HPO4 and 154 mM NaCl) with 2.5 mM potassium persulfate (final concentration) followed by a storage in the dark at room temperature for 16 h before use. The mixture was diluted with ethanol to give an absorbance of 0.70 ± 0.02 units at 734 nm using a spectrophotometer (Helios, Unicam, Cambridge, UK). For each sample, the diluted methanol solution of essential oil (100 μL) was allowed to react with fresh ABTS solution (900 μL), and then the absorbance was measured 6 min after initial mixing. BHT (butylated hydroxytoluene) was used as a positive standard. The capacity of free radical scavenging was expressed as IC50 (mg/L) value, which represents the concentration required to scavenge 50 % of ABTS radicals. The capacity of free radical scavenging IC50 was determined using the same equation previously used for the DPPH method. All measurements were performed in triplicate.
Reducing power assay
The ferric-reducing power of the extracts and references was tested using the assay of Oyaizu (1986). A total of 1 mL of different concentrations of the extracts as well as chlorogenic acid as reference for comparative purposes was added to 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of potassium ferricyanide. Later, the mixture was incubated at 50 °C for 20 min and then 10 % trichloroacetic acid was added. After the mixture was shaken vigorously, this solution was mixed with distilled water and FeCl3 (0.1 %, w/v). After 30 min of incubation, the absorbance was read at 700 nm. The analyses were achieved in duplicate. The increased absorbance of the reaction meant increased reducing power.
Catalase activity
The catalase activity was measured according to Aebi’s (1984) method. Hydrogen peroxide (H2O2) disappearance was monitored kinetically at 240 nm for 1 min at 25 °C. The enzyme activity was calculated using an extinction coefficient of 0.043 mM−1 cm−1. One unit of activity is equal to 1 µmol of H2O2 destroyed/min/mg protein.
Antibacterial activity
Microorganisms
The antibacterial activity was tested against five microorganisms, including reference strains consisting of gram-negative rods: Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853), gram-positive cocci: Staphylococcus aureus (ATCC 25923) and Enterococcus faecalis (ATCC 29212), and clinical strains: Acinetobacter sp. The bacterial strains were cultured over night at 37 °C in Muller Hinton agar.
Micro-well dilution assay
This methodology is applied in order to establish minimum inhibitory concentrations (MIC) of different essential oils, against bacteria.
The MIC was defined as the lowest concentration able to inhibit any visible bacterial growth. MIC values were determined by a microtiter plate dilution method Jabrane et al. (2010b) dissolving the sample in 10 % DMSO solution. Sterile 10 % DMSO solution (100 μL) was pipette into all wells of the micro-titer plate before transferring 100 μL of stock solution to the microplate. Serial dilutions were made to obtain a concentration ranging from 10 to 0.0775 mg mL−1. Finally, 50 μL of 106 colony-forming units (cfu/mL) (according to Mc Farland turbidity standards) of standards microorganism suspensions were inoculated on to microplates and incubated at 37 °C for 24 h. At the end of the incubation period, the plates were evaluated for the presence or absence of growth. MIC values were determined as the lowest concentration of the sample at which the absence of growth was recorded. All the samples were screened three times against each microorganism. The imipenem was employed as a positive control against gram-positive and gram-negative bacteria. The final concentration of DMSO in the well had no effect on bacterial growth.
Anticholinesterase activity
The anticholinesterase activity was studied by the colorimetric method of Ellman et al. (1961) with a slightly modification. The butyrylcholinesterase (BchE) hydrolyzed the butyrylthiocholine (colorless) to butyrate and thiocholine.
Thiocholine reacts with DTNB to form 5-mercapto-2-nitrobenzoic acid (5-MNBA) which is a yellow product. The rate of 5-MNBA formation, measured spectrophotometrically at 405 nm, is proportional to the enzymatic activity of BchE in the sample.
Human plasma (pool plasma from samples designated for biochemical analysis) was used as a source of BchE. In total, 100 µL of extract were added to 100 μL of plasma, and the mixture was incubated at 37 °C for a determined time. After incubation, the enzyme’s activity was measured by Konelab 30® apparatus (1 cm cuvette). The control (plasma and distilled water) was treated in the same conditions. All assays were carried out in duplicate. The anticholinesterase activity was calculated by the following formula:
IC50 as the sample concentration providing 50 % inhibition (IC50) To determine the effect of temperature and incubation times, the assays were carried by the same procedure for the same extract, at 25 °C (fixed incubation times) and for different times at 37 °C.
Cytotoxic activity
Cell cultures
The human A549 lung epithelial carcinoma and Hela cervix cells lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco) containing 10 % (v/v) fetal calf serum, 2 mM glutamine, and antibiotics (200 U L−1 of penicillin and 50 mg L−1 of streptomycin). The cells were maintained at 37 °C in a humidified 5 % CO2 atmosphere.
Cell viability assay
The cytotoxicity was measured by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] test with slight modifications (Yan et al., 2009). The cells were seeded at 5 × 103 cells/well in 100 µL of growth medium and incubated at 37 °C for 24 h to adhere. The cells were treated by essential oils and incubated for 48 h; then 10 µL of MTT (5 mg mL−1) was added to each well and the incubation lasted 2 h. After this, 100 µL of DMSO were added to each well. The absorbance (A) was measured at 540 nm by a Multiskan Ascent (Ascent Software version 2.6) microplate reader. This assay was conducted in triplicate and the percentages of cell growth were calculated as follow:
Cytotoxicity was expressed as the concentration of extract inhibiting cell’s growth by 50 %.
Results and discussion
Chemical composition
The hydrodistillation of the dried aerial’s parts (flowers, leaves, and stems), roots of F. communis, gave yellow essential oils in yields ranging from 0.024 % (roots), 0.11 % (leaves), 0.15 % (stems) to 0.18 % (flowers).
The chemical compositions of the oils were analyzed by GC and GC–MS. More than 20 constituents were identified according to their retention indices (L.R.I). The identified compounds are presented in Table 1, and some significant differences were noted in the quantitative and qualitative composition of the oils obtained from stems and leaves. The main constituents of both oils were sesquiterpenoids (56 % and 48.8 %, respectively) with α-, β-, γ-eudesmol.
The oil obtained from flowers of F. communis present 32 compounds, which represented 97.3 % of the total amount of the oil, containing mainly camphor (18.3 %), α-pinene (15.3 %), β-eudesmol (9.3 %), caryophyllene oxide (8.0 %), and myrcene (5.0 %).
The dillapiole (7.9 %) was found as the major component of root’s essential oil, followed by guaiol (7.3 %), spathulenol (6.8 %), myristicin (6.0 %), and T-cadinol (5.9 %).
The present essential oil is also characterized by the presence of sesquiterpenoids compounds only; the essential oil of the flowers presents camphor (18.3 %) and α-pinene (15.3 %) as major compounds. The last constituents seem to be the main component of the essential oils of F. communis ssp glauca (leaves and flowers, respectively) (Maggi et al., 2009), F. lycia (Kose et al., 2010), F. badrakema (Asili et al., 2009), F. szovitsiana (Dehghan et al., 2007), F. ovina (Ghannadi et al., 2002), F. gummosa (gum and latex) (Ghannadi and Amree, 2002), F. gummosa (fruits) (Sayyah et al., 2001), F. flabelliloba (Rustaiyan et al., 2001a), F. stenocarpa (Rustaiyan et al., 2001b), F. jaesekheana (collected in May), F. jaesekheana (collected in July) (Kapahi et al., 1985), and F. penninervis (Goryaev et al., 1968) (11.7, 24.2, 59.9, 10.9, 8.0, 5.7, 18.3, 10.0, 48.8, 9.5, 30.0, 4.7 %, respectively).
Literarily, the composition of F. communis volatiles from the Mediterranean area has been reported: The leaves’ oil from Corsica was characterized by the presence of myrcene (53.5 %) and aristolene (8.5 %) (Ferrari et al., 2005); in the inflorescences oil from Sardinia, the main metabolites were the sesquiterpenes α- and β-gurjunene (40.7 and 7.1 %, respectively) (Marongiu et al., 2005); the oils from the aerial parts of the Sardinian populations had as major volatiles in the poisonous chemotype the sesquiterpenes aristolene (47.1 %) and (E,E)-farnesol (21.2 %), while in the non-poisonous chemotype, the main component was the sesquiterpene allohedycaryol (53.7 %) (Rubiolo et al., 2006). The essential oil’s composition of F. glauca (formerly considered as subspecies of F. communis) from central Italy appear to be different having as major volatiles in leaves (E)-caryophyllene and caryophyllene oxide, in flowers α-pinene, myrcene, and germacrene D and in fruits α- and β-pinene (Maggi et al., 2009).
The essential oils of Greek F. communis subsp. Communis from the leaves collected from three different regions in Chania (Crete) at the flowering stage (May 2007) (samples le1, infl1: 3 km SW Chania) and at the fruiting stage (April 2008) (samples le2, infr2: along the road between airport and Ag. Triada, samples le3, infr3: 5 km SW Chania) were characterized by the abundance of sesquiterpenes (85.7–88.7 %). Specifically, in sample le1 the main constituents were α- and β-eudesmol (12.6, 9.7 %), in sample le2 δ-cadinene (10.8 %) and germacrene B (10.1 %) and in the sample le 3 α-eudesmol (16.1 %) and δ- and γ-cadinene (13.6, 12.5 %). The inflorescences’ oil (sample inf1) was also abundant in sesquiterpenes with γ- and ar-curcumene (14.0, 8.5 %) being the main ones, while γ-terpinene (10.8 %) was the major component in monoterpenes’s fraction. on the contrary the infructescences oils (samples infr2, infr3) possessed high levels of monoterpenes, with α-pinene (35.2 and 13.6 %, respectively) and β-pinene (40.6, 10.2 %) being the major compounds (Manolakou et al., 2013).
Antioxidant activity
Free radical scavenging activity (DPPH assay)
The antioxidant measurement by DPPH method showed that the essential oil of roots exhibited a moderate activity (IC50 = 0.068 ± 0.003 mg mL−1) than flowers’ essential oil (IC50 = 0.060 ± 0.001 mg mL−1). Stems’ essential oil is the most potent radical scavenger with IC50 = 0.03 ± 0.001 mg mL−1, than leaves (IC50 = 0.030 ± 0.002 mg mL−1). The essential oil of F. communis has an antioxidant activity that is related to its chemical composition. The germacrene B which is present only in relative amounts in stems’ oil can be partly responsible for such activity, but it is difficult to attribute this activity to a single compound as a synergy between the different compounds that can occur.
ABTS radical-scavenging assay
According to the ABTS assay results, the essential oils of the four parts of F. communis showed weak antioxidant activity. The most active essential oil plant’s part was the stem, with an IC50 value of 0.229 ± 0.01 mg mL−1, followed by the leaves (IC50 = 0.26 ± 0.01 mg mL−1), then the flowers with IC50 = 0.51 ± 0.03 mg mL−1 and the roots (IC50 = 0.569 ± 0.02 mg mL−1).
Reducing power assay
Another method to evaluate the antioxidant ability is based on the reduction of Fe3+ to Fe2+ in which the yellow color of the test solution changes to various shades of green and blue, depending on the reducing power of each sample. The presence of the reducing agents causes the conversion of Fe3+/ferricyanide complex to the ferrous form that may be followed at 700 nm due to the formation of Perl’s Prussian blue Fe4[Fe(CN)6]3. Increasing the absorbance at 700 nm indicates an increase in the reductive ability (Joshi et al., 2010). The values of the reducing power at different organs of essential oils (flowers, leaves, stems and roots) of F. communis are shown in Table 2. The antioxidant activity of F. communis stem oils showed moderate ability for scavenging DPPH free radicals, when compared to this reference of (BHT). As well as, the same oils had the highest antioxidant capacity (IC50 = 0.097 ± 0.01 mg mL−1) relative to leaves (IC50 = 0.234 ± 0.01 mg mL−1), flowers (IC50 = 0.253 ± 0.002 mg mL−1), and roots (IC50 = 0.466 ± 0.02 mg mL−1, which had the weakest activity against the three antioxidant assays), when measured through the reducing power’s assay.
Catalase activity
In the present study we demonstrated that the activity of the enzyme catalase from stems’ essential oils (303.03 units) was significantly higher compared with that in leaves (151.51 units). The decrease in catalase activity may cause the accumulation of the O2 −, H2O2 or accumulation of hydrogen peroxide correlates with cancer metastasis (Tsai et al., 2014). Loss of catalase activity results in oxygen intolerance and triggers a number of deleterious reactions such as protein and DNA’s oxidation, and cell’s death (Halliwell and Gutteridge, 1999). The catalase plays a protective role against cardiovascular diseases.
These results confirm the correlation between our results in antioxidant activities: Stems show an interesting DPPH, ABTS, reducing power, and catalase activities, followed by the leaves of F. communis’s essential oils.
Antibacterial activity
As far as the in vitro antibacterial activity results are concerned, in general the essential oils possessed extremely moderate activity potential (Table 3).
In the presence of leaves F. communis essential oils, the strongest activity was observed against P. aeruginosa with an MIC at 0.156 mg mL−1, followed by Acintobacter, S. aureus, and E. coli, with MIC at 0.31; 2.5; and 5 mg mL−1, respectively. The weakest activity was observed against E. faecalis (MIC = 10 mg mL−1). Stems’ essential oils were active against P. aeruginosa and Acintobacter with MIC = 0.312 mg mL−1 and weakly active against S. aureus and E. faecalis (MIC = 10 mg mL−1) and had no effect on the growth of E. coli. The most prominent inhibitory action of roots oil was observed against E. coli with MIC = 1.25 mg mL−1. However, the S. aureus showed weak activity with MIC values of 2.5 mg mL−1. The results show also that flowers’ essential oils were active only against P. aeruginosa (MIC = 1.25 mg mL−1). The essential oils evaluated in this study presented a great variety of sesquiterpenes that could be considered as answerable for the antimicrobial activity (Maggi et al., 2009; Del-Vechio-Vieira et al., 2009; Chalchat and Garry, 1997). The antibacterial activity of different organs of F. communis essential oils could be associated with the presence of oxygenated sesquiterpenes components, and α- and β-eudesmol are well-known substances with pronounced antimicrobial properties (Yu et al., 2008).
Anticholinesterase activity
Plasma cholinesterase was butyrylcholinesterase (BchE) or pseudo-cholinesterase. In fact, the acetylcholinesterase (AchE) hydrolyzes only acetylcholine, whereas BchE metabolizes acetylcholine and butyrylcholine (Kluge et al., 2001). For this reason, we used in this work plasma as a source of BchE to study the anticholinesterase potential of F. communis L. extracts. In a primary assay, the anti-butyrylcholinesterase activity of the essential oils was tested with different incubation times (10, 15, 25 and 30 min) as presented in Fig. 1.
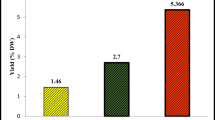
At 30 min of incubation, all organs (flowers, leaves and stems) of essential oils had the high percentages of inhibition (58,328 % from the essential oil of flowers, 55,718 % from the essential oil of leaves and 54,718 % from the stems’ essential oils). The important inhibition was detected in the leaves’ essential oils at the incubation time of 25 min, but the interesting inhibition was that of flowers at 30 min, compared with that of parathion used as a standard drug for the treatment of mild Alzheimer’s disease. So for this reason and in a secondary assay, the anti-cholinestrase activity of essential oils of F. communis was tested at 30 min of incubation for four concentrations (20, 10, 5, 2.5 and 1.25 mg mL−1). The inhibitory effects of concentrations on BchE are listed in Fig. 2. Flowers’ essential oil presents a quite good inhibition (64.623 %) with the concentration of 10 mg mL−1, which was the highest percentage of inhibition.
Several essential oils, as well as some of their components, have been reported as having acetylcholinesterase inhibitor ability. However, it is the first time that this property is reported for F. communis oils. Sesquiterpenes, the dominant constituents of the essential oils of F. communis can partly explain the best activity of these oils, since some studies have revealed that these monoterpenes are potent acetylcholinesterase inhibitors (Smail et al., 2011; Orhan et al., 2008).
Cytotoxic activity
The cytotoxicity of F. communis essential oils of different organs (flowers, roots, leaves and stems) was evaluated on A549 lung epithelial carcinoma and Hela cervix cells lines. The cytotoxic effect of the total essential oils was overall important. The inhibition percentage (%) was summarized in Fig. 3.
The activity increased proportionally with the concentration and we noted the most important activity of samples on the two kinds of cells at 500 µg mL−1. All organs present an interesting cytotoxic activity on Hela cells more than on A 549 cells which could suggest a specificity of action toward cells’ type.
The best inhibition % on Hela cells was shown with essential oils from stems (79.05 %) and (77.52 %) at the concentrations of 500 and 250 µg mL−1, respectively. The flowers’ essential oils present 74.89 % of inhibition % on Hela’s cells. These results present the first report on the cytotoxic activity of F. communis essential oil.
Conclusions
In conclusion, we have identified the essential oils of different organs (flowers, leaves, stems, and roots), also we have shown that these oils and their associated antioxidants, antimicrobial, anticholinesterase, and cytotoxic possess interested potential to develop a chemopreventive agent against various diseases. These studies have shown a relationship between results which could be also related to the presence of the same sesquiterpene in the flowers, stems and leaves of essential oils of F. communis.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing, Carol Stream IL
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Abrash ASA, Al-Quobaili FA, Al-Akhras GN (2000) Catalase evaluation in different human diseases associated with oxidative stress. Saudi Med J 21(9):826–830
Appendino G, Cravotto G, Sterner O, Ballero M (2001) Oxygenated sesquiterpenoids from a nonpoisonous Sardinian chemotype of giant fennel (Ferula communis). J Nat Prod 64:393–395
Aragno M, Tagliapietra S, Nano GM, Ugazia G (1988) Experimental studies on the toxicity of Ferula communis in the rat. Res Commun Chem Pathol Pharmacol 59:399–402
Asili J, Sahebkar A, Fazly Bazzaz BS, Sharifi S, Iranshahi M (2009) Identification of essential oil components of Ferula badrakema fruits by GC–MS and 13C-NMR methods and evaluation of its antimicrobial activity. J Essent Oil Bear Plants 12:7–15
Bellakhdar J (1978) Ferula communis L. In: Tech. Nord-Africaine (ed) Médecine Traditionnelle et Toxicologie Ouest Sahariennes. Tech. Nord-Africaine, Rabat, pp 287–288
Chalchat JC, Garry RP (1997) Correlation between chemical composition and antimicrobial activity. VI. Activity of some african essential oils. J Essent Oil Res 9:67–75
Davies NW (1990) Gas chromatographic retention indexes of monoterpenes and sesquiterpenes on methyl silicone and carbowax 20 M phases. J Chromatogr 503:1–24
Dehghan G, Solaimanian R, Shahverdi AR, Amin G, Mo Abdollahi, Shafiee A (2007) Chemical composition and antimicrobial activity of essential oil of Ferula szovitsiana DC. Flavour Fragr J 22:224–227
Del-Vechio-Vieira G, Sousa OV, Yamamoto CH, Kaplan MAC (2009) Chemical composition and antimicrobial activity of the essential oils of Ageratum fastigiatum (Asteraceae). Rec Nat Prod 3(1):52–57
Ellman GL, Courtney KD, Andres V, Featherstone M (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90
Ferrari B, Tomi F, Casanova JJ (2005) Composition and chemical variability of Ferula communis essential oil from Corsica. Flavour Fragr J 20:180–185
Ghannadi A, Amree S (2002) Volatile oil constituents of Ferula gummosa Boiss. From Kashan, Iran. Essent Oil Res J 14:420–421
Ghannadi A, Sajjadi SE, Beigihasan A (2002) Composition of the essential oil of Ferula ovina (Boiss.) Boiss. From Iran. Daru 10:164–167
Goryaev MI, Sharipova FS, Tikhonova LK, Elchibekova LA (1968) Components of essential oils, XXXI. Essential oil of Ferula penninervis (Stalks). Russ J Appl Chem (Zhurnal Prikladnoi Khimii) 41:2745–2750
Halliwell B, Gutteridge JMC (1999) The chemistry of the free radicals and related reactive species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine, 3rd edn. Oxford Science Publications, Oxford, pp 36–104
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root; their relative astringency and radical scavenging effects. J Chem Pharm Bull 36:2090–2097
Jabrane A, Ben Jannet H, Mighri Z, Mirjolet JF, Duchamp O, Harzallah-Skhiri F, Lacaille-Dubois MA (2010a) Two new sesquiterpene derivatives from the tunisian endemic Ferula tunetana Pom. J Chem Biodivers 7:392–399
Jabrane A, BenJannet H, Mastouri M, Mighri Z, Casanova J (2010b) Chemical composition and in vitro evaluation of antioxidant and antibacterial activities of the root oil of Ridolfia segetum (L.) Moris from Tunisia. J Nat Prod Res 24(6):491–499
Jennings W, Shibamoto T (1980) Qualitative analysis of favor and fragrance volatiles by glass capillary chromatography. Academic Press, New-York, p 472
Joshi SC, Verma AR, Mathela CS (2010) Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. J Chem Toxicol 48:37–40
Kapahi BK, Thappa RK, Aggarwal SG, Sarin YK (1985) Essential oil of Ferula jaesekheana Valtke. PAFAI J 7:23–24
Keskin M, Biçer O, Gül S, Can E (2004) A study on using Ferula communis (Chakshir) for oestrus synchronization in Shami (Damascus) goats under East-Mediterranean Condition of Turkey. In: Book of Abstract of the 55th Annual Meeting of the European Association for Animal Production. Bled, Slovenia, Theatre S3.6, p. 234
Kluge WH, Kluge HH, Bauer HI, Pietsch S, Anders J, Venbrocks RA (2001) Acetylcholinesterase assay for cerebrospinal fluid using bupivacaine to inhibit butyrylcholinesterase. BMC Biochem 2:1–8
Kose ED, Aktas O, Deniz IG, Sarikurkcu C (2010) Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J Med Plants Res 4:1698–1703
Maggi F, Cecchini C, Cresci A, Coman MM, Tirillini B, Sagratini G, Papa F (2009) Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy). Fitoterapia 80:68–72
Manolakou S, Tzakou O, Yannitsaros A (2013) Volatile constituents of Ferula communis L. subsp. communis growing spontaneously in Greece. Rec Nat Prod 7(1):54–58
Marongiu B, Piras A, Porcedda S (2005) Comparative analysis of the oil and supercritical CO2 extract of Ferula communis L. J Essent Oil Res 17:150–152
Massada Y (1976) Analysis of essential oils by gas chrotography and mass spectrometry. Hirokawa Publication, Tokyo, pp 43–285
Nagatsu A, Isaka K, Kojima K, Ondognii P, Zevgeegiin O, Gombosurengyin P, Davgiin K, Irfan B, Iquibal CM, Ggihara Y (2002) New sesquiterpenes from Ferula ferulaeoids (stard.) korovin.VI. Isolation and identification of three new dihyrofuro [2,3-b] chromones. Chem Pharm Bull 50(5):675–677
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Nicholas Au, Rettie AE (2008) Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev 40(2):355–375
Orhan I, Kartal M, Kan Y, Sener B (2008) Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z Naturforsch C 63:547–553
Oyaizu M (1986) Studies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rubiolo P, Matteodo M, Riccio G, Ballero M, Christen P, Fleury-Souverain S, Veuthey JL, Bicchi C (2006) Analytical discrimination of poisonous and non poisonous chemotypes of giant fennel (Ferula communis L.) through their biologically active and volatile fractions. Agric Food Chem J 54:7556–7563
Rustaiyan A, Monfared A, Masoudi S (2001a) The essential oil of Ferula flabelliloba Rech. F. Essent Oil Res J 13:403–404
Rustaiyan A, Assadian F, Monfared A, Masoudi S, Yari M (2001b) Composition of the volatile oil of Ferula stenocarpa Boiss & Hausskn. Essent Oil Res J 13:181–182
Sayyah M, Kamalinejad M, Hidage RB, Rustaiyan A (2001) Antiepileptic potential and composition of the fruit essential oil of Ferula gummosa boiss. Iran Biomed J 5:69–72
Smail A, Lyoussi B, Miguel MG (2011) Antioxidant and anticholinesterase activities of some commercial essential oils and their major compounds. Molecules 16:7672–7690
Stenhagen E, Abrahamsson S, McLafferty FW (1974) Registry of mass spectral data. Wiley, New York
Stravroula M, Olga T, Artemios Y (2013) Volatile constituents of Ferula communis L. subsp. communis growing spontaneously. Rec Nat Prod J 7:54–58
Swigar AA, Silverstein RM (1981) Monoterpenes. Aldrich Chem Comp, Milwaukee
Tsai JY, Lee MJ, Dah-Tsyr Chang M, Huang H (2014) The effect of catalase on migration and invasion of lung cancer cells by regulating the activities of cathepsin S, L, and K. Exp Cell Res 323(1):28–40
Valle MG, Appendino G, Nano GM, Picci V (1987) Prenylated coumarins and sesquiterpenoids from F. communis. Phytochemistry 26:253–256
Yan R, Yang Y, Zeng Y (2009) Cytotoxicity and antibacterial activity of Lindera strychnifolia essential oils and extracts. Ethnopharmacol J 121:451–455
Yu F, Haradab H, Yamasakia K, Okamotoa S, Hirasec S, Tanakac Y, Misawab N, Utsumia R (2008) Isolation and functional characterization of a β-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett 582:565–572
Znati M, Jabrane A, Hajlaoui H, Harzallah-Skhiri F, Bouajila J, Casanova J, Ben Jannet H (2012) Chemical composition and in vitro evaluation of antimicrobial and anti-acetylcholinesterase properties of the flower oil of Ferula lutea. Nat Prod Commun 7(7):947–950
Acknowledgments
Thanks are due to Dr. Fethia Harzallah SKHIRI (Higher Institute of Biotechnology of Monastir, Tunisia) for the botanical identification, and Dr. Ali OTHMANE (Biophysics Laboratory, Faculty of Medicine, Monastir), and Dr. Maha MASTOURI (Laboratory of Microbiologie, Fattouma BOURGUIBA Hospital, Monastir, Tunisia) for their help in performing the cytotoxic and antimicrobial tests, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nguir, A., Mabrouk, H., Douki, W. et al. Chemical composition and bioactivities of the essential oil from different organs of Ferula communis L. growing in Tunisia. Med Chem Res 25, 515–525 (2016). https://doi.org/10.1007/s00044-016-1506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1506-1