Abstract
The study was aimed at exploring cytotoxic activity of Oldenlandia umbellata and its chemical constituents. Cell viability assay of crude methanolic extract of aerial parts of O. umbellata (HUM), its ether soluble fraction (HUM-E) and butanol soluble fraction (HUM-B) against colon cancer HT-29, lung epithelial A549 and breast adenocarcinoma MDA-MB-231 cell lines showed HUM-E to be significantly cytotoxic with IC50 values of 25.7, 67.7 and 69.3 μg/mL, against HT-29, A549 and MDA-MB-231, respectively. Chemical investigation of HUM-E and HUM-B resulted in the isolation of a novel symmetrical coumarin dimer named oledicoumarin (1), together with eleven known compounds, hedyotiscone B (2), cedrelopsin (3), pheophorbide A methyl ester (4), deacetyl asperuloside (5), scandoside methyl ester (6), asperulosidic acid (7), scandoside (8), nicotinic acid (9), 6α-hydroxy geniposide (10) anthragallol 1,2-dimethyl ether (11) and anthragallol 1,3-dimethyl ether (12). All compounds were isolated for the first time from O. umbellata except anthragallols. This is the foremost report exploring the presence of coumarin derivatives in O. umbellata. Testing of cytotoxicity of isolated constituents revealed that compounds 3, 4, 11 and 12 showed significant inhibition against A549 cells with IC50 values of 3.6–5.9 μg/mL. Compounds 4, 11 and 12 showed marked inhibitory effect against MDA-MB-231 cells (IC50 3.6–9.1 μg/mL). Compounds 4 (IC50 1.7 μg/mL) and 7 (IC50 6.1 μg/mL) were highly active against HT-29 cells. In summary, the less polar fraction of O. umbellata and its constituents were found to be cytotoxic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to WHO report, annual cancer cases are expected to rise from 14 million in 2012 to 22 million within the next two decades (Ferlay et al., 2014). Among the different types of cancers, lung, liver, colorectal and breast cancers have been identified as the most frequent cause of cancer deaths each year (GLOBOCAN 2012). Hence, there is a necessity for diverse chemical leads which control or stopover the growth of cancer cells.
Some species of the genus Oldenlandia of the family Rubiaceae have shown remarkable anticancer effect. Oldenlandia diffusa (syn. Hedyotis diffusa) is used clinically as an anticancer herb owing to negligible side effects at a dose of 30–60 g/day and included in about 15 % of Chinese anticancer herbal formula (Shao et al., 2011). Oldenlandia corymbosa (syn. Hedyotis corymbosa) is another interesting species exhibiting significant anticancer activity on human leukemia cells K562 and human breast carcinoma-dependent hormone cells MCF-7 (Sivaprakasam et al., 2014).
O. umbellata (syn. Hedyotis umbellata L.), commonly known as Indian madder or Chay root, is widely grown in India, Ceylon, Burma, Pakistan and west Tropical Africa. The leaves and roots of this annual plant are used as expectorant. In the Indian Siddha system of medicine, O. umbellata is extensively used in the treatment of tuberculosis, haemoptysis, bronchitis and asthma (Yoganarasimhan 2000). Pharmacological studies on this plant have proved antitussive (Hema et al., 2007), hepatoprotective and antioxidant (Malaya et al., 2007), antibacterial (Rekha et al., 2006), anti-inflammatory and antipyretic activities (Padhy and Endale 2014). Recently, the ethanolic and aqueous extracts of O. umbellata whole plant have been shown to decrease tumor growth (Sethuramani et al., 2014). Phytochemical review of O. umbellata explored the presence of anthraquinones derivatives (Purushothaman et al., 1968, Ramamoorthy et al., 2009), ursolic acid and kaempferol-3-O-rutinoside (Hema et al., 2009).
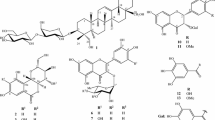
The present paper describes the cytotoxic effect of aerial parts of O. umbellata followed by isolation and structure elucidation of a novel symmetrical coumarin dimer named oledicoumarin (1), besides eleven known compounds, hedyotiscone B (2), cedrelopsin (3), pheophorbide A methyl ester (4), deacetyl asperuloside (5), scandoside methyl ester (6), asperulosidic acid (7), scandoside (8), nicotinic acid (9), 6α-hydroxy geniposide (10), anthragallol 1,2-dimethyl ether (11) and anthragallol 1,3-dimethyl ether (12) (Fig. 1). Also, in vitro cytotoxicity of compounds 3–7, 11 and 12 against human lung epithelial A549, breast adenocarcinoma MDA-MB-231 and colon cancer HT-29 tumor cell lines was tested. This is the first report of cytotoxic studies of O. umbellata extract and its constituents on these cancer cell lines.
Materials and methods
General experimental procedures
NMR spectra were recorded on a Bruker DRX-500 spectrometer in CDCl3 or CD3OD sol. Tetramethylsilane (δ 0.00) was used as an internal standard for 1H NMR shifts, and CDCl3 (δ 77.00) was used as a reference for 13C NMR shifts. Signals of CD2HOD (δ 3.30) and CD3OD (δ 49.00) were used as references for 1H and 13C NMR shifts in the spectra recorded in CD3OD. Optical rotations were measured on a JASCO P-2200 polarimeter. IR spectra were recorded on a JASCO-FT/IR-5300 spectrometer. HRFABMS spectra were measured using a JEOL JMS-700 mass spectrometer. Column chromatography (CC) was performed using silica gel (60–120 μm, spherical neutral, Merck Specialties Private Ltd., Mumbai, India) and Diaion HP-20 (250 μm, Sorbent Technologies, Norcross GA, USA). HPLC separation was performed on a LC 8A instrument (Shimadzu, Japan) equipped with a UV detector (monitored at 254 nm) using a preparative column (Luna, 5 µM, C18 (2), 100 Å, 250 × 21.2 mm). TLC and p-TLC were performed with silica gel 60 F 254 pre-coated glass plates (0.25 mm thickness, Kanto Kagaku, Japan). Cell viability was recorded on a multi-well plate reader (Spectra Max® M4, Molecular Devices, USA). MTT (Sigma-Aldrich, Bangalore, India), phosphate buffered saline (PBS), Rosewell Park Memorial Institute (RPMI) medium and fetal bovine serum (FBS) (Gibco BRL, CA, USA) were purchased from the supplier indicated. Cancer cell lines were procured from National Centre for Cell Science, Pune, India. All the other chemicals and reagents used were of analytical and molecular biology grade.
Plant material
The aerial parts of O. umbellata were collected from Virudunagar District, Tamilnadu, India, during December–January and authenticated by Dr. Chelladurai, Botanical Survey of India. A voucher specimen (HU/2012/12-1) was deposited at Department of Pharmacy, BITS-Pilani Hyderabad Campus, Telangana State, India.
Extraction and isolation
Dried and ground aerial parts of O. umbellata (5.0 kg) were extracted using MeOH under heating at 45–50 °C. The methanolic extract was evaporated under reduced pressure to a dry residue (442 g), and around 440 g was suspended in water and subjected to solvent–solvent partition using 3 × 1.5 L of diethyl ether, and then using 3 × 1.5 L of butanol. The diethyl ether and butanol soluble fractions were evaporated under vacuum and lyophilized to yield dry residues, HUM-E (102 g) and HUM-B (108 g), respectively. Around 96 g of HUM-E was chromatographed on silica gel with solvents of increasing polarity (hexane–toluene, toluene–EtOAc and EtOAc–MeOH). The fractions eluted with toluene–EtOAc (1:1) (51 g) were rechromatographed on silica gel with toluene–EtOAc. Based on the TLC pattern, the fractions eluted with toluene–EtOAc (70:30) were combined and evaporated to give a residue (12.6 g), which was then divided into part A (5.6 g) and part B (7.0 g). Repeated chromatography of part A on silica gel followed by Diaion HP-20 elution with MeOH yielded fractions enriched with compound 1 (3.5 mg). Purification by p-TLC (developed with CHCl3–MeOH (20:1), R f 0.38) gave 1 (1.5 mg) as amorphous solid.
Part B of the residue was rinsed with hexane, and the insoluble residue was treated with EtOAc. The EtOAc soluble portion (2 g) was chromatographed on silica gel with hexane–EtOAc. Elution with hexane–EtOAc (90:10) afforded compound 11 (31 mg), ursolic acid (26 mg) and fractions containing compounds 12 and 2. Further elution with hexane–EtOAc (88:12) gave compound 3 (10 mg) and elution with hexane–EtOAc (75:25) furnished compound 4 (6 mg). The fraction containing 12 was further chromatographed on silica gel with hexane–EtOAc (80:20) to yield compound 12 (5.3 mg). The fraction containing 2 was purified by p-HPLC using a gradient elution of H2O–MeCN (95:05–15:85, 4.0 mL/min) to give compound 2 (1.9 mg).
Around 105 g of n-butanol fraction, i.e., HUM-B was chromatographed on HP-20 Diaion resin column (# 250 μ, 600 g, H2O) and eluted using H2O. About 30 fractions each to a volume of 100 ml were collected. Fractions 3–12 were lyophilized, treated with MeOH and filtered. The filtrate was evaporated under reduced pressure to yield a dry residue (23 g), which was then chromatographed over silica gel (# 100–200, 150 g) using CHCl3 and MeOH as eluent. Early fractions of CHCl3 and MeOH (90:10) eluate (7.5 g) were subjected for prep-HPLC (Luna 5 µM, C18 (2), 100 Å, 250 × 21.2 mm, 10 ml/min) purification using stepwise gradient elution of 0.05 % TFA in H2O and MeCN. Fine purification of compounds (2 g) eluted between 18 and 24 min (MeCN 68–79 %) by rechromatography over silica gel column (# 230–400, 12 g) using gradient elution with EtOAc and MeOH (5–50 % MeOH) mobile phase yielded compounds 5 (6.0 mg), 6 (4.7 mg), 7 (4.0 mg) and 8 (4.1 mg) in series. Flash chromatographic purification of later fractions of CHCl3 and MeOH (90:10) eluate (1.14 g) using silica gel (# 230–400, CHCl3 to MeOH) yielded compound 9 (1.9 mg).
Compound 10 was obtained from CHCl3 and MeOH (80:20) eluate (2.62 g) using flash chromatography (Silica gel, # 230–400, 12 g) with an increasing polarity of MeOH in CHCl3. Eluates of CHCl3:MeOH (20:80) were further purified by prep-TLC (Silica gel GF254, CHCl3:MeOH (8:2), R f: 0.39) to afford 10 (2.2 mg). The purity of isolated compounds was verified using TLC and HPLC–PDA analysis.
8,8′-(1,2-Dimethylcyclobutane-1,2-diyl)bis(6-methoxy-2H-furo[2,3-h]chromen-2-one) ( 1 )
Amorphous powder, [α] 25D = 0 (c = 0.07, CHCl3), UV λ max (MeOH) nm (log ε): 260 (4.28), 307 (3.82) nm, IR (CHCl3) ν max 1722, 1581, 1400, 1330 cm−1. 1H and 13C NMR data (500 and 125 MHz, CDCl3), see Table 1. Positive-ion mode HRFABMS: m/z 513.1469 [M + H]+ (calcd. for C30H25O8: 513.1450).
6-Hydroxy-8-(prop-1-en-2-yl)-8,9-dihydro-2H-furo-[2,3-h]chromen-2-one ( 2 )
Yellow amorphous solid, m.p. 150–152 °C. 1H NMR (500 MHz, CD3OD): δ 7.79 (1H, d, J = 9.5 Hz, H-4), 6.90 (1H, s, H-5), 6.18 (1H, d, J = 9.5 Hz, H-3), 5.45 (1H, dd, J = 9.3, 7.9 Hz, H-2′), 5.14 (1H, s, H-4′a), 4.95 (1H, brs, H-4′b), 3.56 (1H, dd, J = 15.9, 9.3 Hz, H-1′a), 3.17 (1H, dd, J = 15.9, 7.9 Hz, H-1′b), 1.80 (3H, s, H3-5′). 13C NMR (CD3OD): δ 163.7 (C, C-2), 153.6 (C, C-7), 146.3 (C, C-8a), 146.3 (CH, C-4), 144.9 (C, C-6), 140.5 (C, C-3′), 115.5 (C, C-8), 114.6 (C, C-4a), 114.5 (CH, C-3), 113.0 (CH2, C-4′), 112.6 (CH, C-5), 89.4 (CH, C-2′), 32.8 (CH2, C-1′), 17.0 (CH3, C-5′). Positive-ion mode FABMS: m/z 245 [M + H]+. The physical and spectral data were in agreement with those reported in the literature (Chen et al., 2006).
7-Hydroxy-8-(3-methylbut-2-en-1-yl)-6-methoxy-2H-chromen-2-one ( 3 )
Yellow amorphous solid, m.p. 171–172 °C. 1H NMR (500 MHz, CDCl3): δ 7.58 (1H, d, J = 9.5 Hz, H-4), 6.72 (1H, s, H-5), 6.25 (1H, d, J = 9.5 Hz, H-3), 6.20 (1H, s, 7-OH), 5.29 (1H, brt, J = 7.5 Hz, H-2′), 3.94 (3H, s, O–CH3), 3.57 (2H, d, J = 7.5 Hz, CH2-1′), 1.85 (3H, s, H3-4′), 1.68 (3H, s, H3-5′). 13C NMR (125 MHz, CDCl3): δ 161.6 (C, C-2), 148.4 (C, C-8a), 147.4 (C, C-7), 143.7 (CH, C-4), 143.7 (C, C-6), 133.2 (C, C-3′), 120.7 (CH, C-2′), 116.2 (C, C-8), 113.1 (CH, C-3), 111.2 (C, C-4a), 105.1 (CH, C-5), 56.3 (CH3, O–CH3), 25.8 (CH3, C-4′), 22.2 (CH2, C-1′), 18.0 (CH3, C-5′). Positive-ion mode FABMS: m/z 261 [M + H]+. The physical and spectral data were in accordance with those reported in the literature (Simonsen et al., 2004), although the 1H and 13C NMR data reported in the paper (Patre et al., 2011) contain several errors.
3-Hydroxy-1,2-dimethoxyanthracene-9,10-dione ( 11 )
Yellow needles, m.p. 227–230 °C. 1H NMR (500 MHz, CDCl3): δ 8.26 (1H, brd, J = 7.5 Hz, H-5 or H-8), 8.22 (1H, brd, J = 7.5 Hz, H-8 or H-5), 7.78 (1H, brt, J = 7.5 Hz, H-6 or H-7), 7.72 (1H, brt, J = 7.5 Hz, H-7 or H-6), 7.72 (1H, s, H-4), 6.38 (1H, s, 3-OH), 4.12 (3H, s, 1-OCH3), 4.00 (3H, s, 2-OCH3). 13C NMR (125 MHz, CDCl3): δ 182.5, 181.6 (C each, C-9 and C-10), 154.0, 153.8 (C each, C-1 and C-3), 145.5 (C, C-2), 135.0 (C, C-12), 134.1, 133.3 (CH each, C-6 and C-7), 132.6, 131.6 (C each, C-8a and C-10a), 127.1, 126.7 (CH each, C-5 and C-8), 120.9 (C, C-9a), 110.4 (CH, C-4), 61.7 (CH3, 1-OCH3), 61.6 (CH3, 2-OCH3). Positive-ion mode FABMS: m/z 285 [M + H]+. The physical and spectral data were in accordance with those reported in the literature (Zhu et al., 2009, Wijnsma et al., 1984), although no 13C NMR data have been reported.
Cell culture and maintenance
Cells were grown in RPMI-1640 supplemented with 10 % heat inactivated FBS, 100 IU/mL penicillin, 100 mg/ml streptomycin and 2 mM l-glutamine. Cultures were maintained in a humidified atmosphere with 5 % CO2 at 37 °C. The cultured cells were subcultured twice a week, seeding at a density of about 2 × 103 cells/mL.
In vitro cytotoxic assay
HUM, HUM-E, HUM-B and the isolated compounds 3–7, 11 and 12 were evaluated for cytotoxic activities on three human cell lines (A549, MDA-MB-231 and HT-29) using MTT method. Briefly, a limited number of cancer cells (5000/well) were seeded onto a 96-well microplate and became attached to the bottom of the well overnight. On the second day of the procedure, 50 µL of new medium containing the test substances dissolved in DMSO was added. After an incubation period of 72 h, the living cells were assayed by the addition of 15 µL of 5 mg/mL MTT solution. After 4 h incubation at 37 °C, the medium was removed and the precipitated formazan was dissolved in 150 µL of DMSO. Finally, the reduced MTT was assayed at 545 nm, using a microplate reader. Untreated cells were taken as the negative control, and 5-fluorouracil (Sigma-Aldrich, Bangalore, India) was used as a positive standard. All cell lines were procured from National Centre for Cell Science (Pune, India). All concentrations of the tested compounds were assayed in triplicates and the IC50 value of each compound was calculated by GraphPad Prism 5.0 (GraphPad Software; San Diego, CA, USA).
Results and discussion
In vitro cytotoxicity of O. umbellata extract against cancer cell lines
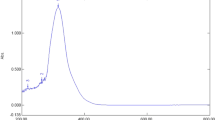
The crude methanolic extract (HUM) was divided into ether soluble less polar fraction (HUM-E) and butanol soluble polar fraction (HUM-B) and were tested against a panel of cell lines (A549, MDA-MB-231 and HT-29) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. HUM-E showed dose-dependent effect against HT-29, A549 and MDA-MB-231 with IC50 values of 25.7, 67.7 and 69.3 μg/mL, respectively, after 72 h (Fig. 2). On the contrary, HUM and HUM-B were less active against MDA-MB-231 (7 and 18.4 % growth inhibition, respectively, at 200 μg/mL) and not cytotoxic against HT-29 and A549.
Isolation and characterization of the constituents of HUM-E
Chromatographic separation of HUM-E afforded six compounds 1–4, 11 and 12. A novel compound, named as oledicoumarin (1), was obtained as amorphous solid and its molecular formula was determined to be C30H24O8 by positive HRFABMS (m/z 513.1469 [M + H]+), requiring 19° of unsaturation. The IR spectrum showed the presence of carbonyl (1722 cm−1) and aromatic (1581 cm−1) groups. Signals of 15 carbons and 12 hydrogens in the NMR spectra (Table 1) suggested a symmetrical nature of 1. Interpretation of the HMBC correlations (Fig. 3) led to the assignment of the 6-methoxy-furocoumarin substructure (Fig. 1). The presence of NOE correlations between H-4 (δ 7.75) and H-5 (δ 6.77) and between H-5 and methoxy methyl (δ 4.09) further supported the angular type furo-coumarin substructure. The remaining three carbon residue, C-3′, C-4′ and C-5′, were found to be quaternary, methylene and methyl carbons, respectively, by DEPT spectrum. Further analysis of HMBC correlations and requirement of one more unsaturation in the molecule necessitated to make a cyclobutane ring by connecting the quaternary carbon to the other quaternary carbon (head-to-head dimeric structure) or to the methylene carbon. The head-to-head dimeric structure was assigned for 1, because the coupling pattern of C-4′ methylene protons were not AA′ type. The relative and absolute configurations at both C-3′ chiral centers were determined based on a structurally related cyclobutane, ligulacephalin A, which had been isolated as a racemic mixture previously (Toyoda et al., 2005). Close similarity of the 13C shifts of C-2′, C-3′, C-4′ and C-5′ (δ 164.1, 45.7, 27.1 and 23.0, respectively, in CDCl3) of 1 with the respective signals of ligulacephalin A (δ 163.4, 46.8, 27.9 and 23.5 in CD3OD) gave evidence that 1 has the same relative stereochemistry (S,S/R,R configuration) as ligulacephalin A rather than S,R configuration (meso-form). Compound 1 was optically inactive. Thus, oledicoumarin was determined to be 8,8′-(1,2-dimethylcyclobutane-1,2-diyl)bis(6-methoxy-2H-furo[2,3-h]chromen-2-one) as shown in Fig. 1. Compound 1 is suggested to be a racemic mixture. Oledicoumarin could be formed from a putative precursor, 2′-isopropenyl-6-methoxyfurocoumarin via [2 + 2], cycloaddition without participation of enzymes, as previously suggested for ligulacephalin A.
The more polar HUM-B fraction on chromatographic purification yielded five iridoid glycosides (5-8, 10) and nicotinic acid (9). In all, the isolated compounds were identified as hedyotiscone B (2) (Chen et al., 2006), cedrelopsin (3) (Patre et al., 2011, Simonsen et al., 2004), pheophorbide A methyl ester (4) (Rho et al., 2003), deacetyl asperuloside (5) (Lopes et al., 2004), scandoside methyl ester (feretoside) (6) (Guvenalp et al., 2006), asperulosidic acid (7) (Kamiya et al., 2002), scandoside (8) (Kamiya et al., 2002), nicotinic acid (9) (Mukaiyama and Funasaka 2007), deacetylasperulosidic acid methyl ester (6α-hydroxy geniposide) (10) (Kamiya et al., 2002), anthragallol 1,2-dimethyl ether (11) (Zhu et al., 2009) and anthragallol 1,3-dimethyl ether (12) (Banthorpe and White 1995, Fraga et al., 2009) by comparison of their spectroscopic data with those published in literature. The 1H and 13C NMR data of 3 recorded in CDCl3, which was assigned by 2D-NMR including HMBC spectrum, are also presented here since the reported data recorded in the same solvent were somewhat inconsistent with ours. Although compound 11 is known, its 13C NMR data are not found in the literature and hence are reported in the present paper.
Compounds 1–10 were isolated from O. umbellata for the first time. The prenyl coumarin, cedrelopsin (8-(3-methyl-2-butenyl)-7-hydroxy-6-methoxycoumarin) (3) and pheophorbide A methyl ester (4) were isolated for the first time from Oldenlandia genus. Notably, O. umbellata became a second natural source for a dihyrofurocoumarin derivative, hedyotiscone B (2) (Chen et al., 2006). The study also unveiled the occurrence of iridoid glycosides 5–10 in O. umbellata which was earlier unknown. Also the study uncovered the presence of some unique constituents such as oledicoumarin (1), hedyotiscone B (2), cedrelopsin (3) and pheophorbide A methyl ester (4) in O. umbellata, which were not identified in its congener such as O. diffusa and O. corymbosa.
In vitro cytotoxicity of the isolated compounds
Compounds 3–7, 11 and 12 were assayed for in vitro cytotoxicity against same set of cancer cell lines, and the results are presented in Table 2. The purity of selected compounds was verified using RP-HPLC–PDA analysis before the assay. Compounds 1, 2 and 8–10 were not tested as they were obtained only in small quantity.
Cedrelopsin (3) showed potent cytotoxicity against A549 cells with IC50 value of 3.7 μM, but was much less toxic against MDA-MB-231 and HT-29 cell lines (IC50 > 100 μM). While the cell viability of HT-29 cells was reduced significantly by compound 7 (IC50 6.1 µg/mL), it was not so by compound 5 and 6. Cytotoxic results of tested iridoids (5, 6 and 7) suggested that the activity is tumor specific, as no toxicity was observed against A549 and MDA-MB-231 cells up to 100 μM. Hence, presence of –COOH group at C-4 and esterification of hydroxyl group at C-10 in the iridoid nucleus might be crucial for the demonstrated antiproliferative effect. Anthragallol 1,2-dimethyl ether (11) and anthragallol 1,3-dimethyl ether (12) expressed similar trends of tumor specificity, being cytotoxic against A549 and MDA-MB-231 cells, but having negligible activity against HT-29 (IC50 > 100 μM).
Compound 4 exhibited the most strong cytotoxicity against all three cell lines with IC50 values of 3.6 (A549), 3.6 (MDA-MB-231) and 1.7 µg/mL (HT-29). Although there are reports on the cytotoxic effects of pheophorbide A methyl ester (4) (Cheng et al., 2001), the study against HT-29 cells was found to be inadequate, i.e., tested only at three broad dose levels, i.e., 62.5, 125 and 250 μg/mL, (Sowemimo et al., 2012). Also in order to give chemical corroboration for the effect of HUM-E on HT-29 cells, MTT assay of pheophorbide A methyl ester (4) was performed with lower concentration levels which proved its potent activity (IC50 1.7 μg/mL). Another molecule responsible for the higher activity of HUM-E against HT-29 cells could be rationalized as ursolic acid, which was also tested and found to have IC50 value of 4.9 μg/mL.
Conclusions
The present study demonstrated cytotoxic effect of O. umbellata extract against three tumor cell lines, A549, MDA-MB-231 and HT-29. The difference in the inhibitory effect of ether fraction and n-butanol fraction proved that less polar constituents are accountable and imperative for cytotoxic effect. Further, diverse phytochemicals along with a novel compound, oledicoumarin (1), were isolated and characterized. The active principles responsible for the cytotoxicity of HUM-E fraction against tested cell lines were rationalized. Similarly the poor inhibitory property of HUM-B was also validated. The current report will add evidence supporting O. umbellata as one of the interesting species that is worthy of continuing anticancer research.
References
Banthorpe DV, White JJ (1995) Novel anthraquinones from undifferentiated cell cultures of Gallium verum. Phytochemistry 38:107–111
Chen YH, Chang FR, Wu CC, Yen MH, Liaw CC, Huang HC, Kuo YH, Wu YC (2006) New cytotoxic 6-oxygenated 8,9-dihydrofurocoumarins, Hedyotiscone A–C, from Hedyotis biflora. Planta Med 72:75–78
Cheng HH, Wang HK, Ito J, Bastow KF, Tachibana Y, Nakanishi Y, Xu Z, Luo TY, Lee KH (2001) Cytotoxic Pheophorbide related compounds from Clerodendrum calamitosum and C. cyrtophyllum. J Nat Prod 64:915–919
Ferlay J, Soerjomataram I, Dikshit R, Esr S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2014) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Fraga BM, Quintana N, Diaz CE (2009) Anthraquinones from natural and transformed roots of Plocama pendula. Chem Biodivers 6:182–192
GLOBOCAN (2012) Estimated cancer incidence, mortality, and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 15 Feb 2015
Guvenalp Z, Kilic N, Kazaz C, Kaya Y, Demirezer LO (2006) Chemical constituents of Galium tortumense. Turk J Chem 30:515–523
Hema V, Venkidesh R, Maheswari E (2007) Antitussive activity of Oldenlandia umbellata. Int J Chem Sci 5:2480–2484
Hema V, Vasudev A, Prameela RA (2009) Phytochemical screening of Oldenlandia unbellata. Int J Chem Sci 7:2096–2102
Kamiya K, Fujita Y, Saiki Y, Hanani E, Mansur U, Satake T (2002) Studies on the constituents of Indonesian Borreria latifolia. Heterocycles 56:537–544
Lopes S, Poser GLV, Kerber VA, Farias FM, Konrath EL, Moreno P, Sobral ME, Zuanazzi JAS, Henriques AT (2004) Taxonomic significance of alkaloids and iridoid glucosides in the tribe Psychotrieae (Rubiaceae). Biochem Sys Ecol 32:1187–1195
Malaya G, Mazumder UK, Thamilselvan V, Manikandan L, Senthilkumar GP, Suresh R, Kakotti BK (2007) Potential hepatoprotective effect and antioxidant role of methanol extract of Oldenlandia umbellata in carbon tetrachloride induced hepatotoxicity in wistar rats. Iran J Pharmacol Ther 6:5–9
Mukaiyama T, Funasaka S (2007) Pyridine-3-carboxylic anhydride (3-PCA): a versatile, practical, and inexpensive reagent for condensation reaction between carboxylic acids and alcohols. Chem Lett 36:326–327
Padhy IP, Endale A (2014) Evaluation of anti-inflammatory and anti-pyretic activity of Oldenlandia umbellata Linn. roots. Int J Pharm Healthc Res 02:12–14
Patre RE, Parameswaran PS, Tilve SG (2011) Synthesis of the naturally occurring prenylated coumarins balsamiferone and cedrelopsin by domino reactions. ARKIVOC 9:68–76
Purushothaman KK, Saradambal S, Narayanaswami V (1968) Isolation and identification of some anthraquinone derivatives from Oldenlandia umbellata. Leather Sci 15:49–51
Ramamoorthy S, Gaurav M, Rajesh D, Nawaz KF, Vijayakumar V, Rajasekaran C (2009) Characterization of novel pH indicator of natural dye Oldenlandia umbellata L. Nat Prod Res 23:1210–1217
Rekha S, Srinivasan V, Vasanth S, Gopal RH (2006) In vitro antibacterial activity of Hedyotis umbellata. Indian J Pharm Sci 68:236–238
Rho MC, Chung MY, Song HY, Kwon OE, Lee SW, Baek JA, Jeune KH, Kim K, Lee HS, Kim YK (2003) Pheophorbide A-methyl ester, Acyl-CoA: cholesterol acyltransferase inhibitor from Diospyros kaki. Arch Pharm Res 26:716–718
Sethuramani A, Jagadeesan M, Kavimani S (2014) Antitumor activity of ethanolic and aqueous extract of Oldenlandia umbellata and Oldenlandia corymbosa against Dalton’s Ascitic Lymphoma in mice. Int J Biol Pharm Res 5:150–155
Shao J, Gong G, Trombetta L (2011) An evidence based perspective of Hedyotis diffusa or Oldenlandia diffusa (spreading Hedyotis) for cancer patients. In: Cho WCS (ed) Evidence based anticancer material medica. Springer, Berlin, pp 179–192
Simonsen HT, Adsersen A, Bremner P, Heinrich M, Smitt UW, Jaroszewski JW (2004) Antifungal constituents of Melicope borbonica. Phytother Res 18:542–545
Sivaprakasam SSK, Karunakaran K, Subburaya U, Kuppusamy S, Subashini TS (2014) A Review on phytochemical and pharmacological profile of Hedyotis corymbosa Linn. Int J Pharm Sci Rev Res 26:320–324
Sowemimo A, Venter MVD, Baatjies L, Koekemoer T, Adesanya S, Lin W (2012) Cytotoxic compounds from the leaves of Combretum paniculatum Vent. Afr J Biotechnol 11:4631–4635
Toyoda K, Yaoita Y, Kikuchi M (2005) Three new dimeric benzofuran derivatives from the roots of Ligularia stenocephala MATSUM. et KOIDZ. Chem Pharm Bull 53:1555–1558
Wijnsma R, Verpoorte R, Mulder-Kieger T, Svendsena AB (1984) Anthraquinones in callus cultures of Cinchona ledgeriana. Phytochemistry 23:2307–2311
Yoganarasimhan SN (2000) Medicinal Plants of India. Vedams Books (P) Ltd, Bangalore
Zhu L, Li H, Liang Y, Wang X, Xie H, Zhang T, Ito Y (2009) Application of high-speed counter-current chromatography and preparative high-performance liquid chromatography mode for rapid isolation of anthraquinones from Morinda officinalis How. Sep Purif Technol 70:147–152
Acknowledgments
We thank Ms. Satsuki Itoh, Tokyo Institute of Technology, for her measurements of FABMS. Author S. Mahibalan thanks Council for Scientific and Industrial Research, New Delhi, India, for granting Senior Research Fellowship (F. No. 09/1026(0005)/2012). Authors also acknowledge Research Initiation Grant from BITS-Pilani Hyderabad, Telangana State, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have any conflicts of interest to declare.
Ethical statement
Prior approval from Institutional Biosafety Committee was obtained to carry out cytotoxicity study.
Rights and permissions
About this article
Cite this article
Mahibalan, S., Rao, P.C., Khan, R. et al. Cytotoxic constituents of Oldenlandia umbellata and isolation of a new symmetrical coumarin dimer. Med Chem Res 25, 466–472 (2016). https://doi.org/10.1007/s00044-015-1500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1500-z