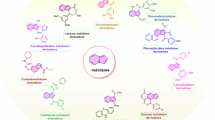
Abstract
Past few decades have witnessed the dawn of new diseases in which cancer is a major problem and the race ensued to eradicate cancer by charting out various effective therapeutic regimens. Circumventing resistance issues and combating the toxicity and selectivity problems are matter-of-concern in cancer treatment. Persistent failure to ensure complete remission and eradication of cancer instigated the researchers to exploit the strategies of combining pharmacophores as targeted therapeutic agents. Momentous improvement in the pharmacokinetic as well as pharmacodynamic profile resulting in the enhancement of bioavailability was seen with the introduction of these pharmacophores. The scope of molecular hybridization can be clearly exemplified through the US-FDA approved estramustine and others such as CUDC-101, CBLC-137, PLX3397, E-3810, and CUDC-907 that are currently in different phases of clinical trials. This review seeks to highlight and discuss anti-proliferative activity of some important hybrid, dual, and multi-targeted pharmacophores reported to date along with their designs, structure activity relationships, scope, and limitations. Further, an emphasis has been made to summarize US-FDA approved as well as drugs currently undergoing clinical trials of anticancer drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is not just a single disease but a group of multiple diseases characterized by inappropriately controlled cell proliferation and replication eventually resulting in disruption of normal physiology, metabolism, or structure (Abogye and Kaliszczak, 2012; Center, 2013; Hainaut and Plymoth, 2013; Hanahan and Weinberg, 2011; Patrick, 2009; Yance, 2010). It is one of the leading diseases claiming numerous lives and consequently responsible for high mortality rates across the globe (Siegel et al., 2012). Although a number of anticancer drugs are known, most of them have failed to yield the desired result. In most cases, the reason for such failure can be attributed to the lack of selectivity (Folger et al., 2011), less efficaciousness, more side effects (Zitvogel et al., 2013), and multi-drug resistance (MDR) (Daniel and Rauch, 2013). Various strategies have been adopted for combating above-mentioned problems via synergistic action and dose reduction involving either combining two pharmacophores, (2) using a dual pharmacophore in a single molecule, or (3) utilizing the multi-targeted pharmacophore approaches (Bolognesi, 2013). Hence, the effectiveness of such a single molecule having more than one pharmacophore in which each pharmacophore possesses a different mode of action is enhanced several folds (Descoteaux et al., 2012; Meunier, 2007; Morphy and Rankovic, 2005). The pharmacophore concept was introduced by Ehrlich (1909) and defined as, ‘a molecular framework that carries (phoros) the essential features responsible for a drug’s (pharmacon) biological activity’ (Yang, 2010). According to IUPAC, “a pharmacophore is a single molecule containing a group of steric, electrostatic and hydrophobic properties indispensable for supramolecular interactions with a biological receptor in order to modify or inhibit a biological effect” (Dietis et al., 2009; Wermuth et al., 1998). Moreover, it must obey the Lipinski’s rule of five (Zhang and Wilkinson, 2007), should be able to achieve stability in the effective conformation, contain functional groups (proton donor/acceptor, hydrophobic parts) in addition to possessing minimum three of pharmacophoric points (Lipinski, 2004; Proudfoot, 2005; Veber et al., 2002).
An exclusive review was put forward about the latest advances in design and development of hybrid anti-proliferative agents by Fortin and Berube. During the course of preparing the present manuscript, reviews by Nepali et al., Bansal et al., and Fortin et al., which covered the approaches and strategies on hybrids drugs were published (Bansal and Silakari, 2014; Fortin and Berube, 2013; Nepali et al., 2014). In 2007, Junior et al., (Viegas-Junior et al., 2007) and Gediya and Njar (Gediya and Njar, 2009) in 2009 also made successful attempts to compile a review on the design of hybrid anticancer agents. The present review is a concerted effort to bring altogether the different types of pharmacophores; dual/multi-targeted in addition to some important hybrid pharmacophores along with their designs, structure activity relationships, scope, and limitations. Further, an emphasis has been made to summarize US-FDA approved as well as drugs currently undergoing clinical trials of anticancer drug development.
Classification
In this review, we have organised the numerous anticancer agents based on the hybrid, dual, and multi-targeted designs employed for the association of the pharmacophores and the sites they target.
-
Class I: chimeric/hybrid pharmacophore designs
-
HDAC and c-Src inhibitor
-
Chlorambucil-testosterone hybrids
-
Estradiol-chlorambucil hybrids
-
Isoflavene-propranolol hybrids
-
Coumarin-stilbene hybrids
-
Resveratrol-coumarin hybrids
-
Isatin-benzothiazole hybrids
-
Class II: dual pharmacophore/inhibitors design
-
Dual aromatase steroid sulfatase inhibitors
-
MAHA and SAHA analogs
-
Isoindolo [2, 1-a] quinoxaline inhibitiors
-
Dual tubulin and Hsp 27 inhibitiors
-
Dual-acting HDAC-topoisomerase inhibitors
-
CUDC–907: a dual inhibitor of PI3 K and HDAC
-
HDAC and RTK dual inhibitors
-
Dual inhibitors of Raf 1 and JNK 1
-
Class III: multi-targeted Hybrids in cancer
-
Multi-targeted pharmacophores based on HDAC inhibitors
-
CUDC-101
Class I: chimeric/hybrid pharmacophore designs
Hybrid anticancer agents are of great therapeutic interest as they can potentially overcome most of the pharmacokinetic drawbacks encountered when using conventional anticancer drugs (Fortin and Berube, 2013). A chemical entity constituting two structural domains, depicting dissimilar action as an outcome of acting through different modes of actions are called as hybrid drugs, thus indicating that a hybrid molecule acts as two distinct pharmacophores. Hybrid anticancer drugs are designed on the basis of combining a haptophoric moiety or merging two or more drugs (Fortin and Berube, 2013; Hulsman et al., 2007).
Ko et al. recently revealed and synthesized the hybrid histone deacetylase (HDAC) inhibitor and c-Src inhibitor (non-receptor tyrosine kinase) (Ko et al., 2013). c-Src has been known to play a crucial role in regulating a number of cellular processes in cancer (Fan et al., 2013). The lower efficacy of c-Src inhibitors led the researchers to select an HDAC inhibitor which was seen to arrest and consequently result in apoptosis of cancer cells with fewer side effects. Further, an HDAC inhibitor, panobinostat, was found to have action synergistic with c-Src inhibition. The researchers found this chimeric inhibitor of c-Src and HDAC (Fig. 1) to be more efficacious in NCI-60 cell lines. Further, the downregulation of c-Src levels through suppression of Src transcription was observed to be brought about by HDAC inhibitors. Therefore, such a chimeric/hybrid inhibitor proved to be advantageous in which a single molecule could accommodate inhibitors against c-Src kinase as well as HDAC.
Androgens are not only found to be critical for the growth and development of male sexual organ and function but their role have also been highlighted in the prostate cancer (Boorjian and Tindall, 2013). Principal androgen in the blood is the testosterone (Morgentaler, 2006), whereas in the cells most potent androgen is the DHT (Wright et al., 1996). Increased levels of androgen receptor have been observed in prostate cancer cells (Lunardi et al., 2013). Androgen stimulates proliferation of prostate cells which in turn initiates the progression of malignant tumor (Chang et al., 2013; Zhang et al., 2013). In addition, androgens are also involved in hormone-dependent cancer progression which makes the use of androgen deprivation therapy in prostate cancer patients. Chlorambucil is a potent alkylating agent used in cancer chemotherapy but its side effects including bone marrow suppression, anemia, and weak immune system limits its use (Chabner and Longo, 2011; Denny, 2001). Aiming to explore better alternatives led researchers to the synthesis of hybrid pharmacophore of the hormonal drugs like testosterone with chlorambucil (Fig. 2) (Bastien et al., 2013). These exhibited potent inhibitory activity against the hormone-dependent LNCaP (androgen-sensitive human prostate adenocarcinoma cells derived from the left supraclavicular lymph node) cancer cell lines and PC3 cell lines.
Gupta et al., established a series of estradiol-chlorambucil hybrids (Fig. 3) which exhibited moderate to significant cytotoxic activity in hormone-dependent (MCF-7) as well as hormone-independent (MDA-MB-436 and MDA-MB-231) breast cancer cell lines (Gupta et al., 2010).
Coumarins are a group of naturally occurring compounds (Garcia-Beltran et al., 2014; Singer et al., 2003). They have myriad biological activities including antitumor effects exhibited by the inhibition of cellular proliferation (Ali, 2014; Croteau et al., 2000; Do et al., 2014). Also, stilbenes like resveratrol have been seen to play a crucial role in numerous biological activities especially in cancer (Khan et al., 2013; Kiselev, 2011) and anti-aging by initiating the sirtuin [silent mating type information regulation two homologue (SIRT1)] (Chakraborty, 2013). SIRT1 is an enzyme that deacetylates proteins that help in cellular regulation. The cytotoxic activities exhibited by such compounds may be attributed to their ability to inhibit cell cycle or to induce differentiation and apoptosis. It has also been observed that in physiological quantities, resveratrol possesses the ability to modulate multiple cellular pathways critical for tumorigenesis, DNA-synthesis, and various inflammatory responses (Tili and Michaille, 2011). The activities exhibited by these naturally occurring compounds prompted the researchers to synthesize molecules using them in combinations.
Belluti and coworkers synthesized and assessed the hybrid molecules comprising of coumarin scaffold and resveratrol (Fig. 4) for their potential as anti-proliferative agent against H460 lung carcinoma cells (Belluti et al., 2010). The synthesized analogs were also claimed to possess proapoptotic activity which could be attributed to their ability to arrest G2 phase and inhibition of G2/M transition of cell cycle resulting in activation of the apoptotic signals.
Xiao et al. synthesized novel coumarin-stilbene (Fig. 5) hybrid and tested them for their cytotoxic potential against a panel of cancer cell lines comprising of KB (derived from an epidermal carcinoma of the mouth), MCF-7, and MCF-7/ADR (Xiao et al., 2010).
Isatin (1H-Indole-2,3-dione), portraying potent anticancer activity, has been known to be well tolerated by humans (Pandeya et al., 2005). This further highlights the fact that combining together molecules possessing different bioactivities renders the final pharmacophore more active than the combining molecules. Isatin-benzothiazole hybrids (Fig. 6) can be used as a prototype of a new class of anti-breast cancer agents.
In 2009, Solomon and coworkers designed and synthesized isatin-benzothiazole analogs using the hybrid pharmacophore approach (Fig. 7). The anticancer activity was tested against a panel of human breast cancer cell lines-MDA-MB231, MDA-MB468, and MCF7 of which anticancer activity against the MCF7 cell lines was maximum (Solomon et al., 2009).
In 2010, the same research group synthesized a series of hybrid molecules comprising of 4-piperazinylquinoline based on isatin scaffold i.e., 4-piperazinylquinoline-isatin analogs, 4-piperazinylquinoline-isatin-thiosemicarbazone analogs, and isatin-thiosemicarbazone analogs (Solomon et al., 2010). These analogs were evaluated for their cytotoxic activity against human breast tumor cell lines-MDA-MB468 and MCF7 in addition to two non-cancer breast epithelial cell lines, 184B5 and MCF10A. 4-piperazinylquinoline-isatin analogs and 4-piperazinylquinoline-isatin-thiosemicarbazone analogs exhibited potent cytotoxic activity. They also designed and synthesized 4-piperazinylquinoline and isatin-based hybrid pharmacophore by merging 4-aminoquinolines with isatin moiety via molecular hybridization (Fig. 8). These analogs have shown potent activity against MDA-MB468 and MCF7 cell lines, and these analogs were also virtually screened. IC50 value against MDA-MB468 and MCF-7 cancer cell lines has been shown in the range of 10.34–40.36 µM (Solomon et al., 2010).
Isoflavones, naturally present in soy products, have been observed to reduce the prevalence of cancer especially that of breast and lung cancer (Mahoney et al., 2012; Messina and Barnes, 1991). Numerous bioactivities exhibited by natural isoflavones led to synthesis of their analogs with increased potency and enhanced bioavailability. Further, the synthetic isoflavene derivatives were also seen to exhibit significant anti-proliferative activity essentially against drug resistant ovarian cancer and prostate cancer at clinical level (Polynesia, 2012). Recently, non-selective β-blocker, propranolol commonly indicated in the treatment of cardiovascular diseases has been shown to enhance the anti-angiogenic and anti-proliferative properties (Ji et al., 2012). Yee et al., explored isoflavene-propranolol hybrids and also evaluated them for their viability assays against SHEP neuroblastoma and MDA-MB-231 breast adenocarcinoma cell lines (Fig. 9). In addition, hybrids were also seen to exhibit anti-angiogenic and anti-proliferative activities against human microvascular endothelial cell lines (HMEC-1). The potency of the final hybrid molecules was found to be superior as compared to individual compounds (Yee et al., 2013).
Recently, our research group synthesized and evaluated a series of hybrids of pyrazolopyrimidinones and imine against a panel of cancer cell lines. The compounds were found to inhibit cell growth at G2/M phase of the cell cycle through multiple mechanisms involving DNA damage, free radical scavenging, and topoisomerase-II inhibition (Fig. 10) (Baviskar et al., 2013).
Class II: dual pharmacophore/inhibitors design
Dual pharmacophore/inhibitors are a group of compounds comprising of one structural domain exhibiting dual action by acting through different modes of action but inhibiting both targets simultaneously (Park et al., 2008; Woo et al., 2003; Wood et al., 2005).
Aromatase inhibitors are mainly indicated in hormone-dependent breast cancer (HDBC). These were developed to inhibit the catalytic action of aromatase enzyme known to play a crucial role in the biosynthesis of estrogens involved in the conversion of androgens to estrogens (Amaral et al., 2013; Bachelot et al., 2012). Clinical application of these aromatase inhibitors for treating advanced
stages of HDBC is well founded, especially in suppressing estradiol levels in plasma to virtually undetectable concentrations (Hanamura et al., 2013). This reduction in the estradiol levels brought about by the aromatase inhibitors can be further accentuated by combining them with steroid sulfatase (STS) inhibitors (Fig. 11) (Nussbaumer and Billich, 2004; Suzuki et al., 2003). STS plays a central role in catalyzing the hydrolysis of steroid sulfates which is mainly responsible for estrogens in cancer and also brings about estrogenic stimulation of hormone-dependent breast tumors as a consequence of modulation of the 5-androstene-3α-17β-diol production (Stanway et al., 2007). Woo and research team merged aromatase inhibitor with STS inhibitor that led to improve the response of hormone-dependent breast tumors to endocrine therapy. This was the result of inhibition of the formation of estrone-3-sulfate in addition to other steroids. Design and synthesis of the dual aromatase steroid sulfatase inhibitor (DASI), possessing the ability to inhibit both the enzymes, resulted in the synthesis of YM511 (Woo et al., 2007, 2003).
Docking, homology modeling, and SAR of DASIs and their corresponding parent phenolic compounds were performed and analyzed by Favia and co researchers (Favia et al., 2006). In addition, DASIs was also docked, by Hernandez-Guzman et al. into the crystal structure of STS to gain insight into the interactions of the compound with the enzyme (Hernandez-Guzman et al., 2003).
Wood and research group proposed and synthesized new structural class of DASI obtained by introducing sulphamate moiety as the pharmacophore (Fig 12) (Wood et al., 2005).
Mycophenolic acid (MPA) (Fig 13) is an inhibitor of Inosine-5′-monophosphate dehydrogenase (IMPDH), employed largely in transplantation (Fukuda et al., 2011; Glander et al., 2012; Sintchak and Nimmesgern, 2000). This has recently shown to play a central role in cancer treatment as evident from the clinical trials undergoing in patients suffering from advanced multiple myeloma (Chen and Pankiewicz, 2007; Takebe et al., 2006). Moreover, inhibitors of IMPDH are accredited with significant ability of differentiation and apoptosis (Dun et al., 2013). On the other hand, SAHA (Suberoylanilide hydroxamic acid) (Gupta et al.) is the HDAC inhibitor that has been recently approved for the cutaneous T cell lymphoma treatment (Rangwala et al., 2012; Tan et al., 2010; Thurn et al., 2011). SAHA brings about alteration in gene transcription in addition to exerting antitumor effects as a consequence of apoptosis, differentiation, and inhibition of tumor angiogenesis (Marks, 2010).
Chen and coworkers carried out the synthesis of compounds exhibiting inhibition against both IMPDH and HDAC (Chen et al., 2013). Merging of MPA to SAHA resulted in the formation of mycophenolic hydroxamic acid which was observed to inhibit both IMPDH and HDAC. SAHA was further modified with moieties that could better interact with IMPDH, thus resulting in compound that could inhibit both IMPDH and HDAC to the same extent as above. Analogs of both MAHA and SAHA were seen to exhibit commendable activity as an anti-proliferative agent in addition to their potential to act as differentiation inducers (Fig 14).
In 2013, Su et al. evidenced that heat shock protein 27 (Hsp 27) and tubulin inhibitors brought about an increased expression of responses such as stress (Su et al., 2013) using SAR. Hsp 27 helps in the survival and inhibits caspase 3 activity through interaction with the pro-caspase 3, thus exhibiting anti-apoptotic activity. Although, it has a molecular weight of 27 kDa, it can form oligomeric complexes. Hsp27 overexpression results in hyperactive chaperone activity which contributes to drug resistance in cancer chemotherapy (Fig 15).
Topoisomerase-I enzyme is known to play a central role in relieving the torsional strain on DNA by cutting one strand of the DNA double helix and passing one strand over the other (Wang, 1996). Inhibitors of topoisomerases bring about DNA strand breaks, cell cycle arrest, and apoptosis, all of which greatly impede replication, one of the important characteristic of cancer, in addition to these activities that would hinder the cell proliferation in cancer. Research supported the fact that topoisomerase I and HDAC inhibitors act synergistically promoting apoptosis in the cancer cells (Johnson et al., 2001; Marchion et al., 2004; Tsai et al., 2000) and both these inhibitors have been found to be concentrated in the nucleus to a higher therapeutic level (Chauhan and Kumar, 2013; Guerrant et al., 2012). On the similar lines, Guerrant et al. evaluated and synthesized dual inhibitors (Fig. 16) by merging together the compounds that possessed the ability to inhibit HDAC as well as topoisomerase I.
Further, Aboagye et al. described that CUDC-907 (N-Hydroxy-2-[[[2-(6-methoxy-3-pyridinyl)-4-(4-morpholinyl) thieno [3, 2-d] pyrimidin-6-yl] methyl] methylamino]-pyrimidinecarboxamide) (Fig. 17) has the ability to target both PI3 k and HDAC simultaneously.(Abogye and Kaliszczak, 2012) (Delcuve et al., 2013; Flinn et al., 2013; Pursell et al., 2013).
In 2013, Zhang et al. accounted the synthesis and evaluation of compounds that could inhibit both HDAC and receptor tyrosine kinases (RTK) (Zhang et al., 2013). RTK inhibitors (human epidermal growth factor receptor (HER) family) which includes erlotinib, gefitinib, and lapatinib consists of quinazoline moiety and used in treatment of solid tumor cancers (Fig. 18). The combination of the compounds belonging to the two classes resulted in growth arrest, differentiation, and apoptosis in cancer cells.
Diana and group synthesized and evaluated the isoindolo [2, 1-a]quinoxaline (Fig. 19) derivatives for their ability to bring about dual inhibition of tubulin polymerisation and topoisomerase (Diana et al., 2008). They successfully merged polycyclic nitrogen heterocycles with quinoxalines. Polycyclic nitrogen heterocycles such as anthracyclins, camptothecin, and amsacrine possessing planar structures have proven to be good pharmacophores owing to their ability to intercalate between the base-pairs of the double stranded DNA. These drugs form ternary complex between the drug, DNA, and enzyme and inhibit the activity of topoisomerase I and II. A large number of quinoxalines and quinoxalinones are reported to act either by inhibiting P-glycoprotein or the topoisomerases. The isoindoloquinoxalines displayed inhibitory potential in the micromolar to nanomolar range against a group of 60 human cancer cell lines. Further, polymerization assay carried out in in vitro revealed their microtubule depolymerizing ability.
Jin and coworkers employed the scaffold-based drug design (Fig. 20) for the production of dual inhibitors of Raf1 and JNK1 kinases as anticancer agents. Ability of the flavone scaffold to bring about the inhibition of MAPKs led to the synthesis of compound 6-ethyl-2′-chloro-4-aminoflavone. This compound was assessed as a low-affinity scaffold and was seen to inhibit 14 % p38-alpha activity at 100 mM (Merkel et al., 2013) and 27 % Raf1 activity at 50 mM (Merkel et al., 2013). Using this scaffold, the compounds were generated by merging 6-ethyl-2′-chloro-4′-aminoflavones and diphenylurea derivative. The synthesized compounds were evaluated for their activity against hepatocellular carcinoma. In addition, docking studies performed revealed their binding sites at Raf1 and JNK 1. The synthesized compounds were also found to be less toxic against normal liver cell-lines QSG7701 and HL7702 (Jin et al., 2013).
Class III: multi-targeted hybrids in cancer
The design of multi-targeted hybrids involves linking of pharmacophores possessing different modes of action supported by in silico approaches. This involves the development of rational methods which combine the different structural features from ligands to produce multi-targeted hybrids (Ai et al., 2012; Wang et al., 2013). The multitargeting approach also involves merging of different inhibitors that target a single specific target which could offer a successful means to treat certain diseases such as cancer, type 2 diabetes mellitus, and viral as well bacterial infections (Melisi et al., 2013).
Cai et al. presented the synthesis of series of novel CUDC-101(Jin et al., 2013; Zhang et al., 2014) compound (Fig. 21) and in the process identified multi-targeted hybrid 7-(4-(3-ethynylphenylamino)-7-methoxy quinazolin-6-yloxy)-N-hydroxyheptan-amide as a potential drug candidate. CUDC-101 possessed the potential to inhibit simultaneously three different targets comprising of HDAC, EGFR, and HER2. The synthesis involved the introduction of a HDACI moiety into the pharmacophore of the epidermal growth factor receptor (EGFR) and HER2 inhibitors. The results obtained as a consequence of the biological screening suggested that a single compound CUDC-101 shows promising in vitro inhibitory activity against HDAC, EGFR, and HER2 with their corresponding IC50 values in nanomolar ranges. Further this compound was observed to exhibit anti-proliferative activity to an extent greater than most of the HDACIs, in vitro. In vivo, CUDC-101 exhibited tumor growth inhibition in various cancer xenograft models such as non-small cell lung cancer (NSCLC), liver, breast, head, and neck, colon, and pancreatic cancers. Phase I study of CUDC-101 has recently been completed in patients with refractory solid tumors (Cai et al., 2010; Lai et al., 2010).
Mahboobi and coresearchers manifested a novel multi-targeting strategy in which the structural features of the Abl, PDGFR-β, and c-Kit inhibitors imatinib were combined with the HDAC inhibitory compounds. This resulted in synergistic inhibition of the different therapeutic targets as well as overcame resistance to imatinib. In addition, this group also reported a similar strategy which involved the merging of EGFR/HER2 kinase inhibitors with the inhibitors of HDAC class 1 or 2 enzymes. In the pursuit to achieve this, a lapatinib scaffold was linked to an HDAC inhibitory head group. This merging resulted in the anti-proliferative activity and proapoptotic, transcriptional reprogramming activity. Thus, hydroxamic acid and benzamide moieties were merged with 4-arylquinazoline core structure of lapatinib (Fig. 22) (Mahboobi et al., 2010).
Clinical trials
The above-mentioned approaches have become an area of interest as a number of agents of hybrid, dual, and multi-targeted pharmacophores are under clinical trials (Table 1). CUDC-907, a dual inhibitor of PI3 k and HDAC that has been discussed above is in phase II clinical trials for non-Hodgkin’s lymphoma and multiple myeloma. Further, the studies are conducted on its safety, toxicity, ADME, and biomarker activity. Other drugs in clinical trials are CUDC-101, PLX3397, and E3810. NO-ASA is a chimeric inhibitor consisting of NO and acetylsalicylic acid and had been proposed for chronic lymphocytic leukemia (CLL). Further, it has been studied for its use against prostate and colon cancer. The clinical trial has been halted due to genotoxicity caused by its benzyl nitrate metabolite (Fig. 23).
Curaxin is efficiently linked to the heterodimers of protein i.e., facilitating chromatin transcription (FACT) complex, which results in initiation of p53 activation and NF-κβ inhibition pathways (Fig. 24) ultimately resulting in death of cancer cells (Di Bussolo and Minutolo, 2011; Gasparian et al., 2011).
Metastatic breast cancer Phase I, Phase II clinical trials of PLX3397 for advanced castration-resistant prostate cancer (CRPC) has been completed. Further combination of PLX3397 and paclitaxel is in phase I study for advanced solid tumors and phase 1b study of PLX3397 in combination with vemurafenib in V600-mutated BRAF melanoma is under process (Lin et al., 2013). E-3810 is a multi-targeting inhibitor of VEGF receptor-1,-2, and -3 and fibroblast growth factor receptor-1 tyrosine kinases. This is presently in phase I clinical trials for solid tumors. Further clinical trials on combination of E-3810-paclitaxel have been completed recently (Bello et al., 2013; Molina et al., 2014). A phase I study to evaluate the safety, tolerability and ADME profile of orally administered CUDC-101 in cancer patients has been carried out and currently is in active stage. Further, Phase Ib expansion study for the safety, efficacy, and pharmacokinetics of intravenous CUDC-101 against advanced head and neck, gastric, breast, liver, and NSCLC tumors has been completed (Wang et al., 2013). Estramustine is the hybrid FDA approved drug for the prostate cancer (Fig. 25) (Caffo et al., 2010; Noguchi et al., 2010; Picus et al., 2011; Ravery et al., 2011). This is an antimicrotubular agent and combination of nitrogen mustard and estradiol. It is also named as estradiol 3-[bis(2-chloroethyl)carbamate]-17-(dihydrogen phosphate), disodium salt, monohydrate.
Conclusions
From the literature search, it is anticipated that anti-proliferative compounds emerged through either of the above-mentioned designs acting synergistically at target site(s), with increased selectivity, potency, lesser resistance, and side effects will definitely find their scope at the clinical level. This has been corroborated by the recently FDA approved estramustine and the drugs undergoing clinical trials. However, following issues need to be addressed or rectified when designing the hybrid, dual or multi-target inhibitors:
-
(a)
Physicochemical properties, drug interactions, manufacturing and regulatory challenges may come into picture and limit their uses which may be due to the reasons that these agents owing to their large size have high molecular mass and increased lipophilicity, thus violating Lipinski’s rule.
-
(b)
Molecular hybridization sometime fail to maintain balance between potency and safety as seen in case of NO-ASA, a chimeric inhibitor which was proposed for CLL, prostate and colon cancer. But the clinical trials have been halted due to genotoxicity which was also present in parent molecules.
-
(c)
Chemical stability of the compounds should be taken into consideration as they may get cleaved before they reach the site of action.
-
(d)
An understating and knowledge of various pathways, receptors, targets, and mediators involved in cross-talk of cancer cell signaling may definitely support in rationale designing of inhibitors.
Further, a lot of work has been done and is being carried out using hybrids of inhibitors of histone deacetylases, kinases, tubulins, hormones, etc. but other targets like chemokines and cellular pathways involving cross talks appear to be unexplored.
References and notes
Abogye EO, Kaliszczak MA (2012) Combination treatment comprising a HDAC6 inhibitor and an AKT Inhibitor. WO Patent 2,012,175,973
Ai T, Cui H, Chen L (2012) Multi-targeted histone deacetylase inhibitors in cancer therapy. Curr Med Chem 19:475–487
Ali MB (2014) Physiological mechanisms and adaptation strategies in plants under changing environment. Springer, London
Amaral C, Varela C, Borges M, da Silva ET, Roleira FM, Correia-da-Silva G, Teixeira N (2013) Steroidal aromatase inhibitors inhibit growth of hormone-dependent breast cancer cells by inducing cell cycle arrest and apoptosis. Apoptosis 18:1426–1436
Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard JC, Debled M, Spaeth D (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clinic Oncol 30:2718–2724
Bansal Y, Silakari O (2014) Multifunctional compounds: smart molecules for multifactorial diseases. Eur J Med Chem 76:31–42
Bastien D, Hanna R, Leblanc V, Asselin E, Bérube G (2013) Synthesis and preliminary in vitro biological evaluation of 7α-testosterone-chlorambucil hybrid designed for the treatment of prostate cancer. Eur J Med Chem 64:442–447
Baviskar AT, Banerjee UC, Gupta M, Singh R, Kumar S, Gupta MK, Kumar S, Raut SK, Khullar M, Singh S, Kumar R (2013) Synthesis of imine-pyrazolopyrimidinones and their mechanistic interventions on anticancer activity. Bioorg Med Chem 21:5782–5793
Bello E, Taraboletti G, Colella G, Zucchetti M, Forestieri D, Licandro SA, Berndt A, Richter P, D’Incalci M, Cavalletti E (2013) The tyrosine kinase inhibitor E-3810 combined with paclitaxel inhibits the growth of advanced-stage triple-negative breast cancer xenografts. Mol Cancer Ther 12:131–140
Belluti F, Fontana G, Bo LD, Carenini N, Giommarelli C, Zunino F (2010) Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: identification of novel proapoptotic agents. Bioorg Med Chem 18:3543–3550
Bolognesi ML (2013) Polypharmacology in a single drug: multitarget drugs. Curr Med Chem 20:1639–1645
Boorjian SA, Tindall DJ (2013) Management of Prostate Cancer. Springer, Berlin
Caffo O, Sava T, Comploj E, Giampaolo MA, Segati R (2010) Estramustine plus docetaxel as second-line therapy in patients with hormone-refractory prostate cancer resistant to docetaxel alone. Urol Oncol: Semin Ori 28:152–156
Cai X, Zhai H-X, Wang J, Forrester J, Qu H, Yin L, Lai C-J, Bao R, Qian C (2010) Discovery of 7-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a potent multi-acting HDAC, EGFR, and HER2 inhibitor for the treatment of cancer. J Med Chem 53:2000–2009
Center NC (2013) Cancer facts and figures
Chabner BA, Longo DL (2011). Wolters Kluwer Health
Chakraborty C (2013) Sirtuins family-recent development as a drug target for aging, metabolism, and age related diseases. Curr Drug Targets 14:666–675
Chang C, Lee S, Yeh S, Chang T (2013) Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast, and liver. Oncogene 1:1–10
Chauhan M, Kumar R (2013) Medicinal attributes of pyrazolo [3, 4-d] pyrimidines: a review. Bioorg Med Chem 21:5657–5668
Chen L, Pankiewicz KW (2007) Recent development of IMP dehydrogenase inhibitors for the treatment of cancer. Curr Opin Drug Discov Devel 10:403
Chen J-B, Chern T-R, Wei T–T, Chen C–C, Lin J-H, Fang J-M (2013) Design and synthesis of dual-action inhibitors targeting histone deacetylases and 3-Hydroxy-3-methylglutaryl coenzyme A reductase for cancer treatment. J Med Chem 56:3645–3655
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). Biochem Mol Niol Plants 24:1250–1318
Daniel C, Rauch C (2013) Molecular mechanisms of tumor cell resistance to chemotherapy. Springer, London
Delcuve GP, Khan DH, Davie JR (2013) Targeting class I histone deacetylases in cancer therapy. Expert Opin Ther Pat 17:29–41
Denny WA (2001) DNA minor groove alkylating agents. Curr Med Chem 8:533–544
Descoteaux C, Brasseur K, Leblanc V, Parent S, Asselin E, Berube G (2012) Design of novel tyrosine-nitrogen mustard hybrid molecules active against uterine, ovarian and breast cancer cell lines. Steroids 77:403–412
Di Bussolo V, Minutolo F (2011) Curaxins: a new family of non-genotoxic multitargeted anticancer agents. Chem Med Chem 6:2133–2136
Diana P, Martorana A, Barraja P, Montalbano A, Dattolo G, Cirrincione G, Dall’Acqua F, Salvador A, Vedaldi D, Basso G (2008) Isoindolo [2, 1-a] quinoxaline derivatives, novel potent antitumor agents with dual inhibition of tubulin polymerization and topoisomerase I. J Med Chem 51:2387–2399
Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham D, Lambert D (2009) Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br J Anaesth 103:38–49
Do TKT, Hadji-Minaglou F, Antoniotti S, Fernandez X (2014) Secondary metabolites isolation in natural products chemistry: comparison of two semipreparative chromatographic techniques (high pressure liquid chromatography and high performance thin-layer chromatography). J Chromatogr A 1325:256–260
Dun B, Sharma A, Teng Y, Liu H, Purohit S, Xu H, Zeng L, She J-X (2013) Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways. PLoS One 8:e81702
Fan P, Griffith OL, Agboke F, Anur P, Zou X, McDaniel RE, Creswell K, Kim SH, Katzenellenbogen JA, Gray JW (2013) c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res 73:4510–4520
Favia AD, Cavalli A, Masetti M, Carotti A, Recanatini M (2006) Three-dimensional model of the human aromatase enzyme and density functional parameterization of the iron-containing protoporphyrin IX for a molecular dynamics study of heme-cysteinato cytochromes. Protein Struct Funct Bioinfo 62:1074–1087
Flinn IW, Oki Y, Copland A, Fattaey A, Lai C-J, Laliberte R, Voi M, Berdeja JG (2013) A first-in-man phase 1 study of CUDC-907, a first-in-class chemically-designed dual inhibitor of PI3 K and HDAC in patients with refractory or relapsed lymphoma and multiple myeloma. Blood 122:4363
Folger O, Jerby L, Frezza C, Gottlieb E, Ruppin E, Shlomi T (2011) Predicting selective drug targets in cancer through metabolic networks. Mol Syst Biol 7:1–10
Fortin S, Berube G (2013) Advances in the development of hybrid anticancer drugs. Expert Opin Invest Drugs 8:1–19
Fukuda T, Goebel J, Thøgersen H, Maseck D, Cox S, Logan B, Sherbotie J, Seikaly M, Vinks AA (2011) Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol 51:309–320
Garcia-Beltran O, Areche C, Cassels BK, Cuca Suarez LE (2014) Coumarins isolated from Esenbeckia alata (rutaceae). Biochem Syst Ecol 52:38–40
Gasparian AV, Burkhart CA, Purmal AA, Brodsky L, Pal M, Saranadasa (2011) Curaxins: anticancer compounds that simultaneously suppress NF-kB and activate p53 by targeting fact. Sci Transl Med 3:1–12
Gediya LK, Njar VC (2009) Promise and challenges in drug discovery and development of hybrid anticancer drugs. Expert Opin Invest Drugs 4:1099–1111
Glander P, Hambach P, Liefeldt L, Budde K (2012) Inosine 5′-monophosphate dehydrogenase activity as a biomarker in the field of transplantation. Clin Chim Acta 413:1391–1397
Guerrant W, Patil V, Canzoneri JC, Oyelere AK (2012) Dual targeting of histone deacetylase and topoisomerase II with novel bifunctional inhibitors. J Med Chem 55:1465–1477
Gupta A, Saha P, Descoteaux C, Leblanc V, Asselin E, Berube G (2010) Design, synthesis and biological evaluation of estradiol–chlorambucil hybrids as anticancer agents. Bioorg Med Chem Lett 20:1614–1618
Hainaut P, Plymoth A (2013) Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol 25:50–51
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hanamura T, Niwa T, Nishikawa S, Konno H, Gohno T, Tazawa C, Kobayashi Y, Kurosumi M, Takei H, Yamaguchi Y (2013) Androgen metabolite-dependent growth of hormone receptor-positive breast cancer as a possible aromatase inhibitor-resistance mechanism. Breast Cancer Res Treat 139:731–740
Hernandez-Guzman FG, Higashiyama T, Pangborn W, Osawa Y, Ghosh D (2003) Structure of human estrone sulfatase suggests functional roles of membrane association. J Biol Chem 278:22989–22997
Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, de Esch IJ, Richel D, Wijtmans M (2007) Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem 50:2424–2431
Ji Y, Li K, Xiao X, Zheng S, Xu T, Chen S (2012) Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg 47:2216–2223
Jin F, Gao D, Zhang C, Liu F, Chu B, Chen Y, Chen YZ, Tan C, Jiang Y (2013) Exploration of 1-(3-chloro-4-(4-oxo-4H-chromen-2-yl) phenyl)-3-phenylurea derivatives as selective dual inhibitors of Raf1 and JNK1 kinases for anti-tumor treatment. Bioorg Med Chem 21:824–831
Johnson CA, Padget K, Austin CA, Turner BM (2001) Deacetylase activity associates with topoisomerase II and is necessary for etoposide-induced apoptosis. J Biol Chem 276:4539–4542
Khan S, Lopez-Dee Z, Kumar R, Ling J (2013) Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Lett 337:90–95
Kiselev KV (2011) Perspectives for production and application of resveratrol. Appl Microbiol Biotechnol 90:417–425
Ko KS, Steffey ME, Brandvold KR, Soellner MB (2013) Development of a chimeric c-Src kinase and HDAC inhibitor. ACS Med Chem Lett 4:779–783
Lai C-J, Bao R, Tao X, Wang J, Atoyan R, Qu H, Wang D-G, Yin L, Samson M, Forrester J (2010) CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res 70:3647–3656
Lin K, Lasater E, Stewart W, Damon LE, Kasarskis A, Bashir A, Pendleton M, Sebra R, Perl AE, Le MH (2013) Preclinical and clinical resistance mechanisms to the investigational selective FLT3 inhibitor PLX3397 in FLT3-ITD + acute myeloid leukemia (AML). Blood 122:3938
Lipinski CA (2004) Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Tech 1:337–341
Lunardi A, Ala U, Epping MT, Salmena L, Clohessy JG, Webster KA, Wang G, Mazzucchelli R, Bianconi M, Stack EC (2013) A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat Genet 45:747–755
Mahboobi S, Sellmer A, Winkler M, Eichhorn E, Pongratz H, Ciossek T, Baer T, Maier T, Beckers T (2010) Novel chimeric histone deacetylase inhibitors: a series of lapatinib hybrides as potent inhibitors of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and histone deacetylase activity. J Med Chem 53:8546–8555
Mahoney S, Arfuso F, Rogers P, Hisheh S, Brown D, Millward M, Dharmarajan A (2012) Cytotoxic effects of the novel isoflavone, phenoxodiol, on prostate cancer cell lines. J Biosci 37:73–84
Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN (2004) Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem Suppl 92:223–237
Marks PA (2010) The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Invest Drugs 19:1049–1066
Melisi D, Piro G, Tamburrino A, Carbone C, Tortora G (2013) Rationale and clinical use of multitargeting anticancer agents. Curr Opin Pharmacol 13:536–542
Merkel D, Filanovsky K, Gafter-Gvili A, Vidal L, Aviv A, Gatt ME, Silbershatz I, Herishanu Y, Arad A, Tadmor T (2013) Predicting infections in high-risk patients with myelodysplastic syndrome/acute myeloid leukemia treated with azacitidine: aretrospective multicenter study. Am J Hematol 88:130–134
Messina M, Barnes S (1991) The role of soy products in reducing risk of cancer. J Natl Cancer Inst 83:541–546
Meunier B (2007) Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res 41:69–77
Molina AM, Hutson TE, Larkin J, Gold AM, Wood K, Carter D, Motzer R, Michaelson MD (2014) A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC). Cancer Chemother Pharmacol 73:181–189
Morgentaler A (2006) Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol 50:935–939
Morphy R, Rankovic Z (2005) Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 48:6523–6543
Nepali K, Sharma S, Sharma M, Bedi P, Dhar K (2014) Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem 77:422–487
Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, Moriya F, Suekane S, Matsuoka K, Komatsu N (2010) A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 59:1001–1009
Nussbaumer P, Billich A (2004) Steroid sulfatase inhibitors. Med Res Rev 24:529–576
Pandeya SN, Smitha S, Jyoti M, Sridhar SK (2005) Biological activities of isatin and its derivatives. Acta Pharm 55:27–46
Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, Knight Z, Shokat K, Azar N, Viguie F (2008) PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia 22:1698–1706
Pastor N, Domínguez I, Orta ML, Campanella C, Mateos S, Cortés F (2012) The DNA topoisomerase II catalytic inhibitor merbarone is genotoxic and induces endoreduplication. Mutat Res-Fund Mol M 738–739:45–51
Patrick GL (2009) An introduction to medicinal chemistry. Oxford University Press Inc, New York
Picus J, Halabi S, Kelly WK, Vogelzang NJ, Whang YE, Kaplan EB, Stadler WM, Small EJ (2011) A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer. Cancer 117:526–533
Polynesia (2012) UMU applied for screening herb and plant extracts or pure phytochemicals for antimutagenic activity. Pharma Biol 50:537–610
Proudfoot JR (2005) The evolution of synthetic oral drug properties. Bioorg Med Chem Lett 15:1087–1090
Pursell N, Ma A, Atoyan R, Samson M, Borek M, DellaRocca S, Yin L, Wang D-g, Zifcak B, Xu G-x (2013) CUDC-907, a Dual HDAC and PI3 K inhibitor, potentially targets cancer cells and the microenvironment in hematological malignancies. Blood 122:4930
Rangwala S, Zhang C, Duvic M (2012) HDAC inhibitors for the treatment of cutaneous T-cell lymphomas. Futur Med Chem 4:471–486
Ravery V, Fizazi K, Oudard S, Drouet L, Eymard JC, Culine S, Gravis G, Hennequin C, Zerbib M (2011) The use of estramustine phosphate in the modern management of advanced prostate cancer. BJU Int 108:1782–1786
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA-Cancer J Clin 62:10–29
Singer AC, Crowley DE, Thompson IP (2003) Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotech 21:123–130
Sintchak MD, Nimmesgern E (2000) The structure of inosine 5′-monophosphate dehydrogenase and the design of novel inhibitors. Immunopharmacology 47:163–184
Solomon VR, Hu C, Lee H (2009) Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 17:7585–7592
Solomon VR, Hu C, Lee H (2010) Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach. Bioorg Med Chem 18:1563–1572
Stanway SJ, Delavault P, Purohit A, Woo LL, Thurieau C, Potter BV, Reed MJ (2007) Steroid sulfatase: a new target for the endocrine therapy of breast cancer. Oncologist 12:370–374
Su B, Zhong B, Chennamaneni S, lama R, Yi X, Geldenhuys WJ, Pink JJ, Dowlati A, Xu Y, Zhou A (2013) Synthesis and anti-cancer mechanism investigation of dual Hsp27 and tubulin inhibitors. J Med Chem 56:5306–5320
Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H (2003) Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res 63:2762–2770
Takebe N, Cheng X, Fandy TE, Srivastava RK, Wu S, Shankar S, Bauer K, Shaughnessy J, Tricot G (2006) IMP dehydrogenase inhibitor mycophenolate mofetil induces caspase-dependent apoptosis and cell cycle inhibition in multiple myeloma cells. Mol Cancer Ther 5:457–466
Tan J, Cang S, Ma Y, Petrillo RL, Liu D (2010) Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol 3:1–13
Thurn KT, Thomas S, Moore A, Munster PN (2011) Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol 7:263–283
Tili E, Michaille J–J (2011) Resveratrol, microRNAs, inflammation, and cancer. J Nucleic Acids 2011:1–9
Tsai S-C, Valkov N, Yang W-M, Gump J, Sullivan D, Seto E (2000) Histone deacetylase interacts directly with DNA topoisomerase II. Nat Genet 26:349–353
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623
Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852
Wang JC (1996) DNA topoisomerases. Annu Rev Biochem 65:635–692
Wang J, Pursell NW, Samson MES, Atoyan R, Ma AW, Selmi A, Xu W, Cai X, Voi M, Savagner P (2013) Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion. Mol Cancer Ther 12:925–936
Wermuth C-G, Ganellin CR, Lindberg P, Mitscher LA (1998) Glossary of terms used in medicinal chemistry (IUPAC recommendations 1997). Annu Rep Med Chem 33:385–395
Woo LL, Sutcliffe OB, Bubert C, Grasso A, Chander SK, Purohit A, Reed MJ, Potter BV (2003) First dual aromatase-steroid sulfatase inhibitors. J Med Chem 46:3193–3196
Woo LL, Bubert C, Sutcliffe OB, Smith A, Chander SK, Mahon MF, Purohit A, Reed MJ, Potter BV (2007) Dual aromatase-steroid sulfatase inhibitors. J Med Chem 50:3540–3560
Wood PM, Woo L, Humphreys A, Chander SK, Purohit A, Reed MJ, Potter BV (2005) A letrozole-based dual aromatase–sulphatase inhibitor with in vivo activity. J Steroid Biochem Mol Biol 94:123–130
Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS (1996) Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest 98:2558
Xiao CF, Tao LY, Sun HY, Wei W, Chen Y, Fu LW, Zou Y (2010) Design, synthesis and antitumor activity of a series of novel coumarin-stilbene hybrids, the 3-arylcoumarins. Chin Chem Lett 21:1295–1298
Yance D (2010) Cancer and Inflammation: the emerging role of botanical compounds in targeting proinflammatory pathways, with particular attention to the NFκB signaling pathway. Diakses Tanggal 6:1–22
Yang S-Y (2010) Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Discov Today 15:444–450
Yee EM, Pasquier E, Iskander G, Wood K, Black DS, Kumar N (2013) Synthesis of novel isoflavene-propranolol hybrids as anti-tumor agents. Bioorg Med Chem 21:1652–1660
Zhang M-Q, Wilkinson B (2007) Drug discovery beyond the ‘rule-of-five’. Curr Opin Biotech 18:478–488
Zhang X, Su M, Chen Y, Li J, Lu W (2013) The design and synthesis of a new class of RTK/HDAC dual-targeted inhibitors. Molecules 18:6491–6503
Zhang Q, Wen C, Xiang Z, Ma J, Wang X (2014) Determination of CUDC-101 in rat plasma by liquid chromatography mass spectrometry and its application to a pharmacokinetic study. J Pharm Biomed Anal 90:134–138
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39:74–88
Acknowledgments
Authors are thankful to DST Grant (SR/FT/CS-71/2011) for the financial support to the present work. We are also thankful to Prof. P. Ramarao, Dean Academic Affairs, and Acting Vice Chancellor, Central University of Punjab, Bathinda for his constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, A., Alex, J.M., Chauhan, M. et al. A review on pharmacophoric designs of antiproliferative agents. Med Chem Res 24, 903–920 (2015). https://doi.org/10.1007/s00044-014-1196-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1196-5